Keratosminthurus, Zeppelini & Brito & Zampaulo & Lima, 2020
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4729.1.2 |
|
publication LSID |
lsid:zoobank.org:pub:6EA7B4E9-CF60-44BB-A78C-F63141684A6E |
|
persistent identifier |
https://treatment.plazi.org/id/4444F169-FFA7-5E23-2DB3-F940FE6DF80F |
|
treatment provided by |
Plazi |
|
scientific name |
Keratosminthurus |
| status |
gen. nov. |
Keratosminthurus gen. nov. Zeppelini
Figs. 1–4 View FIGURE 1 View FIGURES 2–3 View FIGURE 4
Type species: Keratosminthurus tapigu gen. nov. sp. nov.
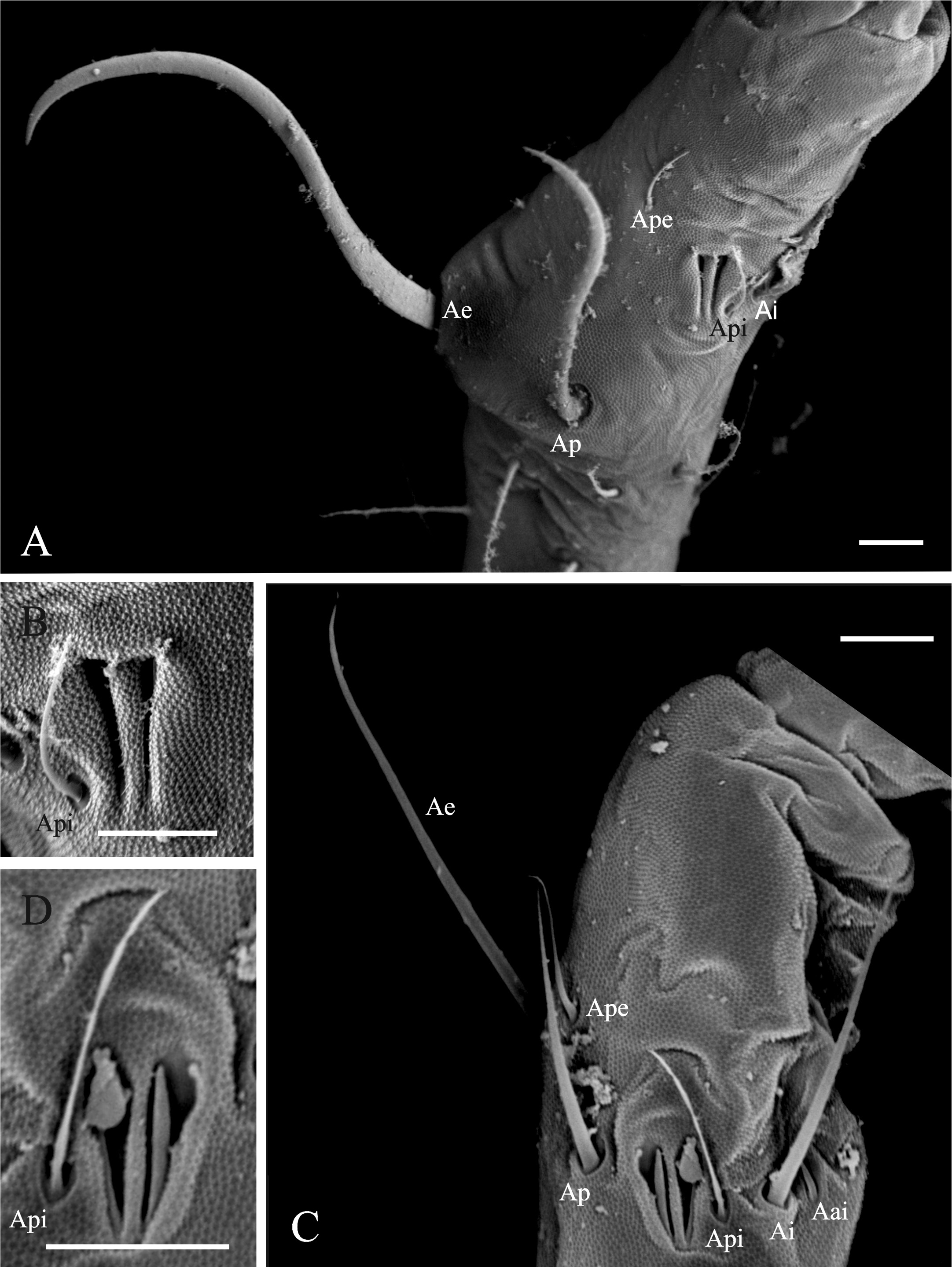
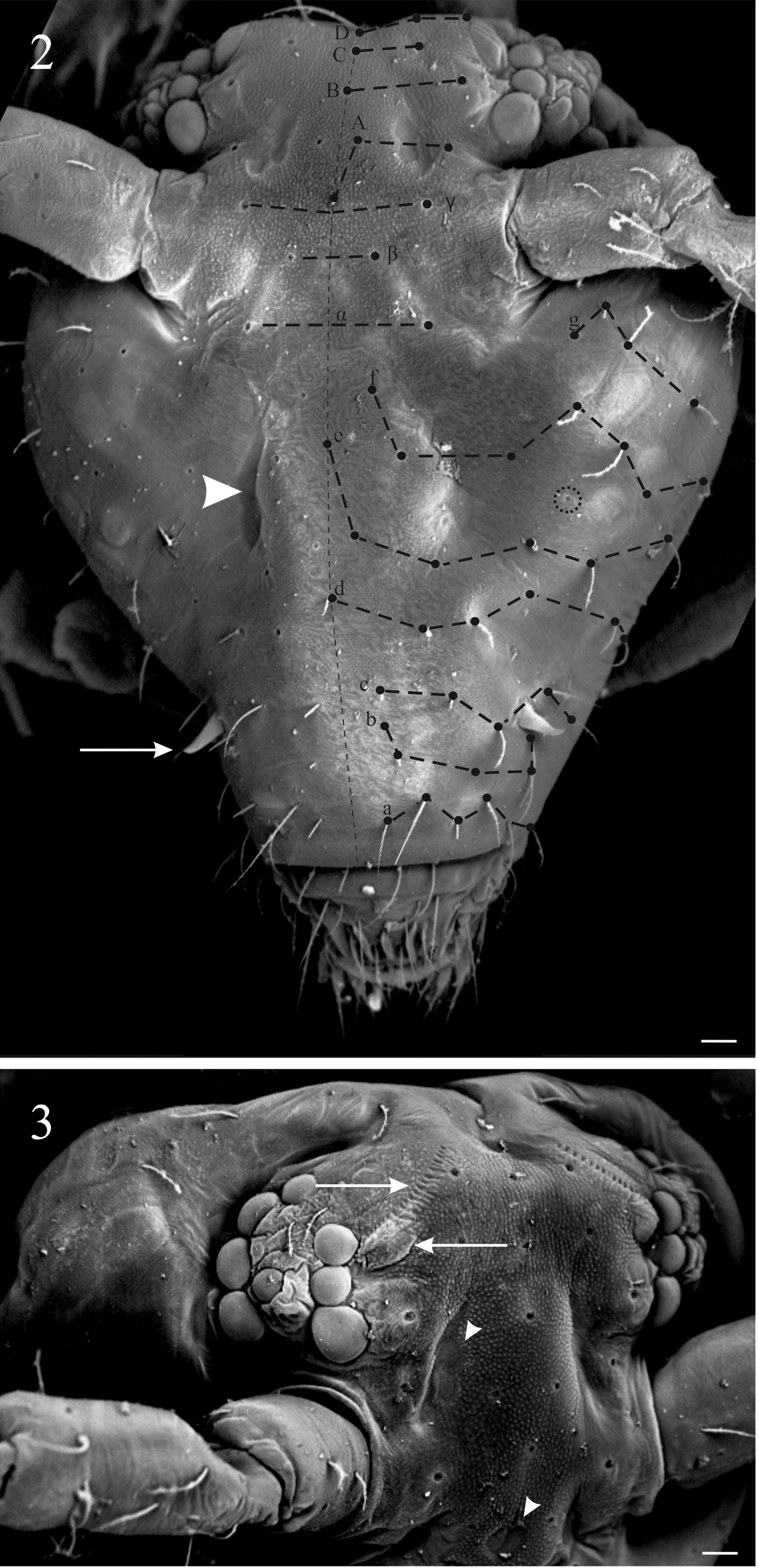
Diagnosis of the new genus. Sminthurinae with Ant. IV clearly subdivided (usually more than 15 subsegments), trochanteral spines present. Tunica present or absent, never filamentous, tenent-hairs acuminated. A pair of neosminthuroid chaetae, apex of mucro asymmetric, mucronal chaeta absent, anterior dens with 3:2:2:2:1:1:1 or more chaetae. Trichobothrial complex ABC forming an obtuse angle opening backwards and AB subequal to BC. Female subanal appendages large and thick, pointing to anal opening. Mature males with a pair of strongly sclerotized, twisted, horn-like Ap and Ae chaetae on AOIII ( Fig. 1A View FIGURE 1 ), a pair of lateral horn-like cuticular spines in the clypeus ( Fig. 2 View FIGURES 2–3 ), and a special organ composed of a vesicle adjacent to the eye patch and a line of crenulated openings at the interocular area ( Fig. 3 View FIGURES 2–3 ).
Etymology. K erato (kἐρατο) is Greek for horn, in allusion to the male dimorphism on antennae and clypeus.
Distribution and biogeographic considerations. C ollections from State of Pará (Carajás) Northern Brazil in Amazon Forest south to Amazon river, State of Pernambuco (Goiana) Northeastern Brazil in Atlantic Forest close to the coast, and in the regions of the “Iron Quadrangle” and northern of Minas Gerais State Southeastern Brazil in Cerrado Forest ( Fig. 4A View FIGURE 4 , C–D), found two related species which seem to be separated from each other by the Tocantins-Araguaia basin and São Francisco river ( Fig 4B View FIGURE 4 ). Despite all the collections done so far, the genus seems to be missing from the areas north to the Amazon river.
Remarks. Keratosminthurus gen. nov. is easily separated from Songhaicinae by means of the structure of the unguis and number of chaetae in the apical whorl of tibiotarsus ( Bernard & Wynne 2017), even the neosminthuroid chaetae, present in the new genus, is seen only in the genus Songhaica Lasebikan, Betsch & Dallai, 1980, among all Songhaicinae . The new genus combines characters from subfamilies Sminthurinae and Sphyrotecinae. The presence of a pair of Neosminthuroid chaetae, the asymmetrical mucronal tip, and the absence of the axial substitutions ams1 and as1 are features that occur in Sphyrotecinae ( Richards 1968; Betsch 1980, 1997; Betsch & Waller 1994; Bretfeld 1999). The numerous anterior chaetae on dens, the long antennae with clearly multidivided Ant. IV and the presence of secondary ams2 and ams3 chaetae on Abd. VI are diagnostic for Sminthurinae ( Betsch 1980, 1997, Betsch & Waller 1994), therefore, we redefined subfamily Sminthurinae to fit the new genus.
Keratosminthurus gen. nov. resembles Temeritas , Caprainea, Novakatianna and Pararrhopalites , all Sminthurinae genera with trochanteral spines and no post-antennal chaetae or dorsal abdominal glands. These genera, but Pararrhopalites, share the presence of 11 or more anterior chaetae on dens, and Ant. IV with at least 13 subsegments. The presence of long thick chaetae on Ant. III, even though in different number and distribution in each genus, is questionable as a diagnostic feature, as in the new genus those chaetae seems to be missing or found only in adult males as part of the AOIII, while females present only ordinary short chaetae in the antennae. In Temeritas there are several long and thick chaetae, either in basal and apical half of Ant. III. However, T. paradoxalis Medeiros & Bellini, 2019 and T. ormondae Arle & Oliveira, 1977 present only short ordinary chaetae on Ant. III, as exceptions for the genus. In contrast, Caprainea presents 4–5 such chaetae placed only in the basal half, while in Novakatianna there are 4 chaetae on the basal and one in the apical third of Ant. III. None of those long thick chaetae are component of the AOIII ( Betsch 1980; Nayrolles 1991), instead, adult males of the Keratosminthurus gen. nov. present a single pair of thick, twisted, horn-like Ae and Ap on AOIII ( Fig. 1A View FIGURE 1 ). The genus Pararrhopalites also lacks long chaetae on Ant. III, but usually has less than 11 chaetae on anterior dens (often 3,2,1…1 or 3,2,2,1…1), and most Neotropical species present about 8–11 Ant. IV subsegments. Nevertheless, P. palaciosi Zeppelini & Brito, 2014 stands as an exception. This is the only species of Pararrhopalites known to be epigean in the Neotropical region, with 8+8 eyes, Ant. IV 18-subsegmented, and anterior dens showing 3,2,2,2,2,1,1 chaetae, just like other Sminthurinae . The genus differs from Keratosminthurus gen. nov. by lacking the neosminthuroid chaeta and the sexual dimorphism.
Many aspects of the chaetotaxy, Ant. IV subsegmentation ( Table 1 View TABLE 1 ), antenna/body ratio, foot complex structure will differentiate those genera from Keratosminthurus gen. nov. (see Betsch 1980, 1997; Bretfeld 1999; Sánchez- García & Engel 2016; Bernard & Wynne 2017). The posterior cephalic chaetotaxy of the new genus is a point for discussion, Keratosminthurus gen. nov. presents axial chaetae at rows A, B, C and D as 5,3,3,5. The axial chaeta B is absent in all Sminthuridae , it is normally seen in Arrhopalitidae ( Betsch & Waller 1994) and some species of Pararrhopalites, e.g. Pararrhopalites palaciosi, P. hennigii ( Palacios-Vargas & Zeppelini, 1995), as exception in Sminthuridae .
Notwithstanding, the most striking features that differentiate the new genus from all other Sminthuridae are those related to its sexual dimorphism. The females are mostly alike all Sminthuridae , with a pair of large subannal appendages (mi5) pointing to anal opening. Males, at the other hand, present striking and unique sexual dimorphism, with modification in the Ap and Ae chaetae of the AOIII, a pair of horn-like spines in the clypeus and an unknown organ in the interocular area, supposed to be a sort of pheromone dispersant, as it is only present in males and has a glandular aspect.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.