Hamacreadium mutabile Linton, 1910
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4254.2.1 |
|
publication LSID |
lsid:zoobank.org:pub:0BDF72E4-5330-4EE7-8560-DF44E71C1F41 |
|
DOI |
https://doi.org/10.5281/zenodo.6048914 |
|
persistent identifier |
https://treatment.plazi.org/id/436E87B5-BE65-554B-FF67-FC54FA964AC3 |
|
treatment provided by |
Plazi |
|
scientific name |
Hamacreadium mutabile Linton, 1910 |
| status |
|
Hamacreadium mutabile Linton, 1910 View in CoL
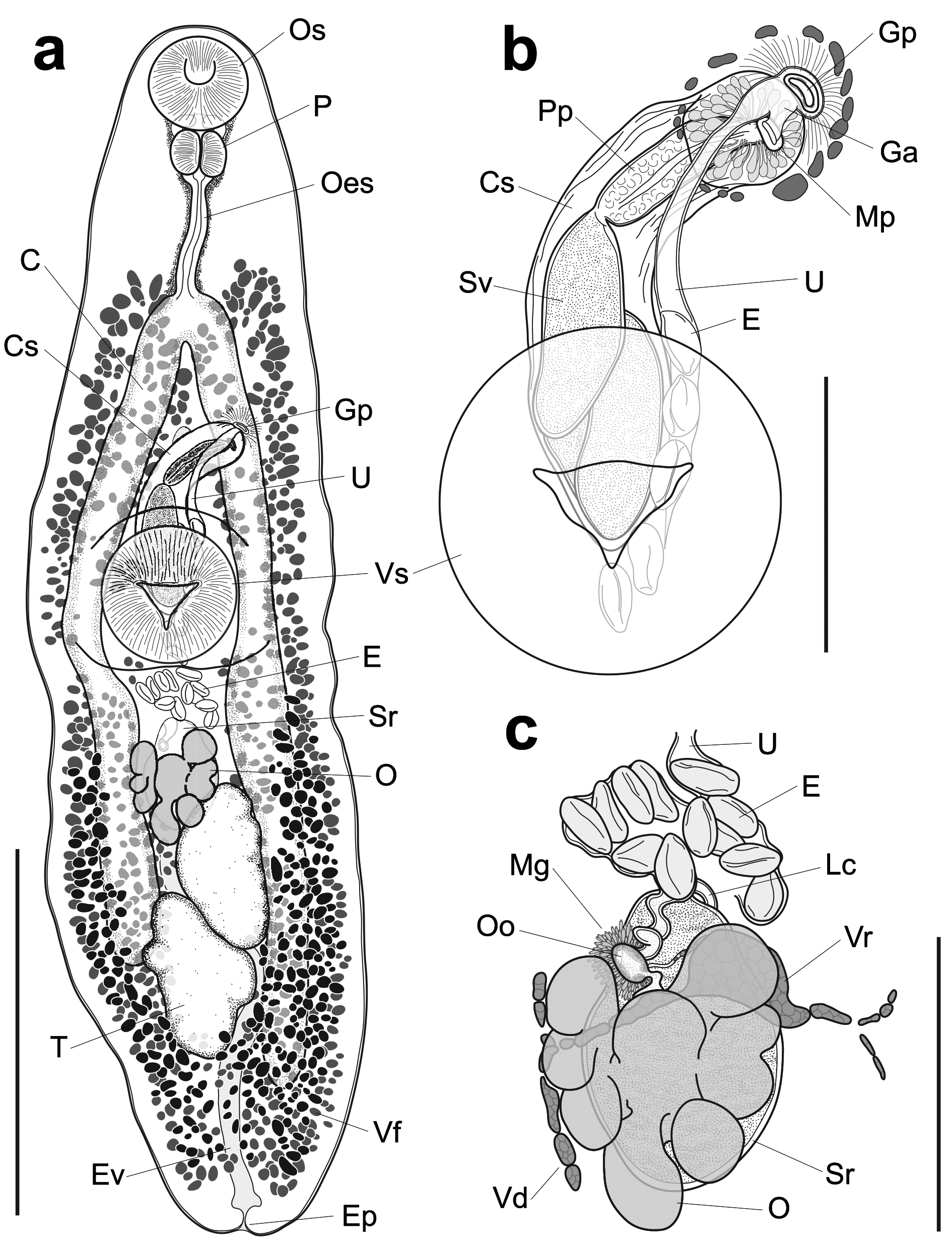
( Figure 1 View FIGURE 1 )
Records. see Table 1.
Description. [Based upon a single specimen, SI NMNH IZ #1402929. Measurements in micrometres]. Body elongate linguiform, tapering anteriorly, bluntly rounded and slightly emarginate posteriorly, 3,300 long, 857 wide at level of ventral sucker. Forebody 1,356 long, occupying 41.1% of body length. Oral sucker round, subterminal, 250 × 267. Ventral sucker round, 379 × 380, 1.42 times width of oral sucker. Pre-pharynx short, entirely dorsal to oral sucker, surrounded by gland cells. Pharynx 128 × 155. Oesophagus straight, thick-walled, lined by gland cells, 323 long, 9.8% of total body length. Intestine bifurcates into blind-ending caeca mid-forebody (53.4%), 724 from anterior extremity, at angle of approximately 45°. Intestinal caeca uneven in length; left extending posteriorly beyond testes, terminating 410 from posterior extremity; right not extending posteriorly beyond posterior testis, terminating 800 from posterior extremity. Excretory vesicle simple, tubular, extends anteriorly to roughly half-way between intestinal bifurcation and anterior margin of ventral sucker.
Testes two, oblique, inter-caecal, oval to wedge-shaped, lightly indented, margins abutting, left (453 × 233) anterior to right (476 × 290). Post-testicular length 492, 14.9% of body length. Cirrus-sac well-developed, curved, extends posteriorly beyond half ventral sucker length (57.8%), occupies 15.4% body length, 508 × 129. Seminal vesicle large, tubular, sigmoid, occupies slightly more than half cirrus-sac length, narrows to distinct pars prostatica. Ejaculatory duct simple. Genital atrium short, indistinct. Genital pore distinctly sinistral, ventral to left caecum, 267 anterior to ventral sucker and 1,061 from anterior extremity (32.3% of body length).
Ovary submedial, inter-caecal, abutting anterior testis, roughly triangular, composed of eight or nine deep lobes, 304 × 252, 186 from ventral sucker (5.6% of body length). Canalicular seminal receptacle similar in size and dorsal to ovary, 307 × 204. Laurer’s canal opens antero-dorsal to ovary. Vitelline reservoir roughly medial, at level of ovary. Vitelline follicles distributed along intestinal caeca, separated into dorsal and ventral fields; ventral field restricted to hindbody, confluent posterior to testes, extends beyond intestinal caeca posteriorly; dorsal field confluent in forebody and hindbody, extends anteriorly just beyond intestinal bifurcation and posteriorly beyond intestinal caeca. Uterus entirely anterior to ovary, coils indistinct, approaches genital atrium from left of cirrus-sac. Eggs about 30, elliptical, operculate, 67–79 (mean 75) × 35–42 (41).
Remarks. Hamacreadium mutabile has been reported in 40 publications, 21 with records from the west Atlantic, three from the east Pacific and 16 from the Indo-west Pacific, infecting 53 species of teleost fishes, from 14 families and three orders (Table 1). Few records are accompanied by an illustration or description, or otherwise justify the identification of the specimens. Although broad geographic distributions spanning multiple ocean basins have been reported for some trematode species infecting fishes, including other opecoelids, these are often for problematic species and are typically treated with scepticism ( Cribb 2005a, Blend & Dronen 2015). No such distribution has been convincingly demonstrated or justified, except for trematodes from highly vagile pelagic fishes ( Aiken et al. 2007). In the case of H. mutabile , recent molecular analyses have provided the first evidence against a multi-ocean distribution. On the basis of these analyses, Bray et al. (2016) considered specimens from the blackspot snapper, Lutjanus fulviflamma (Forsskål) , off New Caledonia, initially identified as H. mutabile , to represent a distinct but uncharacterised species. Importantly, this interpretation suggests that morphological distinctions between genuine Hamacreadium species may be slight. Therefore, previous reports of H. mutabile from the Indo-west Pacific might easily represent this uncharacterised species, or other species known from the region.
Some hosts reported for species of Hamacreadium are ecologically implausible. The life-cycle of H. mutabile suggests piscivorous fishes are the most appropriate definitive hosts ( McCoy 1929, 1930) but kyphosids (reported by Dyer et al. 1992) and siganids (reported by Nagaty 1941) are herbivorous (Table 1). Likewise, the pomacanthid host reported by Linton (1910), the grey angelfish, Pomacanthus arcuatus (Linnaeus) , feeds largely on algae, sponges, cnidarians and tunicates ( Froese & Pauly 2016) and the holocentrids and pempherid hosts reported by Parukhin (1970) are planktivorous or largely predators of benthic invertebrates ( Froese & Pauly 2016). The carangid reported by Parukhin (1970), the bigeye scad, Selar crumenophthalmus (Bloch) , is also largely planktivorous; it may feed on small fishes, but these are usually pelagic post-larvae ( Roux & Conand 2000) and thus unlikely to become infected by the cotylocercous cercariae of H. mutabile (see McCoy 1929). The records of Linton (1910) from a haemulid and a pomacanthid are both based on single specimens, the latter immature. McCoy (1930) considered these records unreliable and experimentally demonstrated that H. mutabile failed to establish in these fishes. There are only two other reports from haemulids ( Sogandares-Bernal 1959; Wang et al. 1992), neither convincing, only one from the West Atlantic, but without a description.
Lutjanids are the most commonly reported hosts for H. mutabile , appearing in 31 publications (Table 1). Lethrinids are frequently reported hosts of other Hamacreadium species, but these fishes are not found in the west Atlantic and there are just five reports of H. mutabile from lethrinids in the Indo-west Pacific ( Nagaty 1941; Durio & Manter 1968; Parukhin 1970; Hafeezullah 1971; Ramadan 1983); none provided explicit illustrations or descriptions of specimens from these fishes. The report of Durio & Manter (1968) is from the type-host and typelocality of H. cribbi Bray & Justine (2016) and probably represents that species.
Ten studies report H. mutabile from serranids, but none are convincing, either depicting a clearly medial genital pore or not explicitly illustrating specimens from these fishes. Four of these records are from the west Atlantic ( Sogandares-Bernal 1959; Nikolaeva & Parukhin 1968; Dyer et al. 1992; Espínola-Novelo et al. 2013), three from the east Pacific ( Manter 1940; Bravo-Hollis & Manter 1957; Lamothe-Argumedo 1969) and three from the Red Sea ( Nagaty 1941; Parukhin 1970; Ramadan 1983). These records probably all represent species of Cainocreadium , except that of Bravo-Hollis & Manter (1957). Their specimen (SI NMNH IZ #1338779), from the leopard grouper, Mycteroperca rosacea (Streets) , off the Pacific coast of Mexico, has an extra-caecal genital pore, a slightly tapered posterior extremity, exceptionally dense vitelline follicles and a distinctly tri-lobed ovary. The anterior extent of the excretory vesicle could not be determined. This specimen is similar to species of Hamacreadium , but also the “maorum” body-type species of Neolebouria (see Dronen et al. 2014).
Manter (1940) was the first to report H. mutabile from other than the west-Atlantic. He identified specimens from a lutjanid [ Lutjanus viridis (Valenciennes) ] and a serranid ( Mycteroperca xenarcha Jordan) collected off the Galápagos. The specimens from the lutjanid (HWML UNL #63), one illustrated by Manter (1940), clearly represent a species of Hamacreadium , seemingly consistent with H. mutabile . However, at least some of the specimens from the serranid (HWML UNL #62a) unambiguously represent a species of Cainocreadium ; the genital pore and cirrus-sac are medial and the body tapers strongly posteriorly to a distinctive teat-like protrusion, a condition seen in some other Caincoreadium species (e.g. Cainocreadium alanwilliamsi Bray, 1990 , Cainocreadium epinepheli Yamaguti, 1934 ). In other specimens from the same fish (HWML #62b,c and 1030), the genital pore is submedial and the cirrus-sac diagonal, but these specimens have been flattened such that the ventral sucker has displaced the cirrus-sac, and otherwise share an overall likeness to the remaining material from the serranid, including in possessing a teat-like posterior protrusion.
Nagaty (1941) provided the first reports of ‘ H. mutabile ’ from the Indo-west Pacific, from five fishes across four families, in the Red Sea (Table 1). Four, a lutjanid, a serranid and two lethrinids, are ecologically plausible hosts, the other, a siganid, is herbivorous and therefore unlikely. Like Manter (1940), Nagaty (1941) described considerable variation, most importantly in the position of the genital pore. Only one specimen was illustrated, but it has a medial genital pore and clearly represents a species of Cainocreadium .
Four other authors have reported H. mutabile from the Red Sea: Parukhin (1970), Ramadan (1983), Nisreen Ezz El-Dien et al. (1990) and Hossam et al. (2012), of which only Ramadan (1983) provided an illustration or description. Like Nagaty (1941), Ramadan (1983) did not specify from which of multiple hosts the illustration was based, and also depicted a medial genital pore. Ramadan (1983) and Hossam et al. (2012) provided the only reports of Hamacreadium species from labrid fishes. Similarly, Nisreen Ezz El-Dien et al. (1990) are the only authors to report a species of Hamacreadium from a moronid, the European seabass, Dicentrarchus labrax (Linnaeus) [= Labrax lupus (Lacépède) & Morone labrax (Linnaeus) ], which happens to be the type-host of the type-species of Cainocreadium . Nagaty (1941), Parukhin (1970) and Ramadan (1983) all list lethrinids and lutjanids among infected hosts and thus specimens of genuine Hamacreadium species were probably involved, but these could easily represent other Hamacreadium species known from the Red Sea and thus cannot be considered convincing records of H. mutabile .
Further records from the Indo-West Pacific include those from the Persian/Arabian Gulf ( Saoud et al. 1986), Indian waters ( Hafeezullah 1971; Madhavi 1975) and tropical/subtropical Chinese waters ( Shen 1990; Wang et al. 1992). The hosts reported by Hafeezullah (1971), Madhavi (1975) and Saoud et al. (1986) are ecologically plausible (see Table 1), but the blackhead seabream, Acanthopagrus schlegelii (Bleeker) (Sparidae) , reported [as Sparus microcephalus (Basilewsky) ] by Shen (1990) feeds on molluscs and polychaetes ( Froese & Pauly, 2016) and, similarly, the chicken grunt, Parapristipoma trilineatum (Thunberg) (Haemulidae) , reported by Wang et al. (1992), feeds mainly on zooplankton ( Froese & Pauly, 2016). Madhavi (1975) did not provide a description, but in the specimens depicted by Saoud et al. (1986) and Wang et al. (1992), the vitelline follicles do not extend beyond the intestinal bifurcation anteriorly, as is the case for H. mutabile . Hafeezullah (1971) provided two illustrations of his specimens; in one, from Lutjanus fulviflamma , the vitelline follicles terminate bifurcally, but in the other, from Lutjanus rivulatus (Cuvier) , the vitelline follicles extend pre-bifurcally. The latter specimen is the most convincing report of H. mutabile from the Indo-west Pacific, but Hafeezullah (1971) also noted that the shape of the vitelline follicles were distinctive in these specimens. Hafeezullah (1971) also reported that specimens from a third lutjanid host, Lutjanus quinquelineatus (Bloch) , were different again; in these the vitelline follicles did not reach the intestinal bifurcation, the intestine bifurcated in a distinctly broad arch and the excretory vesicle reached at least to the intestinal bifurcation. It can therefore be surmised that the specimens collected by Hafeezullah (1971) may represent as many as three similar but distinct species of Hamacreadium , occurring in sympatry but seemingly from different Lutjanus Bloch fishes.
Finally, there are five reports of H. mutabile from the south-west Pacific ( Durio & Manter 1968; Bray & Cribb 1989; Rigby et al. 1999; Bray & Justine 2007; Justine et al. 2012) and one from Hawaiian waters ( Vignon et al. 2009); all are from Lutjanus fishes [ Durio & Manter (1968) also report a lethrinid], and there is no indication that any does not represent a genuine Hamacreadium species. Of these reports, only Bray & Cribb (1989) provided a description, based on a single mature specimen. Their specimen differs only slightly from the specimen collected by Dr M. J. Andres presented here. The most significant characters potentially distinguishing the two appear to be egg size and anterior extent of the vitelline follicles. In the specimen of Bray & Cribb (1989) the eggs are 59–64 × 40–43 µm and the vitelline follicles extend just to the intestinal bifurcation (they considered their specimen anomalous in that the vitelline follicles terminated at the ventral sucker on one side of the body), whereas in both the present specimen and the original description by Linton (1910), the eggs are longer but narrower [67–79 (75) × 35–42 (41) and 75 × 34] and the anterior extent of the vitelline follicles is pre-bifurcal. Additionally, in the specimen of Bray & Cribb (1989), the post-testicular region is relatively longer (19.2% vs 14.9% of body length) and the oesophagus relatively shorter (6.1% vs 9.8% of body length). It is proposed here that the specimen of Bray & Cribb (1989) might represent the uncharacterised species recognised in molecular analyses by Bray et al. (2016). However, without further replication from either the south-west Pacific or the west Atlantic, it is impossible to distinguish between intra-specific and inter-specific variation. Further close examination of new material is required to determine whether any consistent morphological differences corroborate the molecular distinction detected by Bray et al. (2016).
TABLE]. Hosts and localities reported for Hamacreadium mutabile Linton, 1910 . Separated into plausible records (Part a), records probably representing a species of Hamacreadium but unlikely to represent H. mutabile (Part b), and records from doubtful hosts (Part c).
Host Locality Reference
Lutjanus analis (Cuvier) off the Dry Tortugas Manter (1947)
off the Bahamas Sparks (1957)
off Puerto Rico Siddiqi & Cable (1960)
Lutjanus apodus (Walbaum) View in CoL off the Dry Tortugas Linton (1910) [as Neomaenis apodus (Walbaum) ], McCoy (1930), Manter (1947) off Puerto Rico Siddiqi & Cable (1960), Dyer et al. (1985, 1992, 1998) off Jamaica Nahhas & Cable (1964), Fischthal (1977), Nahhas & Carlson (1994) off Belize
Lutjanus griseus (Linnaeus) View in CoL off the Dry Tortugas Linton (1910) [as Neomaenis griseus Evermann & Bean ], McCoy (1929, 1930), Manter (1947)
off Puerto Rico Siddiqi & Cable (1960), Dyer et al. (1985, 1998) off Jamaica Nahhas & Cable (1964) Biscayne Bay, Florida Overstreet (1969)
off the Florida Keys Schroeder (1970)
off Belize Fischthal (1977), Andres et al. (2014) Gulf of Mexico
Lutjanus jocu (Bloch & Schneider) off the Dry Tortugas Manter (1947)
off Puerto Rico Siddiqi & Cable (1960) off Jamaica Nahhas & Cable (1964)
Lutjanus mahogoni (Cuvier) off Puerto Rico Bunkley-Williams et al. (1996)
Lutjanus synagris (Linnaeus) off the Dry Tortugas Manter (1947)
off the Bahamas Sogandares-Bernal (1959) Biscayne Bay Overstreet (1969)
off Belize Fischthal (1977)
off Jamaica Nahhas & Carlson (1994) off Puerto Rico Bunkley-Williams et al. (1996), Dyer et al. (1998)
Lutjanus viridis (Valenciennes) off the Galápagos Manter (1940)
Ocyurus chrysurus (Bloch) View in CoL off the Dry Tortugas Linton (1910), McCoy (1930) off the Bahamas Sparks (1957)
off Puerto Rico Siddiqi & Cable (1960), Dyer et al. (1992) off Panama, Caribbean Sogandares-Bernal & Sogandares (1961) off Belize Fischthal (1977)
off Mexico, Caribbean Caballero (1990)
off Jamaica Nahhas & Carlson (1994) ……continued on the next page TABLE]. (Continued)
Locality Reference
b: reports probably representing other species of Hamacreadium
Lethrinus enigmaticus Smith Red Sea Parukhin (1970)
Lethrinus mahsena (Forsskål) Red Sea Nagaty (1941) [as “ L. mehsena ”]
Lethrinus miniatus Forster off New Caledonia Durio & Manter (1968)
Lethrinus nebulosus (Forsskål) View in CoL Red Sea Nagaty (1941), Ramadan (1983) Gulf of Mannar Hafeezullah (1971)
Lutjanus adetii (Castelnau) View in CoL off New Caledonia Durio & Manter (1968) [as L. amabilis (De Vis) View in CoL ], Justine et al. (2012) off Heron Island, GBR Durio & Manter (1968), Bray & Cribb (1989) [as L. amabilis (De Vis) View in CoL ]
Lutjanus argentimaculatus (Forsskål) View in CoL off New Caledonia Justine et al. (2012)
Lutjanus bohar (Forsskål) Red Sea Parukhin (1970)
off the Tuamotus Rigby et al. (1999)
Lutjanus fulviflamma (Forsskål) View in CoL Red Sea Nagaty (1941) [as Diacope fulviflamma Rüppell ] Moreton Bay, Australia Durio & Manter (1968) Gulf of Mannar Hafeezullah (1971) [as Lutianus fulviflamma (Forsskål) ] off east India Madhavi (1975)
Persian/Arabian Gulf Saoud et al. (1986) off New Caledonia Bray & Justine (2007), Justine et al. (2012)
Lutjanus fulvus (Forster) View in CoL off the Society Islands Rigby et al. (1999), Vignon et al. (2009) off New Caledonia Justine et al. (2012)
Lutjanus gibbus (Forsskål) View in CoL Red Sea Parukhin (1970)
off the Society Islands Rigby et al. (1999) off the Tuamotus Rigby et al. (1999)
Lutjanus kasmira (Forsskål) View in CoL off New Caledonia Bray & Justine (2007), Justine et al. (2012) off the Society Islands Vignon et al. (2009)
Lutjanus quinquelineatus (Bloch) Gulf View in CoL of Mannar Hafeezullah (1971) [as Lutianis quinquelinearis (Bloch) ] off New Caledonia Justine et al. (2012)
Lutjanus rivulatus (Cuvier) Gulf View in CoL of Mannar Hafeezullah (1971) [as Lutianus rivulatus (Cuvier) ] off east India Madhavi (1975)
Lutjanus russellii (Bleeker) View in CoL off New Caledonia Durio & Manter (1968) Persian/Arabian Gulf Saoud et al. (1986) [as L. russelli (Bleeker) View in CoL ]
Lutjanus vitta (Quoy & Gaimard) View in CoL off New Caledonia Justine et al. (2012)
Lutjanus sp. off New Caledonia Justine et al. (2012)
……continued on the next page TABLE]. (Continued)
Host Locality Reference Part c: reports from doubtful hosts
Myripristis murdjan (Forsskål) View in CoL Red Sea Parukhin (1970) Sargocentron cornutum (Bleeker) View in CoL Red Sea Parukhin (1970) [as Holocentrus cornutus Bleeker View in CoL ] Sargocentron punctatissimum (Cuvier) View in CoL Red Sea Parukhin (1970) [as Holocentrus lacteoguttatus Cuvier View in CoL ] Perciformes View in CoL
Selar crumenophthalmus (Bloch) Red Sea Parukhin (1970) Haemulidae
Anisotremus virginicus (Linnaeus) View in CoL off the Dry Tortugas Linton (1910) Haemulon sciurus (Shaw) View in CoL off the Bahamas Sogandares-Bernal (1959) Parapristipoma trilineatum (Thunberg) View in CoL off Fujian, China Wang et al. (1992) Kyphosidae View in CoL
Kyphosus sectatrix (Linnaeus) View in CoL off Puerto Rico Dyer et al. (1992) Labridae View in CoL
Anampses caeruleopunctatus Rüppell View in CoL Red Sea Ramadan (1983) Thalassoma rueppellii (Klunzinger) View in CoL Red Sea Hossam et al. (2012) Moronidae View in CoL
Dicentrarchus labrax (Linnaeus) View in CoL Red Sea Nisreen Ezz El-Dien et al. (1990) [as Morone labrax (Linnaeus) View in CoL ] Pempheridae View in CoL
Pempheris analis Waite Red Sea Parukhin (1970) Pomacanthidae
Pomacanthus arcuatus (Linnaeus) off the Dry Tortugas Linton (1910) Serranidae
Cephalopholis cruentata View in CoL (Lacépẻde) off the Bahamas Sogandares-Bernal (1959) [as Petrometopon cruentatus (Lacépẻde)] Cephalopholis fulva (Linnaeus) Gulf View in CoL of Mexico Nikolaeva & Parukhin (1968) Cephalopholis miniata (Forsskål) View in CoL Red Sea Parukhin (1970)
……continued on the next page Considering both the modern understanding of biogeographic patterns of fish-infecting trematode distributions, and the deficiencies among reports of H. mutabile View in CoL , it is hypothesised here that no report provides compelling evidence that H. mutabile View in CoL infects any fishes other than lutjanids. It is also hypothesised that no record adequately demonstrates presence of H. mutabile View in CoL anywhere other than the west Atlantic, except perhaps that of Manter (1940) from off the Galápagos. Among the records from lutjanids of the west Atlantic, H. mutabile View in CoL has been reported from off Florida, the Bahamas, Mexico, Belize, Jamaica, Puerto Rico and Panama, in Lutjanus analis (Cuvier) View in CoL , Lutjanus apodus (Walbaum) View in CoL , L. griseus , Lutjanus jocu (Bloch & Schneider) View in CoL , Lutjanus mahogoni (Cuvier) View in CoL , Lutjanus synagris (Linnaeus) View in CoL and Ocyurus chrysurus (Bloch) View in CoL (see Table 1).The recent availability of sequence data from Andres et al. (2014), together with the morphological redescription provided here, means that this new hypothesis can now be tested more readily and objectively than has been previously possible.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.