Aulacophora Chevrolat, 1836
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4932.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:95612386-B43D-44DB-A9A0-D1637F854C81 |
|
DOI |
https://doi.org/10.5281/zenodo.4678579 |
|
persistent identifier |
https://treatment.plazi.org/id/41532456-3F56-E540-708F-C43CFA554131 |
|
treatment provided by |
Plazi |
|
scientific name |
Aulacophora Chevrolat, 1836 |
| status |
|
Aulacophora Chevrolat, 1836 View in CoL
Aulacophora Chevrolat, 1836: 378 View in CoL ; Maulik 1936: 167; Wilcox 1972: 221; Anand & Cox 1986: 81; Barroga & Mohamedsaid 2002; Lee & Beenen 2015
= Raphidopalpa Chevrolat, 1836: 378 ; Gemminger & Harold 1876 (synonymy)
= Rhaphidopalpa Rosenhauer, 1856: 327 ; Hincks 1949: 621 (synonymy)
= Acutipalpa Rosenhauer, 1856: 327 ; Joannis 1866: 99 (synonymy)
= Ceratia Chapuis, 1876: 100 View in CoL ; Maulik 1936: 167 (synonymy)
= Triaplatys Fairmaire, 1879: 113 ; Weise 1924: 9 (synonymy)
= Orthaulaca Weise, 1892: 396 ; Maulik 1936: 167 (synonymy)
= Cerania Weise, 1892: 396 ; Maulik 1936: 167 (synonymy)
= Sphaerarthra Weise, 1896: 396 ; Maulik 1936: 167 (synonymy)
= Pachypalpa Weise, 1896: 392 ; Maulik 1936: 167 (synonymy)
= Paraulacophora Csiki, 1953: 131 ; Wilcox 1972: 222 (synonymy)
Type species: Galeruca quadraria Olivier, 1808 , by subsequent designation ( Duponchel & Chevrolat 1842). This name is currently considered a junior synonym of the south-east Asian species A. analis (Weber, 1801) ( Lee & Beenen 2015) .
Diagnostic description
Generic diagnosis. The following diagnostic description is based on the Australopapuan species and the generic redefinition by Barroga and Mohamedsaid (2002).
Body form elongate pear-shaped in dorsal view, length about 2x width, head, prothorax and elytra at shoulders varying slightly in proportions but generally successively wider by a factor of 1.5; dorsally sparsely and finely punctured (interspaces> puncture diameters), except pronotal anterolateral patch of secretory pits, and dorsally glabrous except trichobothria on frons and pronotal angles, semi-erect setae on clypeus, short erect setae on elytral lateral margins and some males with patch of setae posterior to elytral humeri; closely setose ventrally and on appendages.
Head. Vertex smooth and impunctate, except a single trichobothrium above each eye, without longitudinal median groove; row of five pairs of long setae anterior to antennal sockets; frontoclypeus medially ridged and elevated from between antennal sockets towards anterior; anterior edge frontoclypeus truncate, shallowly concave or medially excavate; interantennal space 0.5–1.0x socket diameter, sockets level with anterior half of eyes and separated from them by 0.5–0.75x socket widths; eyes large and laterally prominent, width of head across eyes about 1.5x width of head at anterior eye margins; gena 0.5–0.7x eye length; postantennal calli distinct, slightly convex and approximately triangular, divided by short median groove, bases truncate and defined by deep postcallal groove extending laterally to posterior edge of eyes, sides defined by rim of antennal sockets, internal margins defined by grooves at base of frontoclypeal ridge; labrum with four pairs of discal setae, apical margin truncate; antennae 0.5–0.8x body length; all antennomeres elongate, 3–11 narrow and of similar width except some may be expanded in males; apical maxillary palpomere conical, shorter and narrower than preapical.
Thorax. Pronotum transverse in dorsal view, widest anterior to middle, basal 2/3 straight edged and slightly contracted to base, anterior 1/3 strongly contracted to obtuse apical angles; pronotal lateral margins sinuate in lateral view; all pronotal sides with raised margins, margination broadest laterally and narrowest anteriorly (may be partly absent); anterior and posterior angles each with a trichobothrium, posterior angles about 90º; pronotum with transverse or sinuate groove at middle; prosternal process triangular, tapering to a thin elevated blade which terminates at about half length of procoxae; procoxae conical; procoxal cavities externally wide open, gap about length of hypomeral process; scutellum flat, approximately triangular with rounded apex; elytra usually at least slightly expanded from base to about 2/3 length, with rounded apex, often leaving pygidium partially exposed; elytra nonstriate, non-carinate, with or without transverse posthumeral depression; epipleura broad at base, abruptly contracted between meso and metacoxae and not defined beyond this point; fully winged; mesoventrite not covered by metaventrite, apex acutely pointed and base without procoxal rests; mesocoxae separated by much less than width of coxa; femora almost parallel-sided; tibiae with or without single apical spur, if present, length about half or slightly less than apical width of tibia metatarsus about 0.8x as long as metatibia; first metatarsomere as long as or slightly longer than metatarsomeres 2+3; tarsal claws bifid, inner teeth convergent and about 2/3 as long as outer teeth.
Abdomen. Ventrites not laterally bordered by raised bead; ventrite I with rounded to broadly triangular intercoxal process; apex of male ventrite V with rectangular lobe defined on either side by slots; apex of female ventrite V truncate or concave; penis symmetrical or slightly asymmetric, apex entire, base without recurved lobes; ovipositor with well-developed apodeme (tignum) at base of sternite VIII; palpi one-segmented; spermathecal receptaculum falcate or C-shaped, transversely grooved at base and with swollen collum at insertion of duct and gland.
Diagnosis of the Aulacophora indica species-complex
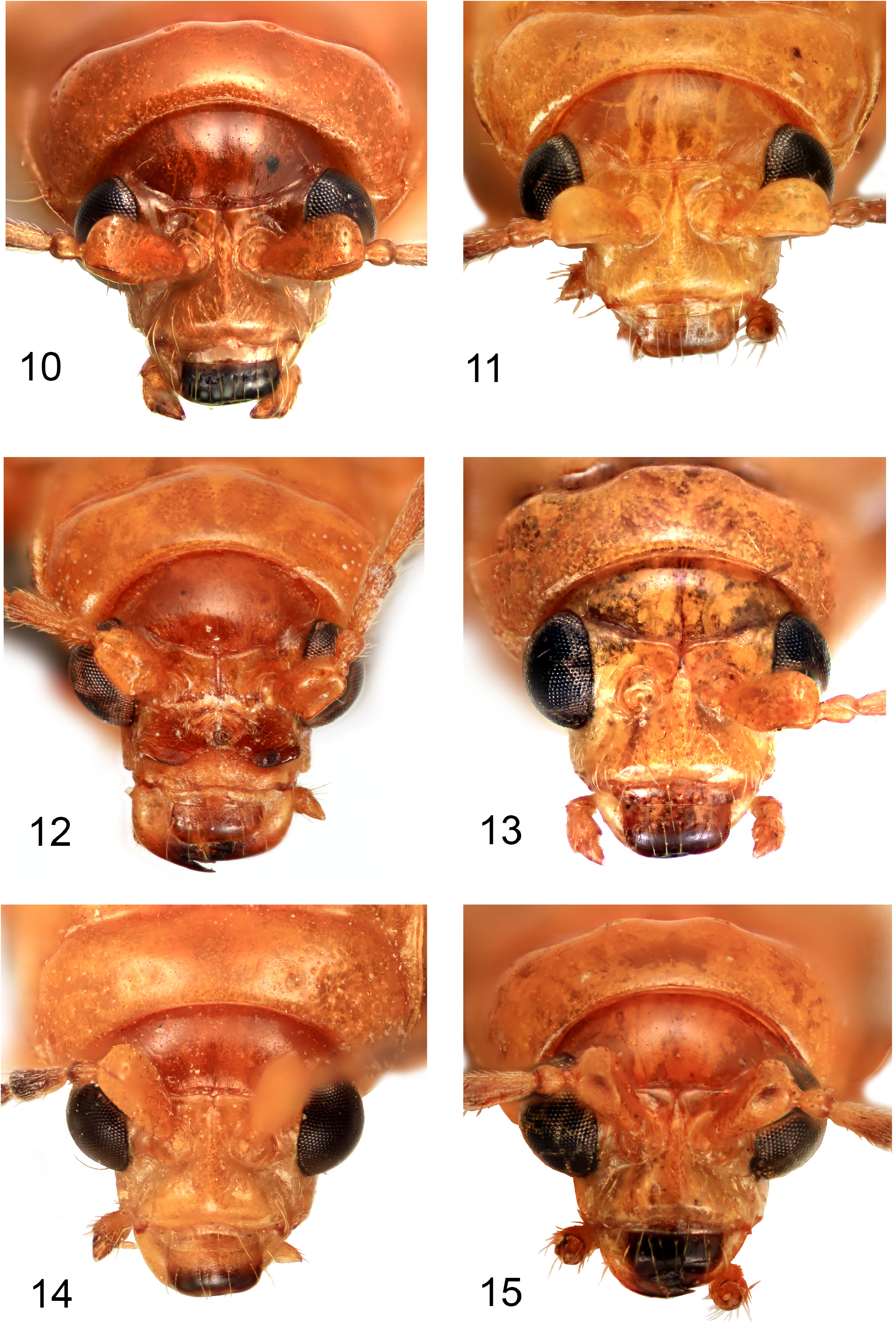
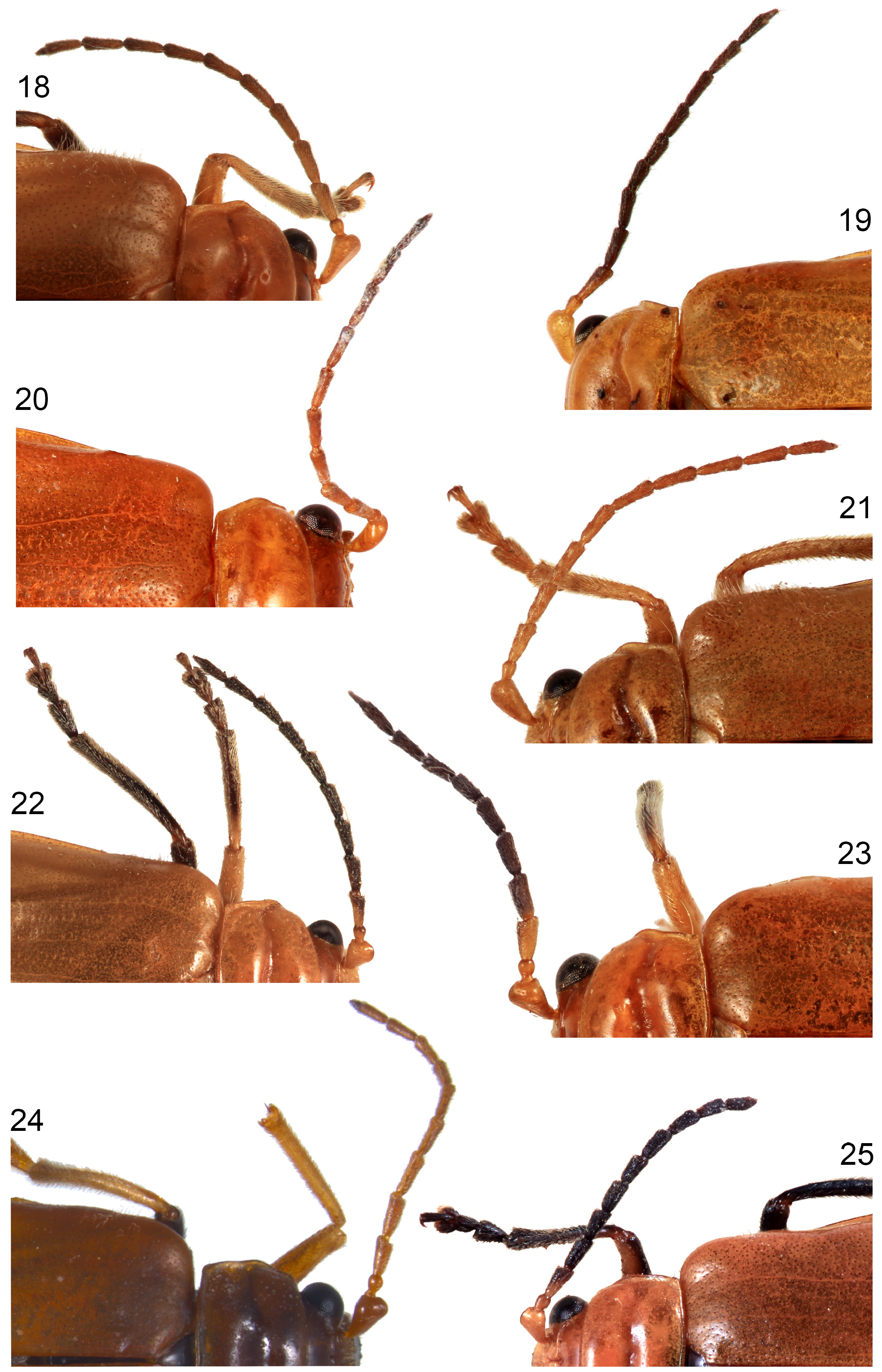
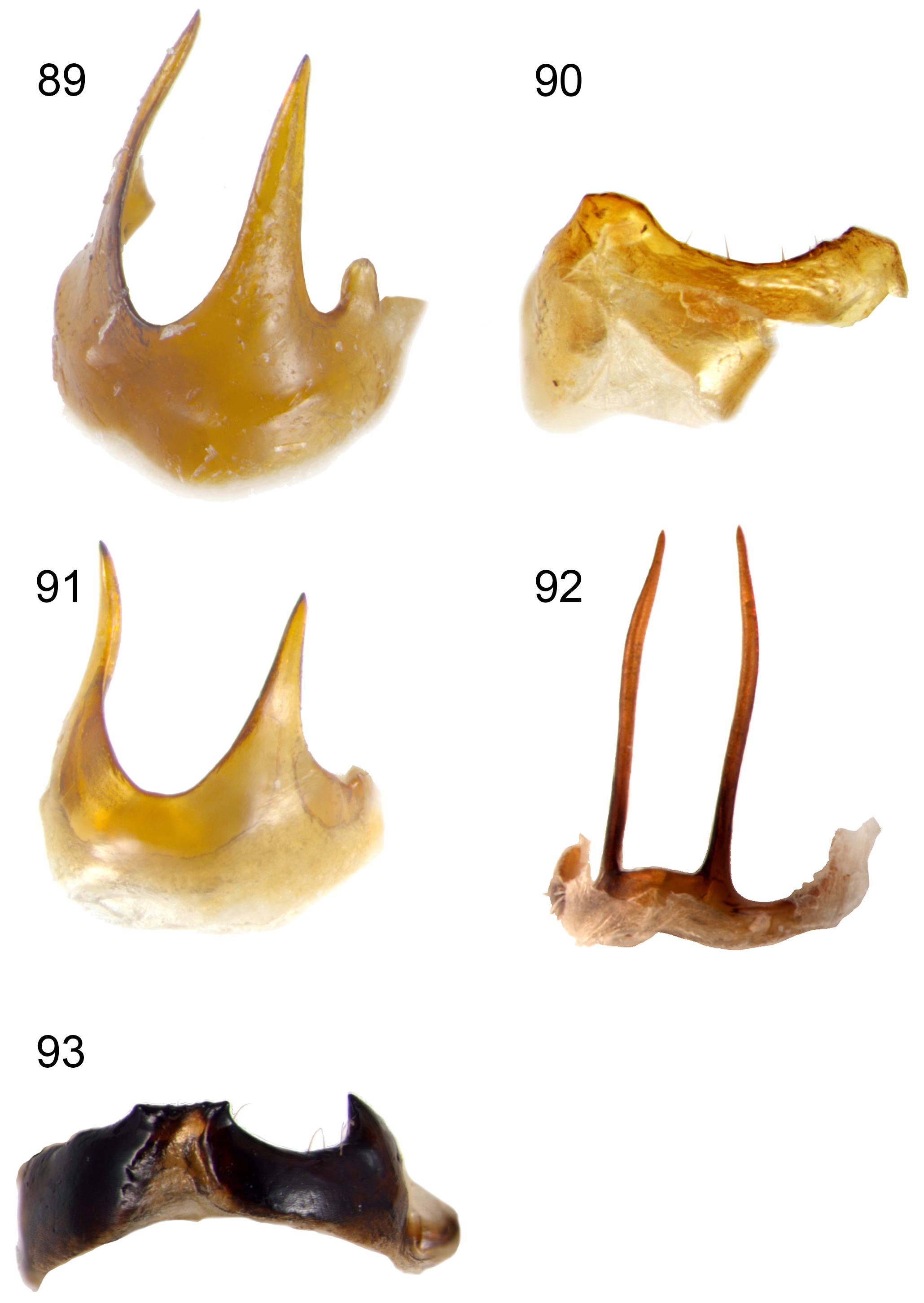
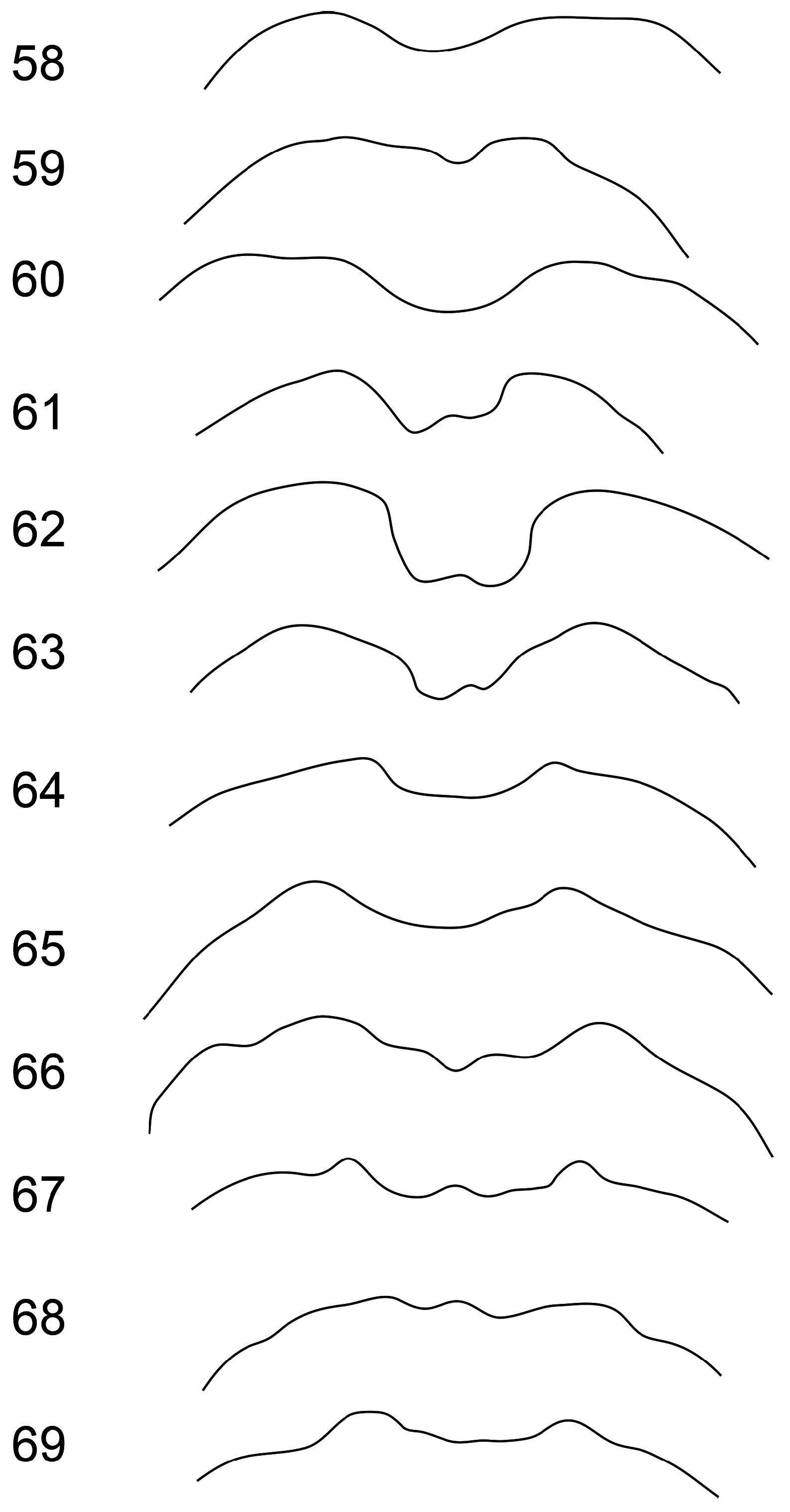
Head capsule, pronotum and elytra entirely pale (brownish-yellow when dead, orange or red when alive) ( Figs 2–9 View FIGURES 2–5 View FIGURES 6–9 ), one Japanese species, A. bipunctata ( Olivier, 1808) , with small black spots; male first antennomere approximately pyramidal in shape, expanded from base to middle, with a flat area on most of its anterior face ( Figs 10–17 View FIGURES 10–15 View FIGURES 16–17 ); male antennomeres 3–11 not modified ( Figs 18–25 View FIGURES 18–25 ); male with deeper transverse prothoracic groove than female ( Figs 28–29 View FIGURES 26–31 ); elytra without posthumeral transverse depression, males of some species with lateral patch of erect setae posterior to humeri ( Figs 18–25 View FIGURES 18–25 ); all tibiae with single apical spur ( Fig. 24 View FIGURES 18–25 ); female pygidium usually elongated beyond apex of apical ventrite ( Figs 44–57 View FIGURES 44–49 View FIGURES 50–55 View FIGURES 56–57 ); apex of male last ventrite with elongate-oval cavity on elongate-rectangular middle lobe, which is defined on either side by narrow slots ( Figs 32–43 View FIGURES 32–37 View FIGURES 38–43 ); male tergite VIII thickened and modified into either a transverse bar or a pair of posteriorly directed prongs ( Figs 83–93 View FIGURES 83–88 View FIGURES 89–93 ); penis asymmetricin dorsal view, except A. wallacii ( Figs 94–108 View FIGURES 94–97 View FIGURES 98–101 View FIGURES 102–105 View FIGURES 106–107 View FIGURES 108–111 ). Hostplants where known are predominantly cucurbits ( Cucurbitaceae ) but may also include legumes ( Fabaceae ).
The group of species designated here as the Pale Pumpkin Beetles (PPB) or A. indica species-complex (Appendix 1) is a group of externally similar species that are difficult to separate. The following described species belong in this complex, based on examination of material, or descriptions: A. abdominalis , A. bipunctata , A. cornuta , A. foveicollis ( Lucas, 1849) , A. indica , A. kotoensis Chujo, 1962 , A. relicta , A. sulaksonoi Mohamedsaid, 2009 , A. wallacii and A. wilsoni . Although the Javanese endemic A. bipunctata has small elytral spots, it otherwise conforms to the definition given above and its male genitalia are similar to those of A. indica ( Barroga & Mohamedsaid 2002) .
The A. indica species-complex is approximately equivalent to the genus Rhaphidopalpa Rosenhauer, 1856 , as defined by Weise (1892) using male and female secondary sexual characters. By including only males with setose humeri, Rhaphidopalpa was constrained to be a smaller group than the definition of PPB included here. Weise erroneously abandoned the name Aulacophora because it had been used for a plant genus, described A. indica under five different names in Rhaphidopalpa ( Barroga & Mohamedsaid 2002; Lee & Beenen 2015), and at the same time made this species (under a sixth name, A. similis ( Olivier, 1808)) the type of his new genus, Orthaulaca Weise, 1892 . Using the current nomenclature for all the named species he listed, Weise’s Rhaphidopalpa only definitely included A. foveicollis and A. indica .
In a morphological analysis of the nearby Sunda fauna, Barroga & Mohamedsaid (2006) identified a clade which included A. indica and A. cornuta , together with A. bipunctata , A. flavomarginata , A. lewisi Baly, 1886 and A. mouhoti Baly, 1886 , defined by the thickening of male tergite VIII. Aulacophora mouhoti , described from the Malay Peninsula and Vietnam, is a poorly understood pale species defined by a variable female secondary sexual character and apparently only reliably known from females ( Baly 1886; Barroga & Mohamedsaid 2002, 2006). We consider it unlikely to be a distinct species and as it is outside the region of interest it is not discussed further. Aulacophora lewisi has black elytra, simple male antennae and a short female pygidium ( Lee & Beenen 2015) and is therefore excluded from our definition of the A. indica species-complex, although there is molecular evidence for its placement in this group ( Fig. 1 View FIGURE 1 ). Aulacophora flavomarginata has mostly black elytra, modified male antennomeres 3–5 and the female pygidium is not produced ( Barroga & Mohamedsaid 2002); this species is also excluded from our definition of the A. indica species-complex. The thickened and modified male tergite VIII does not define the indica species-complex, but may have been a significant precursor for development of the other male and female abdominal characters shared by the group.
Aulacophora nigroscutata Baly, 1886 , described from North Maluku (eastern Indonesia), is dorsally pale, with an excavate male abdomen, but is excluded from the PPB because the female abdomen is structurally quite different ( Baly 1886, 1889). Two other species of Aulacophora in the region, or nearby, A. coffeae (Weber 1801) and A. bicolor (Weber 1801) , may be entirely pale but they have male first antennomere simple, male last abdominal ventrite simple and female without elongated or swollen pygidium. However, these two superficially similar species are included in the key so that they can be separated from the A. indica species-complex (PPB). Further away from the region of interest, there is an entirely pale species in Sulawesi, A. unicolor Jacoby, 1883 , but this lacks the male characters of the PPB complex and has distinct creamy-white antennae and is therefore also excluded from consideration ( Perkins et al. 2019).
The circum-Mediterranean curcurbit pest Aulacophora foveicollis ( Lucas, 1849) clearly belongs in the PPB complex and is particularly similar to A. abdominalis and A. indica . Aulacophora foveicollis differs from these species by the following combination of characters: entirely pale, male pronotum with deeply arcuate central depression divided by a median ridge, penis similar to A. abdominalis but with strongly undulated profile, male tergite 8 elongate with short paired apical projections separated by semicircular concavity, female pygidium produced and sometimes bilobed at apex, apex of female sternite 7 semicircularly excavate ( Maulik 1936; Berti 1990; Borowiec 2013).
Excluded species names (Appendix 1)
There is clearly a problem of identity with the four names coined by Boisduval (1835) for pale species of Galerucinae collected in the region and none have been identified with any certainty by subsequent authors (for example Allard 1888; Weise 1924; Lea 1924, Wilcox 1972). We have been unable to find type material. The best described, Galleruca relicta Boisduval, 1835 , is discussed below, under A. relicta . The other three Boisduval names represent poorly described and unrecognisable species from either New Guinea ( Galleruca flaveola Boisduval, 1835 ), Australia ( G. punctata Boisduval, 1835 ) or the “Pacific Ocean” ( G. scutellata Boisduval, 1835 ).
Galleruca flaveola has been treated as a possible senior synonym of A. aruensis Weise, 1892 ( Weise 1924; Wilcox 1972), itself a junior synonym of something else (see below under A. abdominalis ). The description of G. flaveola is so poor (only “lutea; abdomine, pectore pedibusque nigris”: Boisduval 1835: 558) that is impossible to identify to genus. None of the Aulacophora species in the region have entirely black legs, therefore, if accurately described, G. flaveola is unlikely to be an Aulacophora . Galleruca punctata is also not identifiable to genus. If it is an Aulacophora it is most likely a junior synonym of either A. abdominalis ( Wilcox 1972; Kimoto 1990) or A. relicta , and therefore of no taxonomic significance. Both Galleruca flaveola and G. punctata should be discarded, as nomina dubia, like other unidentifiable Boisduval names ( Reid 2006).
Galleruca scutellata has been treated as a senior synonym of the single Australian Aulacophora species with a black scutellum, A. wilsoni Baly 1888 , because of Blackburn’s tentative synonymy of these two names, which ignored the notable difference in type localities ( Blackburn 1890; Lea 1924; Wilcox 1972; Kimoto 1990). Blackburn noted that Boisduval’s description was “perhaps unworthy of attention” ( Blackburn 1890: 361). Aulacophora wilsoni is a scarce species of high rainfall forests in southeastern Queensland and northeastern New South Wales, a species unlikely to have been encountered by collectors in the early 19 th century, and there are no pale species of Aulacophora with a black scutellum in the Pacific region. With this combination of colour and type locality, G. scutellata is more likely a senior synonym of Candezea palustris ( Perroud & Montrouzier, 1864) , as originally suggested by Allard (1888) and discussed by Baly (1889) and Lea (1924), the latter accepting A. wilsoni as a valid Australian species. Candezea palustris is an abundant species in the West Pacific ( Beenen 2008), surprisingly not otherwise noted by Boisduval. The name Candezea palustris is stable and designates an important pest of sweet potatoes in the region ( Kimoto et al. 1984). In the interests of nomenclatural stability, we think it best to discard Galleruca scutellata and also treat this name as a nomen dubium until it is formally supressed.
The names Galleruca cristovallensis Montrouzier, 1856 and G. flavescens Montrouzier, 1856 are also attached to miserly descriptions, although both are regarded as valid species ( Wilcox 1972). Type material of these species is probably no longer extant. The first was described from San Cristoval Island (now Makira, Solomon Islands) and has a transverse pronotal groove, parallel-sided elytra, is 4.5 “lignes” long (10 mm) and is “testacée, lisse” (smooth and brick-red coloured) ( Montrouzier 1856: 70). This species is too large and wrongly coloured for a PPB, so is not discussed further here. Gallerucella flavescens was described from both Woodlark Island (now eastern Papua New Guinea) and San Cristoval Island (Solomons) and also has a transverse pronotal groove, but has expanded elytra, is 3 “lignes” long (6.8 mm) and is “entièrement jaune, sauf le dessous du corps qui est rembruni” (entirely yellow except the underside which is darkened) ( Montrouzier, 1856: 71). This vague description probably applies to a species of PPB but it is impossible to say which one, and in any case all the evidence from the material to hand is that the currently valid species names for any PPB in the New Guinea and Solomons region are senior to anything described by Montrouzier. In the interests of nomenclatural stability, we think it best to discard Galleruca flavescens and treat this name as a nomen dubium. Another Montrouzier name, Galleruca artensis Montrouzier, 1861 , described from New Caledonia and overlooked in revisions of that fauna ( Beenen 2008, 2013), is equally impossible to identify from its four lines of description. It was originally compared with G. argyrogaster Montrouzier, 1861 (synonymised below with A. abdominalis ), from which it only differed by pale antennae and legs and an unusual golden elytral spot in living specimens ( Montrouzier 1861: 394). Galleruca artensis is probably a junior synonym of Aulacophora abdominalis , but it could possibly be a pale form of A. deplanchei ( Perroud & Montrouzier, 1864) , discussed below. Type material of G. artensis is probably lost. Since there is no reasonable way of determining the species, we think this name should also be set aside, as a nomen dubium.
Entirely pale species in the region that do not belong to A. indica species-group (Appendix 2)
(i) Pale colour morphs of Aulacophora deplanchei ( Perroud & Montrouzier, 1864) and A. bicolor (Weber, 1801) .
We have only had access to typical maculate forms of A. deplanchei , including a photograph of the lectotype, so the following discussion is partly based on the confusing literature. Aulacophora deplanchei ( Perroud & Montrouzier, 1864) was described from New Caledonia with black maculae on the elytra. It was then redescribed by Beenen (2008) but he later entirely rejected his own redescription without explanation ( Beenen 2013). His redescription appears to have been an accidental ‘copy and paste’ of his description of A. montrouzieri Beenen, 2008 in the same paper (a preoccupied name, later renamed A. xavieri Beenen, 2013 ). Beenen (2008) noted that some specimens of A. deplanchei were entirely pale dorsally. This potentially makes them members of the PPB species-complex. The original description of A. deplanchei did not mention structures of the first antennomere, male ventrite 5 and female pygidium, but Beenen associated this species with the maculate species A. xavieri , which was described in detail and lacks all of the PPB diagnostic attributes. Our examination of specimens of A. deplanchei shows that the male antennae are simple, the male apical ventrite is short and flat and the female pygidium is not extended. The male genitalia of A. deplanchei are certainly similar to A. xavieri and dissimilar to any member of the PPB complex treated here ( Beenen 2008). We therefore exclude A. deplanchei from the PPB complex, but as it has entirely dorsally pale morphs we include it in our diagnostic key. The presence of rows of long setae on the male antennomeres suggests that A. deplanchei might be related to both A. bicolor (Weber, 1801) and A. coffeae ( Hornstedt, 1788) . Aulacophora perroudi Baly, 1888 , described from New Caledonia and overlooked by subsequent authors, is probably a synonym of A. deplanchei as it has dark antennae, dark elytral humeri and female pygidium rounded ( Baly 1888: 177). Furthermore, the female pronotal groove is described as absent medially, which is almost true of the small amount of material to hand. However, in the absence of type material and the possibility that there is a valid species on New Caledonia with maculate humeri, we refrain from making this synonym.
Aulacophora bicolor is a widespread and variable south-east Asian species, which usually has black markings on the elytra but has rare entirely dorsally pale colour morphs ( Barroga & Mohamedsaid 2002; Lee & Beenen 2015). Aulacophora bicolor has an unexpanded first antennomere, short flat apical male ventrite and short female pygidium and therefore lacks the characters defining the PPB complex ( Barroga & Mohamedsaid 2002). This species is not recorded from the Pacific Region but is associated with cucurbit crops and is recorded from as far east as Timor (Mohamedsaid 2009), so it is included in our diagnostic key.
(ii) Aulacophora coffeae ( Hornstedt, 1788)
Aulacophora coffeae is another pale species of Aulacophora , which, like A. bicolor , lacks the diagnostic secondary sexual characters of the PPB complex ( Barroga & Mohamedsaid 2002). Aulacophora coffeae is a pest of cucurbits in southeast Asia and Indonesia (Kalshoven 1951; Lewis & Metcalf 1996; Mohamedsaid 2009a), also recorded from Ipomoea ( Barroga & Mohamedsaid 2002) . In the region under review, it has been noted from Fiji feeding on Cucumis and Cucurbita ( Greenwood 1940; Lever 1942), but these records were queried by Bryant & Gressitt (1957) who implied that they referred to A. similis (itself a misidentification of A. abdominalis ). In Papua New Guinea, A. coffeae has been recorded feeding on cucurbits and legumes: mung bean ( Vigna ), rock melon ( Cucumis ) and peanut ( Arachis ) ( Greve & Ismay 1983). All specimens of pale Aulacophora we have examined from Fiji belong to A. abdominalis and all pale Aulacophora we have examined from New Guinea belong to A. abdominalis , A. cornuta or A. indica . Greve & Ismay (1983) did not indicate where their material was deposited but at the time of their report both were based in Port Moresby. Our colleague Al Samuelson has searched the largest holding of New Guinean Chrysomelidae in the world, in BPBM, and has failed to find any specimens of A. coffeae from New Guinea or the Pacific (Samuelson, pers. com. September 2018). We therefore conclude that all records of A. coffeae from the region of interest have been misidentifications, which may have been caused by early misidentification of A. coffeae by Allard (1888), as noted by Baly (1889). The nearest accurate record of A. coffeae is from Sumba ( Mohamedsaid 2009a), only 300 km west of Timor, so it may spread further east, as little agricultural biosecurity is practiced within the Indonesian Archipelago. The species is included in our diagnostic key in case it appears in the region.
Key to species of plain pumpkin-beetles, Aulacophora indica species-complex, in Australopapua, Timor and the west Pacific region
1 Male without enlarged first antennomere, as female; male antennomeres 4–11 with longitudinal row of erect setae on inner margins; male last ventrite with short flat apical median projection; penis symmetrical; female pygidium neither dorsally swollen nor with apex extended beyond apical ventrite (pygidium dark brown to black)................................. 2
- Male first antennomere enlarged, like an inverted pyramid or almost cuboid, with one side partly flattened ( Figs 2–17 View FIGURES 2–5 View FIGURES 6–9 View FIGURES 10–15 View FIGURES 16–17 ), contrasting with almost cylindrical segment of female; male antennomeres 4–11 with 4–6 scattered erect setae on inner margin between base and apex, not forming a single longitudinal row; male last ventrite with a deeply and elongately hollowed median lobe ( Figs 32–43 View FIGURES 32–37 View FIGURES 38–43 ); penis usually asymmetric in dorsal view ( Figs 94–107 View FIGURES 94–97 View FIGURES 98–101 View FIGURES 102–105 View FIGURES 106–107 ); female pygidium with apex usually extended beyond apex of ventrite 5 and often dorsally swollen ( Figs 44–55 View FIGURES 44–49 View FIGURES 50–55 ) ( A. indica species-complex).............................. 4
2(1) Elytra dull, finely microreticulate; antennae entirely pale; pygidium clothed in dense silvery recumbent setae; labrum black; pronotal transverse depression deep at middle............................................................... 3
- Elytra shiny, without microreticulation; antennomeres 4–11 dark brown to black; pygidium with sparser semi-erect brownish setae; labrum pale brown; pronotal depression shallow at middle (elytra usually blotched or maculate; endemic to New Caledonia)............................................................... A. deplanchei (Perroud & Montrouzier)
3(2) Base of head and labrum usually pale; male antennomeres 3–11 each with longitudinal row of about 10 erect setae along one side, setae about as long as width of antennomere, and antennomeres 3–7 parallel-sided; female ventrite V with narrowly notched apex; venter of prothorax partly dark brown (elytra usually spotted or at least partly black; only recorded from Timor in region, widespread in SE Asia).......................................................... A. bicolor (Weber)
- Base of head and labrum dark brown to black; Male antennomeres 3–11 each with longitudinal row of 5–6 short erect setae, setae less than width of antennomere, and antennomeres 3–7 slightly expanded apically; female ventrite V with shallowly concave to truncate apical margin; venter of prothorax pale (not confirmed in region but likely to be adventive, widespread in SE Asia as far east as Flores).............................................................. A. coffeae (Hornstedt)
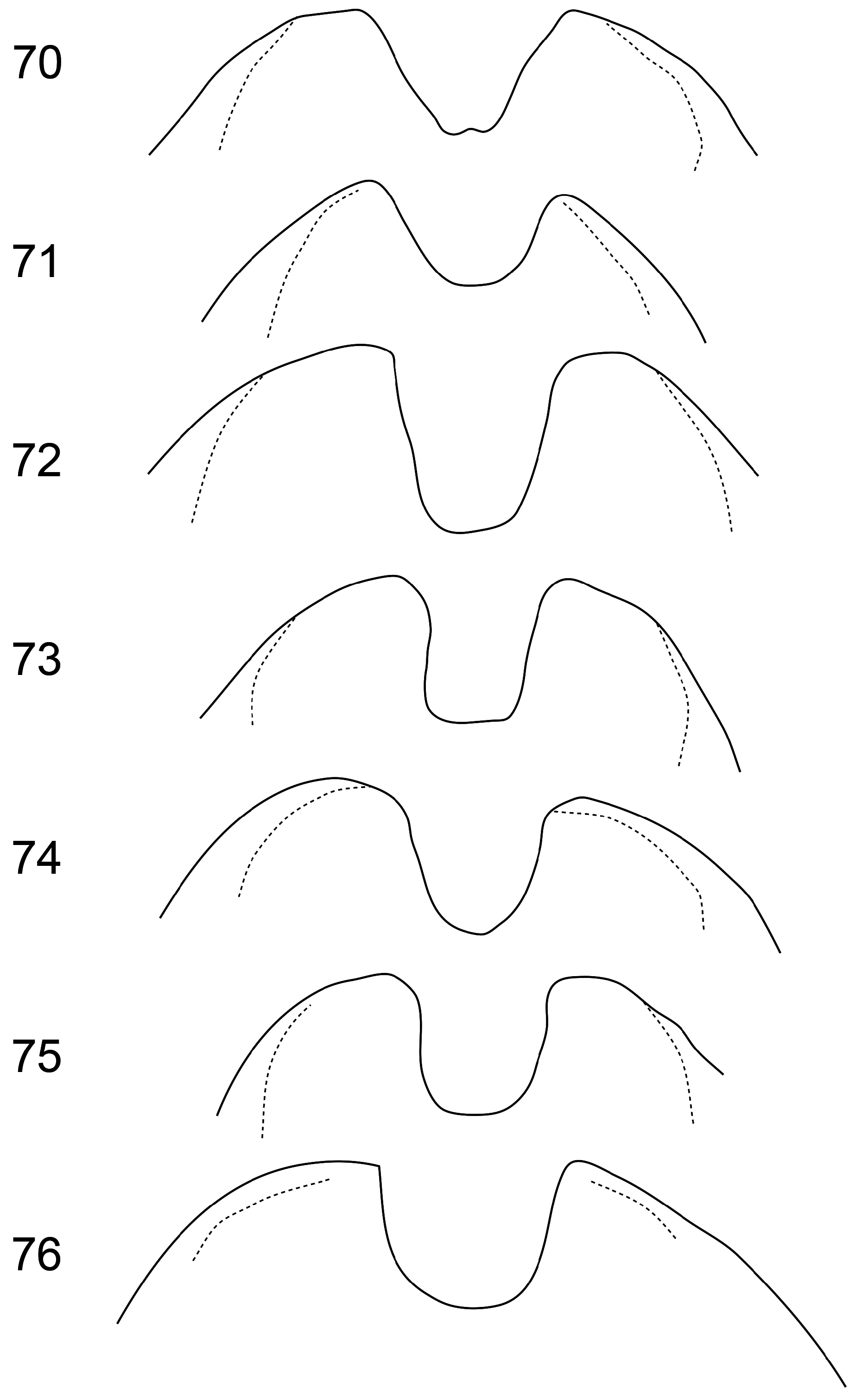
4(1) First antennomere enlarged apically, like an inverted elongate pyramid, with one side partly flattened ( Figs 2–17 View FIGURES 2–5 View FIGURES 6–9 View FIGURES 10–15 View FIGURES 16–17 ); pronotal lateral groove deep ( Fig. 28 View FIGURES 26–31 ); apical ventrite deeply and elongately hollowed ( Figs 32–43 View FIGURES 32–37 View FIGURES 38–43 ); basitarsomeres of anterior and middle legs slightly expanded, with pads of discoidal setae (males).................................................... 5
- First antennomere simple, fusiform; pronotal lateral groove shallow ( Fig. 29 View FIGURES 26–31 ); apical abdominal ventrite without elongate hollow but apex truncate to strongly notched ( Figs 44–57 View FIGURES 44–49 View FIGURES 50–55 View FIGURES 56–57 ); basitarsomeres straight sided, without discoidal setae (females)... 12
5(4) Frontoclypeus with median ridge and without pits or paired lateral ridges ( Figs 10–11, 13–17 View FIGURES 10–15 View FIGURES 16–17 )........................ 6
- Frontoclypeus without median ridge, with pair of arcuate ridges below antennal sockets and median patch of dense short golden setae above these ( Fig. 12 View FIGURES 10–15 ); (length 7.5–9.5 mm; elytral humeri without setae ( Fig. 20 View FIGURES 18–25 ); penis symmetrical, sagittate ( Fig. 99 View FIGURES 98–101 ); SE Asia to Solomon Islands)................................................................ A. cornuta Baly
6(5) Elytral humeri with oval patch of erect setae ( Figs 18–19, 21–22, 24–25 View FIGURES 18–25 )......................................... 7
- Elytral humeri glabrous ( Fig. 23 View FIGURES 18–25 ); (length 6–7.5 mm; femora pale, with meso- and metatibiae darkened, or legs entirely pale; apical segments of antennae usually slightly darkened; pygidium entirely brownish-yellow; pronotum with pair of large pits anterior to transverse groove, anterior half of pronotum more convex than posterior half, pronotal depression in lateral view with anterior slope steeper than posterior ( Fig. 30 View FIGURES 26–31 ); last ventrite with apical excavation asymmetric, deeper and more sharply bordered on left ( Fig. 41 View FIGURES 38–43 ); penis in dorsal view slightly constricted at middle ( Figs 104–105 View FIGURES 102–105 ); Australia)..................................................................................................... A. relicta Boisduval
7(6) Meso- and metafemora usually mostly darkened, but if femora mostly to entirely pale, meso- and metatibiae also mostly to entirely pale ( Fig. 7 View FIGURES 6–9 ); groove in last ventrite usually assymmetric, with swelling at one side and/or deepened groove at one side ( Figs 32–35 View FIGURES 32–37 ); pronotal groove narrower at middle ( Figs 2, 5–6, 8–9 View FIGURES 2–5 View FIGURES 6–9 ); penis without median tooth in lateral view ( Figs 121–124 View FIGURES 121–124 , 127–130 View FIGURES 125–128 View FIGURES 129–132 , 133–134 View FIGURES 133–134 ).................................................................................... 8
- Femora pale except meso- and metafemora with dark apices, meso- and metatibiae dark brown to black; apical groove on ventrite 5 symmetrical ( Fig. 36 View FIGURES 32–37 ); pronotal groove greatly expanded at middle with well-defined sinuate anterior and posterior edges ( Figs 3 View FIGURES 2–5 , 19 View FIGURES 18–25 ); penis with minute median tooth in lateral view ( Fig. 125 View FIGURES 125–128 ) (length 6.5–7 mm; tergite 8 medially lobed ( Fig. 85 View FIGURES 83–88 ); pronotum without pits anterior to median groove, in lateral view groove with posterior angle greater than curving anterior angle ( Fig. 27 View FIGURES 26–31 ); coastal NT and WA).................................. A. barrogae Reid, Halling & Beatson , sp. nov.
8(7) Abdomen with at least apices of both pygidium and ventrite V pale ( Figs 32–35 View FIGURES 32–37 , 38–40, 42 View FIGURES 38–43 ); antennomeres longer, length of 5th more than 2.3x width ( Figs 18, 21–22, 24 View FIGURES 18–25 ); apex of penis usually hooked in lateral view ( Figs 121–124 View FIGURES 121–124 , 127–130 View FIGURES 125–128 View FIGURES 129–132 , 133 View FIGURES 133–134 ); scutellum usually same colour as elytra, rarely darker brown ( Figs 2, 5 View FIGURES 2–5 , 8 View FIGURES 6–9 ) (mesepimeron usually pale)................. 9
- Abdomen entirely black ( Fig. 43 View FIGURES 38–43 ); antennomeres shorter, length of 5th about 2.2x width ( Fig. 25 View FIGURES 18–25 ); penis strongly curved in lateral view, without hooked tip ( Fig. 134 View FIGURES 133–134 ); scutellum always much darker than elytra, brown to almost black ( Fig. 9 View FIGURES 6–9 ) (length 6.5–7 mm; mesepimeron black or dark brown; meso- and metatibiae black and anterior tibiae with black outer edges; antennomeres 4–11 black; last ventrite with apical excavation slightly asymmetric; tergite 8 medially lobed ( Fig. 93 View FIGURES 89–93 ); pronotum without paired pits anterior to median groove; high rainfall areas of NE NSW & SE Qld)............................. A. wilsoni Baly
9(8) Pronotal groove sinuate or almost straight, deep at middle and with pair of pits anterior to median groove ( Figs 2, 5 View FIGURES 2–5 , 8 View FIGURES 6–9 , 18, 21, 24 View FIGURES 18–25 ); elytral setal patch usually large and dense ( Figs 18, 21, 24 View FIGURES 18–25 ); tergite 8 strongly bilobed ( Figs 83–84, 87–89, 92 View FIGURES 83–88 View FIGURES 89–93 ); penis almost straight in lateral view ( Figs 121–124 View FIGURES 121–124 , 127–129 View FIGURES 125–128 View FIGURES 129–132 , 133 View FIGURES 133–134 )........................................................ 10
- Pronotal groove almost straight, and shallow throughout, anterior and posterior slopes shallow and symmetrical in lateral view, and without pits anterior to median groove ( Figs 6 View FIGURES 6–9 , 22 View FIGURES 18–25 ); elytral setal patch small, sparse, not dense ( Fig. 22 View FIGURES 18–25 ); tergite 8 medially lobed ( Fig. 90 View FIGURES 89–93 ); penis strongly curved in lateral view ( Fig. 130 View FIGURES 129–132 ) (length 7–7.5 mm; meso- and metatibiae dark brown to black and anterior tibiae often with dark outer edges; antennomeres 4–11 dark brown to black; inland N Qld)................................................................................ A. mbabaram Reid, Halling & Beatson , sp. nov.
10(9) Pronotal median depression medially deeper, in lateral view with anterior slope steeper than posterior ( Figs 18, 24 View FIGURES 18–25 , 26, 31 View FIGURES 26–31 ); penis sinuately margined in dorsal view, with acute apex ( Figs 94–97 View FIGURES 94–97 , 107 View FIGURES 106–107 )...................................... 11
- Pronotal median depression medially shallower, in lateral view with anterior and posterior slopes about equally steep ( Figs 21 View FIGURES 18–25 , 27 View FIGURES 26–31 ); penis almost parallel-sided in dorsal view, with blunt apex ( Figs 100–102 View FIGURES 98–101 View FIGURES 102–105 ) (length 6–8 mm; antennae usually entirely pale, rarely darkened from antennomere 4 to apex; scutellum usually pale, dark brown in some specimens from Borneo; mesepimeron pale; legs entirely pale or with darkened meso- and metafemora and external edges of meso- and metatibiae; last ventrite with apical excavation asymmetric, more sharply bordered on left; tergite 8 arcuate between narrowly triangular prongs ( Figs 87–89 View FIGURES 83–88 View FIGURES 89–93 ) (S & E Asia to Timor, Micronesia, Guam, and intercepted in Australia but not established)....... A. indica (Gmelin)
11(10) Antennae usually darkened apically ( Fig. 18 View FIGURES 18–25 ), rarely entirely pale (some Timorese specimens); last ventrite with asymmetric deep apical excavation, more sharply bordered on left ( Figs 32–35 View FIGURES 32–37 ); scutellum pale, as elytra; mesepimeron pale; tergite 8 arcuate between elongate triangular prongs ( Figs 83–84 View FIGURES 83–88 ); penis broader, asymmetric, in dorsal view slightly constricted at middle, without ventral ridge ( Figs 94–97 View FIGURES 94–97 , 108–111 View FIGURES 108–111 ) (length 6–7.5 mm; meso- and metafemora and tibiae usually darkened; pygidium with base darker than apex; Timor to Tonga and probably Samoa, including Torres Strait Islands but not Australian mainland)............................................................................... A. abdominalis (Fabricius)
- Antennae entirely pale ( Fig. 24 View FIGURES 18–25 ); last ventrite with symmetrical broad shallow apical excavation, without sharply ridged lateral margins ( Fig. 42 View FIGURES 38–43 ); scutellum often darkened, brown or dark brown; mesepimeron usually dark brown; tergite 8 with pair of long needle-like processes, truncate between ( Fig. 92 View FIGURES 89–93 ); penis thinner, symmetrical, constricted before apex in dorsal view and venter longitudinally ridged ( Figs 107 View FIGURES 106–107 , 119 View FIGURES 116–119 ) (length 7.5 mm; meso- and metafemora usually dark brown or black but tibiae pale); (endemic to Timor)................................................................... A. wallacii Baly
12(4) Frontoclypeus as male ( Fig. 10 View FIGURES 10–15 ), with glabrous median keel from postantennal calli to labrum; apex of pygidium strongly produced, and apex truncate, narrowly rounded, or angulate ( Figs 44–47 View FIGURES 44–49 , 50–55 View FIGURES 50–55 ).................................... 13
- Frontoclypeus without median keel, with elongate patch of dense setae at base between antennae; apex of pygidium slightly produced, and with broadly rounded apical margin ( Fig. 48 View FIGURES 44–49 ) (length 8–9 mm; abdomen dark brown to black; apical swelling of pygidium with median carina)............................................................... A. cornuta Baly
13(12) Apex of abdomen pale, at least apical margins of both pygidium and ventrite 5 ( Figs 44–47, 49–55 View FIGURES 44–49 View FIGURES 50–55 ); apical margin of ventrite 5 medially deeply excavate to shallowly concave; antennomeres longer, length 5th 2.5–3.2x width, 4–11 usually pale or slightly darkened, rarely dark brown to black; scutellum usually pale................................................. 14
- Abdomen entirely black ( Figs 56–57 View FIGURES 56–57 ); apical margin of ventrite 5 truncate; antennomeres shorter, length 5th about 2.4x width, 4–11 black; scutellum dark brown to black (length 6.5–7 mm; mesepimeron black or dark brown; mid and hind legs black and anterior tibiae with black outer edges; apex of pygidium more or less narrowly truncate with apical median tubercle)............................................................................................... A. wilsoni Baly
14(13) Apical excision of ventrite 5 shallowly arcuate to broadly U-, V-, or W-shaped, often asymmetric or irregular in shape, width at least 1.8x depth (deepest in New Ireland specimens of A. abdominalis ), usually much greater, and apex of ventrite 5 usually with strongly reflexed edge ( Figs 45–47 View FIGURES 44–49 , 53–55 View FIGURES 50–55 , 58–69 View FIGURES 58–69 , 77–82 View FIGURES 77–82 ), with subapical transversely arcuate depression or paired transverse depressions not bounded by lateral ridges, apex of pygidium usually thick in lateral view with small apicodorsal tubercle; pygidium usually less or not acutely produced, apical angle more than 70°, or rounded............................. 15
- Apical excision of ventrite 5 deep, width at most 1.8x depth (shallowest in Vietnamese specimens), almost symmetrically U- or V-shaped and apical margin with flat edge and pair of preapical ovate depressions, bounded laterally by a ridge which fuses apically with margin of ventrite ( Figs 49–52 View FIGURES 44–49 View FIGURES 50–55 , 70–76 View FIGURES 70–76 ); pygidial apex usually thin in lateral view, without tubercle; pygidium usually produced as an elongate straight-sided triangular lobe, apical angle usually 50–75°, rarely 76–80° ( Figs 69–72 View FIGURES 58–69 View FIGURES 70–76 ) (length 6–8 mm; antennae usually entirely pale, rarely darkened to almost black from antennomere 4 to apex; legs variable in colour; abdominal ventrite V with basal 1/3–2/3 black)................................................ A. indica (Gmelin)
15(14) Metafemora entirely pale ( Fig. 7 View FIGURES 6–9 ); pygidium produced as a broadly obtuse to narrowly acute triangle ( Figs 44–46 View FIGURES 44–49 , 53–54 View FIGURES 50–55 ); (antennae pale at base including antennomere 4, usually slightly darkened apically)................................ 16
- At least apical third of metafemora dark brown to black ( Figs 2 View FIGURES 2–5 , 8 View FIGURES 6–9 ); pygidium variable in shape...................... 17
16(15) Abdominal tergites brownish-yellow, including whole of pygidium; ventrite 5 with brownish-yellow middle of base ( Fig. 54 View FIGURES 50–55 ) (length 5.5–7.5 mm; pygidium usually produced as ± 90º triangle, rarely rounded, usually with acute apicodorsal tubercle)..................................................................................... A. relicta (Boisduval)
- Base of pygidium darker than brownish-yellow apex and remainder of abdominal dorsum black or dark brown; ventrite 5 with brown base ( Figs 44–46 View FIGURES 44–49 ) (length 7–8 mm; apex pygidium usually produced as slightly elongate triangular lobe, apical angle usually 76–90̊, rarely 90–100º, acute tubercle, if present, usually apical). A. abdominalis (Fabricius) (Timorese colour variety)
17(15) Antennomeres 1–3 pale and 4–11 dark brown to black ( Figs 19, 22 View FIGURES 18–25 ); pygidium narrowly produced, apical margin rounded or almost truncate but with small median tubercle ( Figs 47 View FIGURES 44–49 , 53 View FIGURES 50–55 ); apex of ventrite 5 shallowly arcuate and not or weakly reflexed (meso- and metafemora usually with basal third pale and apical third dark brown, middle third variable; meso- and metatibiae dark brown)........................................................................................ 18
- Antennae entirely pale or gradually apically darkened, sometimes with antennomeres 6–11 brown to dark brown but at least inner surface of 4 pale ( Figs 18, 24 View FIGURES 18–25 ); pygidium triangularly produced, or apex almost rounded ( Figs 44–46 View FIGURES 44–49 , 55 View FIGURES 50–55 ); apex of ventrite 5 variably excised, usually strongly reflexed (metafemora usually entirely dark brown to black; ventrite 5 with at least basal third dark brown or black)............................................................................. 19
18(17) Pronotal groove laterally deepened; abdominal ventrites 1–4 with basal half yellowish-brown, ventrite 5 yellow except for laterobasal dark patches ( Fig. 47 View FIGURES 44–49 ); pygidium brownish-yellow (length 7–8 mm).................................................................................................... A. barrogae Reid, Halling & Beatson , sp. nov.
- Pronotal groove entirely shallow; abdominal ventrites 1–4 black with brown apical margins, basal half or more of ventrite 5 dark brown to black ( Fig. 53 View FIGURES 50–55 ); pygidium partly dark brown (length 7–7.5 mm).................................................................................................... A. mbabaram Reid, Halling & Beatson , sp. nov.
19(15) Venter of pygidial apex flat or shallowly concave, and apex pygidium produced as a triangular lobe, apical angle usually 76–90̊, rarely 90–100º ( Figs 44–46 View FIGURES 44–49 ); metafemora and tibiae dark brown, or tibiae slightly paler ( Fig. 2 View FIGURES 2–5 ); scutellum same colour as ely- tra; ventrite 5 marginal excavation of variable depth ( Figs 58–69 View FIGURES 58–69 ) and apical margin not or weakly reflexed ( Figs 44–46 View FIGURES 44–49 ) (length 7–8 mm)...................................................... A. abdominalis (Fabricius) (typical colour form)
- Venter of pygidial apex deeply notched, and apex of pygidium almost rounded ( Fig. 55 View FIGURES 50–55 ); metafemora black strongly contrasting with yellow metatibiae ( Fig. 8 View FIGURES 6–9 ); scutellum usually darker than elytra; apex of ventrite 5 narrowly excavate at middle and strongly reflexed ( Fig. 55 View FIGURES 50–55 ) (length 8–9 mm;).................................................... A. wallacii Baly
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
SubFamily |
Galerucinae |
Aulacophora Chevrolat, 1836
| Reid, Chris, Halling, Luke & Beatson, Max 2021 |
Paraulacophora
| Wilcox, J. A. 1972: 222 |
Ceratia
| Maulik, S. 1936: 167 |
Sphaerarthra
| Maulik, S. 1936: 167 |
Pachypalpa
| Maulik, S. 1936: 167 |
Triaplatys
| Weise, J. 1924: 9 |
Orthaulaca
| Maulik, S. 1936: 167 |
| Weise, J. 1892: 396 |
Cerania
| Maulik, S. 1936: 167 |
| Weise, J. 1892: 396 |
Rhaphidopalpa
| Rosenhauer, W. G. 1856: 327 |
Acutipalpa
| Rosenhauer, W. G. 1856: 327 |
Aulacophora
| Anand, R. K. & Cox, M. L. 1986: 81 |
| Wilcox, J. A. 1972: 221 |
| Maulik, S. 1936: 167 |
| Chevrolat, L. A. A. 1836: 378 |
Raphidopalpa
| Chevrolat, L. A. A. 1836: 378 |