Phelypera schuppeli (Boheman, 1834)
|
publication ID |
https://doi.org/ 10.5281/zenodo.212200 |
|
DOI |
https://doi.org/10.5281/zenodo.6180551 |
|
persistent identifier |
https://treatment.plazi.org/id/442587EC-FFDF-7258-4CDD-6A14FC04AA67 |
|
treatment provided by |
Plazi |
|
scientific name |
Phelypera schuppeli (Boheman, 1834) |
| status |
|
Phelypera schuppeli (Boheman, 1834) View in CoL
( Figs 1–32 View FIGURES 1 – 7 View FIGURES 8 – 16 View FIGURES 17 – 23 View FIGURES 24 – 32 )
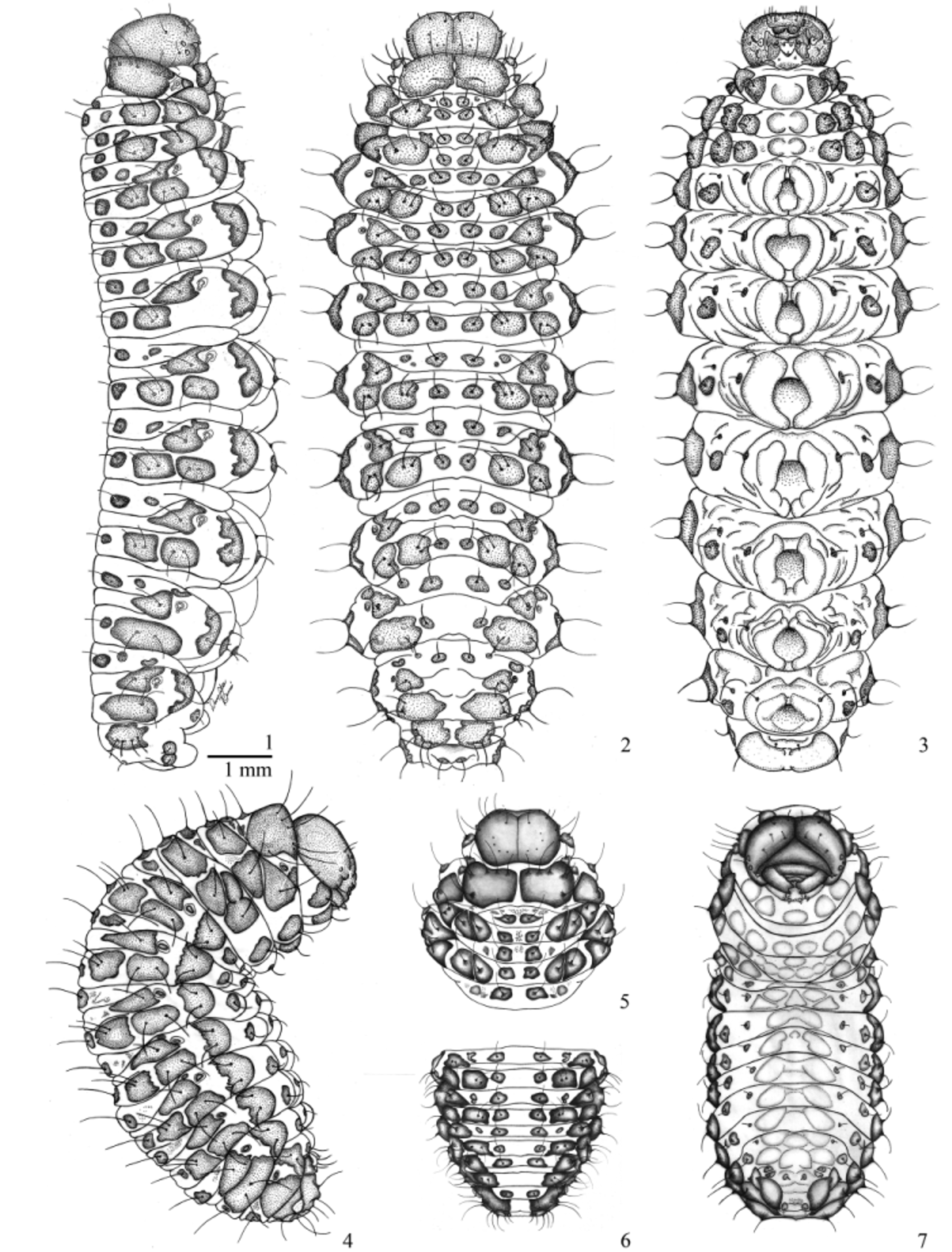
Mature larva description ( Figs 1–3 View FIGURES 1 – 7 ). Length: 13.0−16.0 mm; head width: 1.4–1.5 mm. Body elongate and subcylindrical, slightly flattened dorsoventrally, lateral lobes prominent, abdominal segments I to V or VI widest, lateral margins of thoracic and posterior abdominal segments feebly convergent. Ventral side modified and adapted to crawling. Median region of abdominal segments I–VIII with a pair of semi-circular protuberances which form eight ambulatory ampullae. Coloration: head capsule, mandibles, maxillae, pronotal shield and body sclerites dark brown to black, labrum and clypeus dark brown with two rounded lateral whitish patches, remaining of body bright yellow to greenish yellow with dorsal and lateral sclerotized spots dark brown to black. Dark spots with dark, curved and elongate hair-like setae.
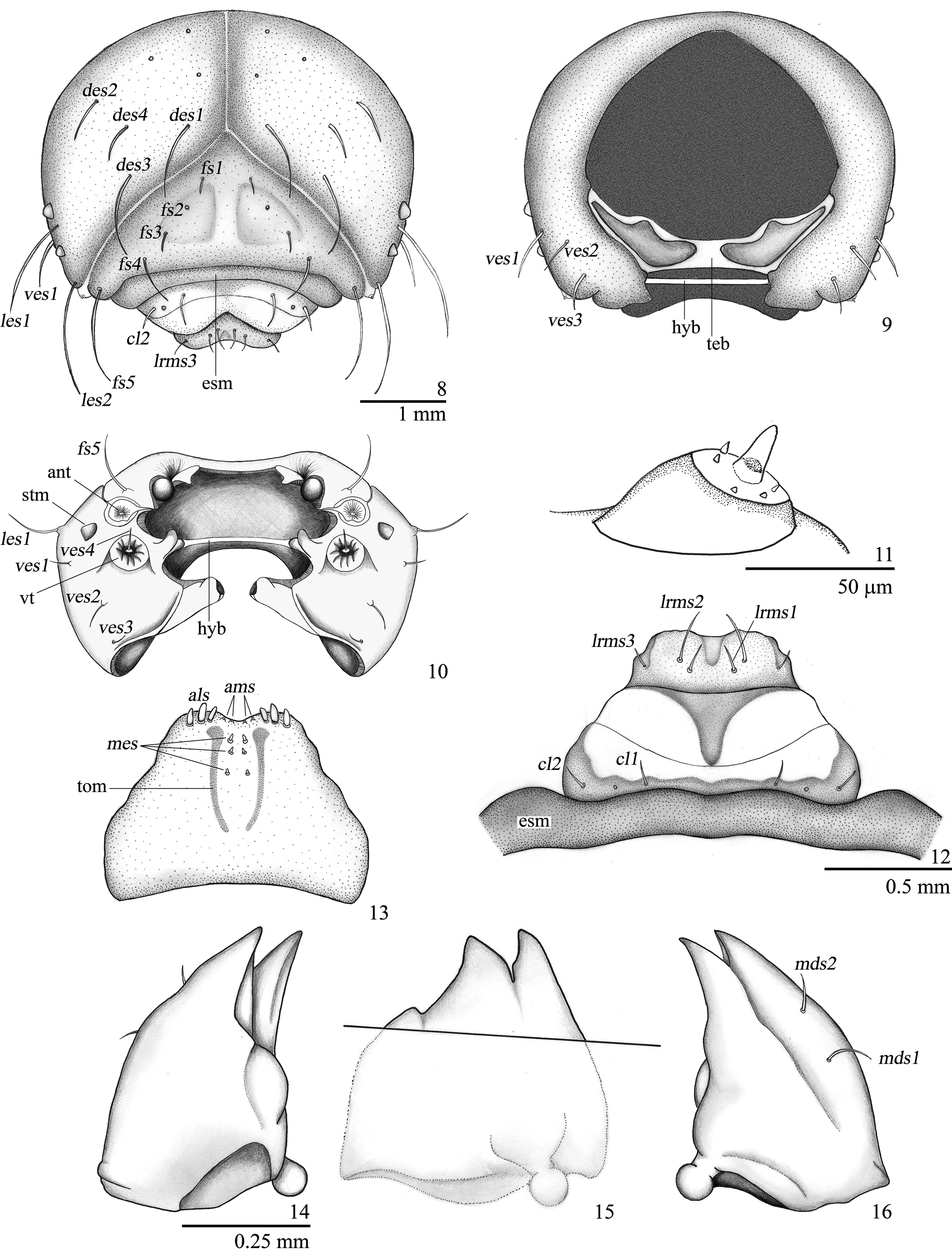
Head ( Figs 8–10 View FIGURES 8 – 16 ) hypognathous, sclerotized, free, head capsule rounded in shape, about 1.4 times as wide as long. Epicranial suture distinct, approximately 0.45 times as long as head capsule. Frontal suture distinct, complete, weakly arcuate, V-shaped. Median endocarina absent. Postoccipital condyles absent. Hypopharingeal bracon (hyb) present, clear ( Fig. 9 View FIGURES 8 – 16 ). One pair of convex stemmata present on each lateral side. Antennae exposed ( Fig. 11 View FIGURES 8 – 16 ), onesegmented, with conical accessory appendage elongate, about two times as long as basal width and with five minute processes. Head capsule with four pairs of dorsal epicranial setae (des 1–4), des1 and des3 positioned along frontal suture, des2 and des4 placed more laterally; four pairs of frontal setae (fs), fs2 missing, fs5 longer than fs4; two pairs of lateral epicranial setae (les), les1 located between stemmata, les2 placed near anterior angle of head capsule; four pairs of ventral epicranial setae (ves), ves 4 placed on a prominent tubercle (vt) ( Fig. 10 View FIGURES 8 – 16 ). Epistoma and frontoclypeal sutures slightly arcuate. Clypeus ( Fig. 12 View FIGURES 8 – 16 ) transverse, with two pairs of clypeal setae (cls 1–2), anterior margin concave. Labrum ( Fig. 12 View FIGURES 8 – 16 ) transverse, with three pairs of labral setae (lrms1–3), anterior margin emarginate (sinuate), lateral margins rounded, posterior margin with median pigmented projection. Epipharynx ( Fig. 13 View FIGURES 8 – 16 ) with two pairs of anteromedian setae (ams 1–2), three pairs of anterolateral setae (als 1–3), 3 pairs of median setae (mes1–3) between dark, thick, elongate, convergent labral rods. Mandibles ( Figs 14–16 View FIGURES 8 – 16 ) symmetrical, stout, apically bidentate, inner margin with a small accessory teeth ( Fig. 15 View FIGURES 8 – 16 ), lateral margin with two long setae (mds). Maxillae ( Figs 17 and 18 View FIGURES 17 – 23 ) with cardo transverse, sub-rectangular; stipes elongate, with one stipal (stps) and two palpiferal setae (pfs1–2) all equal length; mala rounded, with: two malar basiventral setae (mbs), five marginal dorsal short spatulate setae (dms) and five smaller ventral setae (vms) more or less aligned along outer margin; palpifer membranous; maxillary palpus two-segmented, proximal palpomere slightly larger than distal one, with a seta and two sensilla on ventral side. Labium ( Figs 17 and 18 View FIGURES 17 – 23 ): prementum with three pairs of setae, posterior pair very long, two anterior pairs very short; premental sclerite V-shaped, with an anterior median extension; labial palpi two-segmented, proximal palpomere transverse, much wider than distal palpomere, conical, slightly longer than wide; ligula with a V-shaped area bearing asperities; postlabium with lateral pairs of postlabial setae (plbs) much closer together than median pair.
Thorax ( Figs 1–3 View FIGURES 1 – 7 , 24, 27 and 30 View FIGURES 24 – 32 ). Pro-, meso- and metathorax transverse, width increasing backwards. Prothorax: pronotum with two transverse, large, contiguous sclerites, each one with six pronotal setae (prns 1–6); pedal area with one sclerite and two pedal setae (pdas 1–2); ventropleural lobe with an asetose sclerite; mediosternal fold with a sclerite and two mediosternal setae (msts 1–2); ambullatory ampulla lobate, asetose. Meso- and metathorax: prodorsum of meso- and metathorax with two pairs of dark sclerites, inner pair with one prodorsal seta (prs), outer pair of sclerites asetose. Postdorsum of meso- and metathorax with one pair of dark sclerites, each one with a postdorsal seta (pds). Alar area of meso- and metathorax with a rounded sclerite, with two alar setae (as 1–2); dorsopleural lobe of meso- and metathorax with a large irregular-shaped sclerite, each one with a dorsopleural lobe seta (dpls); ventropleural lobe, pedal area and mediosternal fold with one bisetose sclerite each (vpls 1–2, pdas 1–2, msts 1–2); ambulatory ampullae bilobate. Prothoracic spiracle ( Fig. 19 View FIGURES 17 – 23 ) bicameral, peritreme circular, air tubes with nine annuli, directed upwards.
Abdomen ( Figs 1–3 View FIGURES 1 – 7 , 25, 26, 28, 29, 31 and 32 View FIGURES 24 – 32 ) with 8 pairs of lateral spiracles ( Figs 20 and 21 View FIGURES 17 – 23 ), bicameral, peritreme circular, air tubes with nine annuli, obliquely caudad; spiracles of abdominal segments I–VII similar, spiracles of segment VIII smaller, with air tubes directed backwards. Abdominal segments I–VIII each one with three folds, fold indistinct in segment IX. Dark sclerites and setation similar on segments I–VI ( Figs 25, 28 and 31 View FIGURES 24 – 32 ): prodorsum with one pair of sclerites, inner sclerite with a prodorsal seta (prs), outer sclerite asetose; sclerite of spiracular area triangular and unisetose (ss); postdorsal fold with one unisetose sclerite (pds); dorsolateral area with two bisetose sclerites (dls 1–4); dorsopleural lobe with a bisetose sclerite (dpls 1–2); ventropleural lobe, laterosternal lobe and mediosternal fold with a unisetose sclerite each (vpls, lsts, msts); ambulatory ampulla circular. Lobes and chaetotaxy of segment VII similar to I–VI ( Figs 26, 29 and 32 View FIGURES 24 – 32 ), but dorsolateral area with a single bisetose sclerite (dls 1–2). Lobes and chaetotaxy of segment VIII ( Figs 26, 29 and 32 View FIGURES 24 – 32 ) similar to segments I–VII, but spiracular area with two sclerites, the larger bisetose (ss 1–2); segment IX: dorsolateral area( Figs 26, 29 and 32 View FIGURES 24 – 32 ) with a large sclerite with four dorsal setae (ds 1–4); pleural lobe ( Fig. 32 View FIGURES 24 – 32 ) with two contiguous sclerites, similarly sized and unisetose (ps 1–2); ambulatory ampullae transversal. Segment X reduced, placed dorsally to segment IX ( Fig. 26 View FIGURES 24 – 32 ), with a pair of small, dark, unisetose sclerites; anus opening dorsally.
Alimentary canal ( Fig. 22 View FIGURES 17 – 23 ) lacking mycetomes; posterior ventriculus two coiled, with about 36 short rodlike gastric caeca, nearly contiguous, arranged in a sinuous row on each side of lower ventricular coil. Malpighian tubules not thickened.
Pre-pupa description ( Figs 4–7 View FIGURES 1 – 7 ). Body stout, very shortened and curved, C-shaped. Color predominantly orange, not showing greenish shades, dark brown to black sclerites closely grouped and less distinct, last abdominal segments telescoped. Abdominal ambulatory ampullae retracted and almost indistinct.
Pupa description ( Figs 33 and 34 View FIGURES 33, 34 ). Length: 8.0–9.0 mm. Adecticous and exarate. Coloration cream, abdomen pinkish, with weakly curved setae, concentrated mainly on dorsal side. Head completely covered by pronotum when seen from above; on each side with one vertical setae (vs), two approximate short super orbital setae (sos 1–2) and one orbital setae (os) located near eye margin; rostrum with lateral margins arcuate, on each side with two short postantennal setae (pas 1–2) and two short rostral setae (rs 1–2). Pronotum conical, transverse, with two transverse lateral depressions; discal area with a pair of dorsal setae (ds); on each side with two pairs of apical setae (as 1–2), three lateral setae (ls 1–3), and three posterolateral setae (pls 1–3). Mesothorax with two setae located medially near anterior margin, one pair located anterolaterally and eight setae located posteriorly and forming an irregular transversal row. Metathorax with eight anterior setae of similar length and one pair of shorter setae located posteromedially. Abdomen: segments I to VII with five pairs of setae, the median pair longer and placed over a weak protuberance; segment III strongly constricted, much narrower than segments II or IV; segments I–VI with a latero-dorsal dark pink rounded depression on each side. Each leg with two femoral setae (fes 1–2) of similar length. Pterothecae extending up to apex of fourth ventrite. Anterior coxae rounded and prominent, unisetose. Abdomen with six pairs of annular spiracles on segments I–VI, elongate oval, visible in dorsal view. Abdominal segment IX visible only in ventral view, tergal area with a group on four long setae on each side and a pair of micro-setae; sternal area with a pair of micro-setae.
Remarks. The subfamily is divided into two tribes, the Holarctic Hyperini Marseuil, 1863 and the Cepurini Capiomont, 1867, distributed mainly in the Southern Hemisphere (Alonso-Zarazaga & Lyal 1999). Taxonomy and natural history of the Cepurini, is scanty. Only the ecology and behaviour of adults and immatures of P. distigma are well known. Furthermore, no detailed descriptions of larva and pupa of any Cepurini species have been published thus we present a preliminary comparison with the described larvae of species of two genera of Hyperini : Hypera Germar, 1817 and Donus Jekel, 1865 . Larvae of P. schuppeli are similar to that of H. nigricornis (Fabr., 1775) and H. rumicis (L., 1758) described by Scherf (1964), the latter redescribed by Skuhrovec (2006), larva of H. punctata (Fabricius, 1775) described by May (1993), larvae of 19 species of Hypera described or redescribed by Skuhrovec (2004 and 2006), and larvae of Donus described by Skuhrovec (2007). The known last instar larvae of Hypera and Phelypera share the frontoclypeal suture arcuate, premental sclerite sclerotized, V-shaped and well distinct. They differ mainly by the labial palpi which are 1-segmented in Hypera ( May 1993 and Skuhrovec 2004 and 2006) but 2-segmented in P. schuppeli , and by the body hairs which can vary from hair-like to scale-like in Hypera while only hair-like setae occur in P. schupelli . Larvae of Phelypera differ from larvae of species of the genus Donus mainly by the following characteristics present in Donus , according to Skuhrovec (2007) (characters of Phelypera parenthetic): frontoclypeal suture slightly concave medially (frontoclypeal suture arcuate), mandibles with three or four teeth apically (with two apical teeth), premental sclerite indistict (premental sclerite sclerotized, V-shaped and well distinct). Another difference is the presence, in Donus , of club-like, bacciliform and hair-like setae, while in Phelypera the setae are hair-like.
The pupa of P. schuppeli ( Figs 33 and 34 View FIGURES 33, 34 ) is very similar to that of H. rumicis (L.1758), H. nigrirostris (Fabricius, 1775) and H. postica Gyllenhal, 1813 (as H. variabilis Hbst. ) described by Scherf (1964), and H. arundinis (Paykull, 1792) described by Gosik (2007). We compare P. schuppeli with the latter since the description presented by Gosik (2007) is much more detailed than that presented by Scherf (1964). The main differences are the presence of 2 pairs of orbital setae (os1 and os2) in H. arundinis , and one pair (os1) in P. schuppeli ; abdominal segments I- VI with 16 dorsal setae in H. arundinis and 10 ds in P. schuppeli ; and presence of 10 ventral setae in H. rumicis but vs absent in P. shuppeli ; and the third abdominal segment which is constricted in P. schuppeli but not constricted in H. arundinis . The sexual dimorphism is similar in pupae of both species, the gonothecae are undivided in males and semicircularly divided in females.
Marvaldi (2003) published a preliminary but very useful key to larvae of the South American families and subfamilies of Curculionoidea, based on external morphological characters. In the Curculionidae , 18 subfamilies were considered, Hyperinae among them. Due to the lack of larval descriptions of South American Cepurini, characters of the larvae of Palaearctic Hyperini were used in the key. The larva of P. schuppeli would key out to couplet 20 in Marvaldi's key that leads to Hyperinae , agreeing in "frontal seta 5 (fs5) longer than frontal seta 4 (fs4); body pigmented, with ambulatory ampullae; ectophytic on leaves", but disagreeing in the following characters (characters of P. schuppeli parenthetic): head with maculae (head not maculate, uniformly dark colored); some dorsal setae of body short and expanded at apex (dorsal setae hair-like). More descriptions of Cepurini larvae are required to provide a better knowledge of the morphological character variation within the tribe and to supply a diagnosis for the tribe.
Host plant. Pachira aquatica Aubl. (Bombacaceae) is a tropical wetland tree which ranges from Mexico, through Central America and into Amazonian South America. It can grow up to 16 m and the seeds are edible ( Lorenzi 2000). Due to its dense and spreading canopy, it was introduced as an ornamental shade tree for the urbanization of streets in many Brazilian cities (including Bauru, São Paulo; Dourados, Mato Grosso do Sul and Pirenópolis, Goiás, localities where the studied specimens were collected) located in frost-free areas outside its native range. The plant is cultivated in East Asia ( Japan and Taiwan) as an interior decorative green plant and commercially sold on internet web sites under the name “money tree”.
Biological notes on natural history. According to Ferreira & Camargo (1989) and Diniz & Morais (1996), oviposition can occur day or night. The eggs are laid in galleries constructed by females, with the help of rostrum, inside the central vein of the leaf or in the petiole of young leaves of terminal branches. Up to 80 eggs can be laid by one female inside the gallery. The eggs are elongate oval, measuring about 1 mm in length, and are light yellow. The eggs take three days to hatch and the emergence of the larvae is simultaneous. Larval development has four instars and lasts, on average, six or seven days. First instar larvae are about 1.5 mm in length. Fourth instar larvae collected by us in the field varied from 13 to 16 mm in length. Fourth instar larvae remain grouped on the adaxial leaf surfaces, forming groups of up 50 individuals ( Fig. 37 View FIGURES 35 – 40 ). The larvae begin to eat holes in the leaves ( Fig. 35 View FIGURES 35 – 40 ), next they enlarge the feeding area including the lateral margins ( Fig. 36 View FIGURES 35 – 40 ) and eventually almost completely consume the leaf surface, leaving only the main veins untouched ( Fig. 39 View FIGURES 35 – 40 ). The larvae can partially destroy the apical meristemes and can be important defoliators of the host plant, as was observed for Phelypera griseofasciata by Bondar (1943) and for P. d is ti gm a by Janzen (1979). Garcia (1999) sampled 1800 ornamental trees in the urban area of Goiânia (GO) and observed that Pachira aquatica was the most common, representing 62.7% of the trees, and Phelypera schuppeli was one of the five most frequent insects, occurring on 43.4% of the host trees; however, the damages caused by the weevil to its host were considered not as severe as those inflicted by two other beetles, Steirastoma breve (Sulzer, 1776) (Cerambycidae) and Euchroma gigantea (L., 1758) ( Buprestidae ).
The larvae are very conspicuous due to their bright black and yellow coloration ( Fig. 37 View FIGURES 35 – 40 ). It is likely that the coloration of Phelypera larvae is aposematic and directed against vertebrate predators ( Janzen 1979, Costa et al. 2004), mainly insectivorous birds as suggested by Bondar (1943). The grouping behavior probably increases the warning effect against predators. Furthermore, the dorsum of some larvae observed in the field was covered with feces ( Fig. 38 View FIGURES 35 – 40 ), as already observed in P. griseofasciata by Bondar (1943) and in P. schuppeli by Ferreira & Camargo (1989). Feces are produced by the larvae themselves and held above their bodies with the aid of the long and stiff dorsal and lateral setae. The dorsal opening of the anus ( Fig. 23 View FIGURES 17 – 23 ) facilitates placement of the feces. Excremental covering behavior was reported in the Cepurini Isorhinus undatus (Champion, 1902) in Panamá by Aiello & Stockwell (1996) and Haplopodus submarginalis (Boheman, 1840) in Rio de Janeiro, Brazil by Abreu et al. (2003). It is also known in other exophytic beetle larvae such as Cassidinae and Criocerinae (Chrysomelidae) and Cionini (Curculionidae) ( Crowson 1981). This kind of camouflage was not mentioned for P. d i s t i g m a by Fizgerald et al. (2004) and Costa et al. (2004), and was not observed in the Bauru (São Paulo) and Dourados (Mato Grosso do Sul) populations of P. schuppeli , represented by mature larvae. The cycloalexic formation (sensu Jovilet et al. 1990) characteristic for P. distigma (Fizgerald et al. 2004 and Costa et al. 2004) has not been observed in P. schuppeli , neither in the field nor in the laboratory. The circular aggregation reported for P. distigma by Fizgerald et al. (2004) and Costa et al. (2004) has not been observed either.
Three distinct behaviors using mandibles were observed in P. schuppeli : a) larvae can grab the margin of an adjacent leaf with their mandibles, making it possible for their bodies to pass from one leaf to another thus facilitating dispersal or escape; b) larvae can strongly hold onto a leaf with their mandibles making difficult to dislodge them, as was also reported for P. d i s t i g m a by Fizgerald et al. (2004); and c) larvae can try to or actually bite when disturbed by an aggressor. Also, when disturbed, the larvae can wag the head towards the disturbance, if as wanting to strike something. Occasionally, the larvae can raise the anterior fourth of the body backwards, looking like an inverted C-shape. The larva can move alternately the raised portion of the body forwards and backwards, or remain motionless with the head directed backwards. This behavior is different from the head vibration performed by moving larvae of P. d i s t i g m a and may be associated with acoustic or vibrational communication among conspecifics, enciting group activity (Costa et al. 2004). It was also observed that the larva can eject a dark liquid from its mouthparts, a defensive behavior, well known in other insects, and described for P. schuppeli by Ferreira & Camargo (1989) and for P. d i s t i g m a by Fizgerald et al. (2004).
The globular lattice-like pupal cocoons are translucent white ( Figs 40–45 View FIGURES 35 – 40 View FIGURES 41 – 46 ). In the field they were found attached to the adaxial face of the leaves, usually forming clusters, sometimes one cocoon above the other ( Fig.40 View FIGURES 35 – 40 ). A few cocoons were also found attached to the ground. The cocoon is built by the last instar larvae with materials extruded from their anus. According to Crowson (1981), the nature and origin of these substances have scarcely been investigated, and the material in weevils is most probably formed by the peritrophic membrane rather than produced by the Malphigian tubules, the latter being the usual source in the Coleoptera . However, Kenchington (1982) published a X-ray diffraction study on the larval cocoon silk of the weevils Hypera postica (Gyllenhal, 1833) and H. rumicis (L., 1758) and showed using histological preparations of larvae about to spin the cocoon, that the lumen of the Malpighian tubules were filled with silk secretion, and that the silk was most likely secreted by the Malpighian tubules and stored in the rectum. Our observations of the mature larva of P. schuppeli , showed that, with the help of the mouthparts, the larvae gather the liquid secretion extruded from the anus ( Fig. 43 View FIGURES 41 – 46 ) and rapidly place it – as it hardens very quickly – on the adaxial face of the leaf surface such that it forms the attachment point of the cocoon. Next, the larva catches more material from the anus and molds strings with the mandibles, which are stuck to the cocoon base. The larva continues catching secretion, molding strings, and building an irregular and very loose lattice around its body ( Figs 41 and 42 View FIGURES 41 – 46 ). The remarkable aspect of the construction is how the larva gives the final shape to the cocoon, by building, tearing up and rebuilding it. By pressing the body against the newly constructed lattice, over and over, the larva breaks and stretches some parts of the not yet completely hardened cocoon mesh ( Fig. 41 View FIGURES 41 – 46 ). Immediately after, the larva repairs the damage, constructing a new set of strings or reinforcing the old ones. The entire process of cocoon construction takes approximately two hours and agrees well with the observations published for Coniatus repandus by Fornasari (2004).
As soon as the construction of the cocoon is accomplished, the larva defecates. Next, the yellowish shades of the body coloration starts to change to orange, the body bends and shortens, the larva molts and the pupa emerges. The pupa remains free inside the cocoon ( Figs 44 and 45 View FIGURES 41 – 46 ). The pupal phase lasts about four days. The teneral adult remains about 12 hours inside the cocoon and breaks the lattice walls of the cocoon with its mandibles to get free ( Ferreira & Camargo 1989). Just after getting free, the adult feeds on completely the cocoon ( Fig. 46 View FIGURES 41 – 46 ) and disperse. The same behavior was observed in the Cepurini Ishorhinus undatus by Aiello & Stockwell (1996); however, in the Hyperini Donus velutinus (Boheman, 1842) and Coniatus repandus Germar, 1817 , the adults ate their way out of the cocoons but did not consume them completely ( Fornasari 2004; Skuhrovec 2009).
Adults of P. schuppeli feed on the young branches, unlike the larvae which prefer the young expanding leaves. The length of the entire cycle, from egg to adult, under laboratory conditions lasts about 16.6 days ( Ferreira & Camargo 1989). Ferreira & Camargo (1989) and Diniz &Morais (1996) reported many aspects of the biology of Pteromalidae parasitoids that attack pre-pupae and pupae of P. schuppeli . During our study, we discovered some other aspects regarding possible natural enemies of P. schuppeli . We observed larvae of P. schuppeli being fed upon in the field (Pirenópolis, GO) by two species of Heteroptera. Photographs were sent to Dr. Jocélia Grazia Vieira (Universidade Federal do Rio Grande do Sul) who identified the species as Dysdercus sp. ( Pyrrhocoridae ) and Supputius cincticeps (Stål, 1860) (Pentatomidae) . Dysdercus sp. was found feeding on the dead body of a parasitized pre-pupa inside the cocoon ( Fig. 48 View FIGURES 47 – 52 ). The genus Dysdercus comprises phytophagous species, but some are known to resort to cannibalism or necrophagy in search of water (José Antônio Marin Fernandes, pers. comm.; Goodchild 2009). Aiello & Stockwell (1996) also reported Dysdercus sp. feeding on larvae of Isorhinus undatus that were in the process of making their cocoons. According to those authors (l.c.), in Panamá, these bugs are normally seed predators of Pseudobombax spp. ( Malvaceae ). However, attacks by species of Dysdercus on active feeding larvae of Hyperinae were not observed. Supputius cincticeps was observed sucking a weevil larva, holding its body with the rostrum ( Fig. 47 View FIGURES 47 – 52 ). Fizgerald et al. (2004) reported a species of Pentatomidae piercing the larva of P. distigma and dragging it from a small cycloalexic formation. Ferreira & Camargo (1989) also noticed predation of P. schuppeli larvae by unidentified assassin bugs ( Reduviidae ).
We observed micro-wasps passing through the cocoon meshes to lay eggs inside the pre-pupa. The wasps ( Fig. 50 View FIGURES 47 – 52 ) were identified by Dr. Valmir Antonio Costa (Instituto Biológico de Campinas, SP) as Jaliscoa nudipennis Bouček, 1993 (Pteromalidae) . The wasps seemed to select the intersegmental lateral and ventral regions of the abdomen as oviposition sites, usually between the segments III/IV and IV/V. When molested by wasps, the weevil pre-pupa can quickly turn around its body inside the cocoon, thus hampering the parasitoids to lay their eggs. However, this behavior is not effective enough to deter the wasps from ovipositing. According to Bondar (1943), parasitoid wasps can also lay the eggs inside the pupae of P. griseofasciata . The pre-pupal phase lasts 3.8 days in healthy individuals but consumes more time in parasitized ones, according to Ferreira & Camargo (1989). This longer time may be caused by the parasitoids, which can release chemicals that inhibit or delay the ecdysis to the pupal phase ( Sharkey & Fernández 2006). The parasitoidism results in the shrinkage and death of pre-pupae or pupae, as observed by previous authors ( Bondar 1943; Ferreira & Camargo 1989; Diniz & Morais 1996). The wasp larvae leave the host and spin a cocoon which is attached by a posterior peduncle to the weevil cocoon ( Fig. 49 View FIGURES 47 – 52 ), as reported by Lima (1956) and Diniz & Morais (1996). As soon as the adult wasps emerge, they begin to copulate. Wasps are short-lived and die two to three days after the adult emergence.
Jaliscoa nudipennis View in CoL is remarkable in the strong polymorphism of the male head, which varies from small round (as in females, Fig. 51 View FIGURES 47 – 52 ) to large transversely diamond shaped and with eyes more distantly separated ( Fig. 52 View FIGURES 47 – 52 ). Parasitoidism of wasps on pre-pupae of P. schuppeli View in CoL was previously reported by Lima (1920), Ferreira & Camargo (1989), and Diniz & Morais (1996), probably by the same species of Pteromalidae View in CoL . Ferreira & Camargo (1989) sent pteromalid wasps to Dr. E. Eric Grissel (Systematic Entomology Laboratory, U.S. Department of Agriculture, Washington), who identified the species as belonging to a new genus near Trichokaleva Bouček, 1972 View in CoL . According to Dr. Walmir Costa (pers. comm.), Diniz & Morais (1996) have collected the same wasp species on P. schuppeli View in CoL between 1990 and 1992. On that occasion, wasps were sent to Dr. Zdeneck Bouček (The Natural History Museum, London) who identified the pteromalid as belonging to an undescribed genus related to Psilocera Walker, 1833 View in CoL . Dr. Bouček described the genus Jaliscoa View in CoL in 1993, based on a single female from Jalisco ( Mexico) and without host record, but unfortunately he had no time to study and include the Brazilian specimens sent by Dr. Diniz and Dr. Morais in his paper, which was concerned with North and Central American species of Pteromalidae View in CoL and Tetracampidae View in CoL . Voucher specimens of Jaliscoa nudipennis View in CoL were deposited in the MZSP.
Our data support the hypothesis that cocoons provide an anchorage point to accommodate a pupa restraint to the leaf substrate, but not that they afford protection against the attacks of parasitoids and some predators. However, Aiello & Stockwell (1996) reported a failed attempt of an assassin bug to pierce an immature of Isorhinus undatus View in CoL inside a cocoon. They suggested that the cocoon can protect the weevil immatures from some predators, mainly bugs, because the holes are small enough to prevent the predator’s rostrum from reaching the cocoon occupants. Further observations would be desirable to clarify this matter.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Phelypera schuppeli (Boheman, 1834)
| Vanin, Sergio Antonio, Bená, Daniela De Cassia & Albertoni, Fabiano Fabian 2012 |
Trichokaleva Bouček, 1972
| Boucek 1972 |
Psilocera
| Walker 1833 |