Aulacophora barrogae Reid, Halling & Beatson, 2021
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4932.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:95612386-B43D-44DB-A9A0-D1637F854C81 |
|
DOI |
https://doi.org/10.5281/zenodo.4678614 |
|
persistent identifier |
https://treatment.plazi.org/id/104DCAC7-BF6F-4008-A6FD-933A4C9D6A8C |
|
taxon LSID |
lsid:zoobank.org:act:104DCAC7-BF6F-4008-A6FD-933A4C9D6A8C |
|
treatment provided by |
Plazi |
|
scientific name |
Aulacophora barrogae Reid, Halling & Beatson |
| status |
sp. nov. |
Aulacophora barrogae Reid, Halling & Beatson , sp. nov.
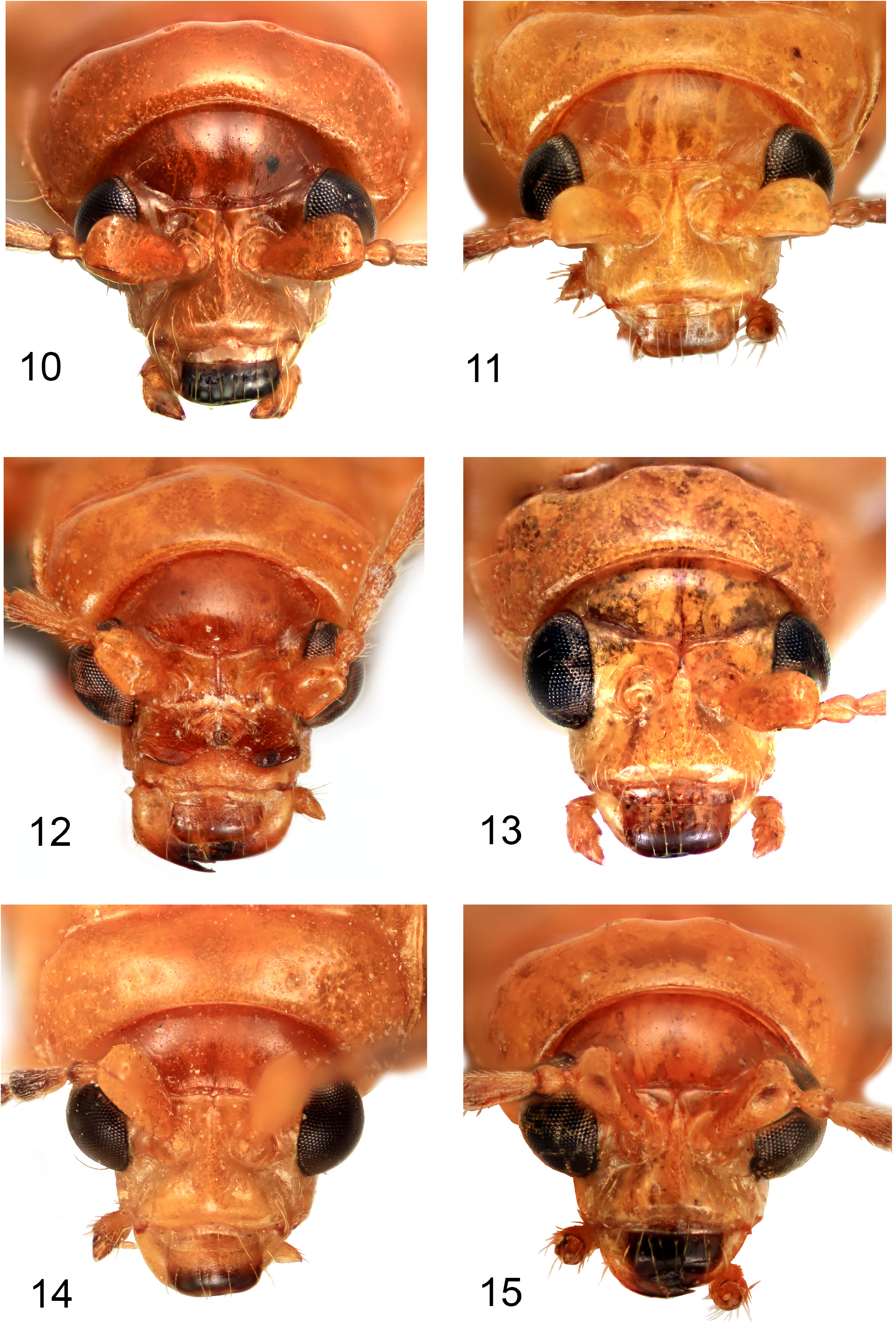
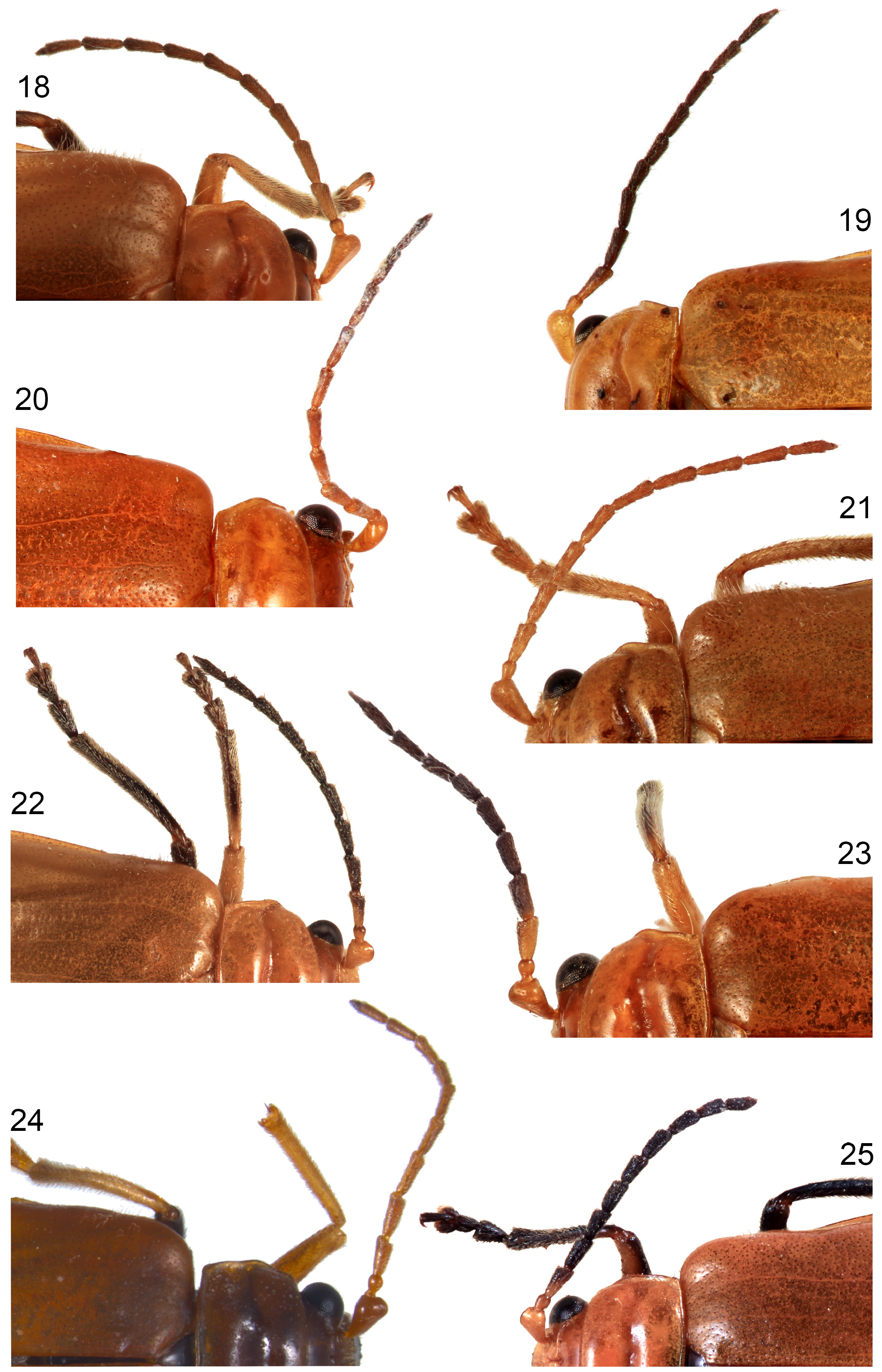
( Figs 3 View FIGURES 2–5 , 11 View FIGURES 10–15 , 19 View FIGURES 18–25 , 27 View FIGURES 26–31 , 36 View FIGURES 32–37 , 47 View FIGURES 44–49 , 85 View FIGURES 83–88 , 98 View FIGURES 98–101 , 112 View FIGURES 112–115 , 125 View FIGURES 125–128 , 139 View FIGURES 135–142 , 153 View FIGURES 150–155 , 168 View FIGURES 164–172 , 183 View FIGURES 182–183 ) http://zoobank.org/ urn:lsid:zoobank.org:act:104DCAC7-BF6F-4008-A6FD-933A4C9D6A8C
Material examined. Types. Holotype: ♁*/ Needle Rock Fls [Flats?], WA, 15:29S 124:29E, 5.iv.1992, N Scullion, J Collins, hand collected/ Chrysomelidae Rhapidiopalpa palmerstoni / Holotype Aulacophora barrogae Reid et al. / ( AMS); Paratypes (3): Australia: 1♁, 1♀ / Port Darwin, N Territory/ Aulacophora palmerstoni Blk, N Territory / on permanent loan from Macleay Museum, University of Sydney/ Paratype Aulacophora barrogae Reid et al. / ( ANIC); 1♀ / Calvert Exped 1896 Fitzroy & Margaret R[iver]s/ Paratype Aulacophora barrogae Reid et al. / ( SAM).
Description. Colour ( Fig. 3 View FIGURES 2–5 ). Head brownish-yellow, except apical half of labrum brown; extreme apices of mandibles dark brown; antennomeres 4–11 dark brown to black, 3 outer edge and apex dark brown to black, 2 yellowish-brown, 1 yellow; pronotum and elytra entirely brownish-yellow; venter of prothorax entirely brownish-yellow; scutellum brownish-yellow; mesanepisternum, mesepimeron and mesoventrite brownish-yellow; metaventrite yellowish-brown with dark brown posterolateral patches or dark brown with yellowish anterior margin; procoxae, mesocoxae and metacoxae brownish-yellow; profemora brownish-yellow; mesofemora brownish-yellow; metafemora yellowish-brown with apical 2/3 or less brown; protibiae inner face brownish-yellow, outer face with dark brown, meso- and metatibiae dark brown with paler bases; protarsi brown, meso- and metatarsi dark brown; tergites brown with yellow margins, pygidium yellowish-brown; abdominal ventrites 1–4 yellowish-brown with dark brown apical margins, ventrite 5 brownish-yellow, with laterobasal brown patches or base narrowly brown.
Male: length 6.5–7 mm; frontoclypeus without arcuate ridges or densely setose patches; first antennomere expanded, oval flat area in apical half defined by sharp ridge; antennae about 0.6x body length; antennomere 2 shortest, about one third length of 1, antennomere 1 longest, comparative lengths: 1>11>4=6>3=5=7=8=9=10>2; length antennomere 5 about 2.5x width; antennomeres 3–7 slightly expanded to apices; antennomeres 3–11 each with only 1–4 erect lateral setae; pronotal transverse depression posteriorly arcuate, deep and broad at middle; in lateral view anterior half of pronotum slightly less convex than posterior half and median depression with anterior slope shallower than posterior slope; without pair of large pits anterior to transverse groove; elytra shining, shallowly microreticulate; elytral humeri with small patch of 10–15 laterally directed erect setae (may be broken off); apical lobe of ventrite V symmetrically sculptured, cavity bounded by a thin ridges on either side; elongate cavity deepened from base almost to apex and deepest on midline, apically bounded by an almost vertical wall; tergite VIII pale brown, strap-like, medially acutely produced (more so in Port Darwin specimen than Needle Rock specimen), slightly membranous on midline, without lateral lobes; penis thick & angularly bent in lateral view with minute ventral hook at tip and sharp tubercle on basal half of dorsal surface; sides penis not conspicuously punctured, smooth and unridged; penis broad and only slightly asymmetric in dorsal view, almost evenly attenuated from middle to acute apex; membranous area about 2/3 penis length.
Female as male, except: length 7–8 mm; antennomeres slightly thinner than male, length antennomere 5 about 2.5x width, length antennomere 8 about 2.5x width; transverse pronotal depression shallower than male but relatively deep at sides compared with all other species; elytral without setal patch; pygidium apically swollen and extended, faintly apically medially ridged; apex pygidium in dorsal view narrowly produced as an almost truncate lobe with a minute median tubercle; pygidial apex in lateral view flat but thick ventrally, with almost straight sides and without tubercle; venter of pygidial apex deeply concave; apex ventrite V unevenly shallowly concave; vaginal palpi broadly elongate ovate, length about 2x width, with 8 pairs of setae in apical half; basal apodemes sinuate, about 0.5 mm long; sternite VIII with tignum separated from weakly sclerotised posterior margin of the sternite by a transparent membranous area, and posterior margin feebly concave, not produced; tignum 1.3mm long, kinked, apex membranous, slightly expanded, not separated from shaft by a band of deeper pigmentation; spermatheca falcate, collum abruptly demarkated from receptaculum, reflexed relative to receptaculum, insertion point of gland (ramus) slightly produced; receptaculum strongly hook-shaped with curved interior bend and large beak-like appendix.
Diagnosis. Male: without paired glands on pronotal disc ( Fig. 11 View FIGURES 10–15 ), pronotal depression broad and deep ( Fig. 26 View FIGURES 26–31 ), humeral setal patch present ( Fig. 3 View FIGURES 2–5 ), scutellum pale ( Fig. 3 View FIGURES 2–5 ), tergite 8 medially lobed ( Fig. 85 View FIGURES 83–88 ), penis smooth sided with acutely attenuated apex ( Fig. 98 View FIGURES 98–101 ) and minute median tooth in lateral view ( Fig. 125 View FIGURES 125–128 ). Female: frontoclypeus medially keeled (as male, Fig. 11 View FIGURES 10–15 ), antennomeres 1–3 pale and 4–11 dark brown to black ( Fig. 19 View FIGURES 18–25 ), scutellum pale, ventrite 5 yellow except laterobasal dark patches ( Fig. 47 View FIGURES 44–49 ), pygidium wholly brownish-yellow with rounded apex ( Fig. 47 View FIGURES 44–49 ), apical margin of ventrite 5 shallowly concave ( Fig. 47 View FIGURES 44–49 ).
Notes. All four known specimens are old and/or damaged. The Needle Rock specimen is missing three legs (one of each pair) and has a large peck mark on the pronotum (we note that peck marks from birds are common on specimens of PPB). The Port Darwin specimens have been affected by mould in the past and were originally pinned.
All localities for this species are somewhat problematic. Needle Rock is a sea stack on the coast of a remote corner of the Kunmunya Aboriginal Reserve, Kimberley Region, Western Australia. This area is only accessible by boat or seaplane. There is nowhere named Needle Rock Flats that we are aware of, but co-ordinates for the single specimen from this locality indicate a low plateau of 90m elevation, about 500m south of the coastline near Needle Rock.
The two specimens labelled Port Darwin, originally from Macleay Museum, were almost certainly collected by Edward Spalding in 1877. Spalding was employed as a collector in the Port Darwin area by WJ Macleay, from May to September 1877 (Musgrave 1932; Rob Blackburn, pers. com. May 2017). Although labelled A. palmerstoni and removed to ANIC as putative type material there is no evidence that they formed part of the syntypic series of that species ( Blackburn 1888). The Calvert Expedition specimens in SAM were collected by the naturalist George Keartland in the vicinity of the modern town of Fitzroy Crossing, at the junction of the Fitzroy and Margaret Rivers, in late 1896, after material collected earlier on the expedition had been dumped in the desert ( Hill 1905).
Etymology. Named for the Philippine entomologist Grace Barroga, to honour her pioneering work on this difficult genus.
Distribution ( Fig. 183 View FIGURES 182–183 ) and biology. Aulacophora barrogae is known from three widely separated localities in northern Australia, from the western Kimberley in Western Australia to the Darwin region of Northern Territory. Only one site, on the semi-arid Kimberley coast, has detailed collecton information. All sites are dominated by savannah woodland with rainfall restricted to the summer months. The distribution of A. barrogae is similar to that of the endemic cucurbit Cucumis umbellatus ( Telford et al. 2011) and it possible that this species is a host. The distribution of A. barrogae overlaps with that of A. relicta and the two may possibly be found together.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.