Ophlitaspongia Bowerbank, 1866
|
publication ID |
https://doi.org/ 10.11646/zootaxa.5297.1.2 |
|
publication LSID |
lsid:zoobank.org:pub:1F89880E-AFBB-4392-87F6-913C99EBCABD |
|
DOI |
https://doi.org/10.5281/zenodo.7990955 |
|
persistent identifier |
https://treatment.plazi.org/id/446ADD2C-FFFB-DA0F-F9B4-FC6337D8F8CD |
|
treatment provided by |
Plazi |
|
scientific name |
Ophlitaspongia Bowerbank, 1866 |
| status |
|
Parent Ophlitaspongia Bowerbank, 1866 View in CoL View at ENA
Orig. name Ophlitaspongia (?) arbuscula Row, 1911:347-349 , pl. 39, fig. 22, pl. 40, fig. 25, text fig. 22 Accepted name Clathria (Clathria) arbuscula ( Row, 1911)
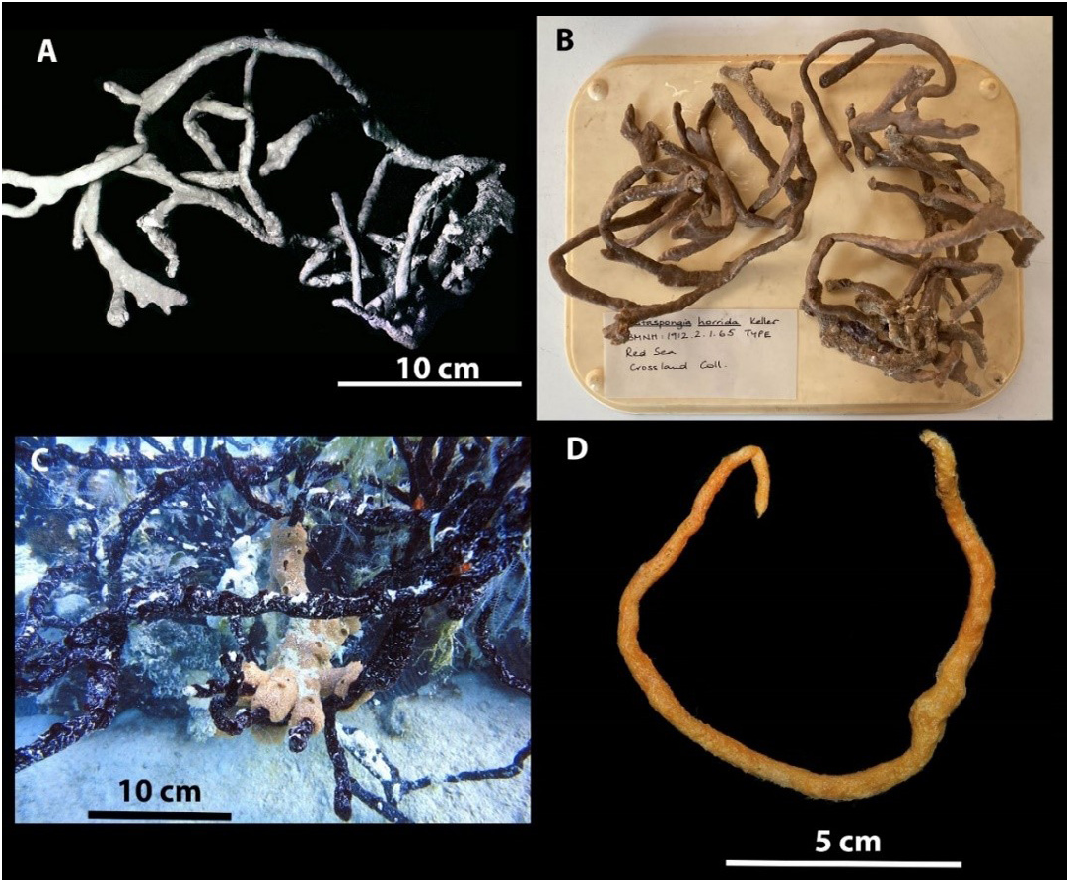
Material examined: Holotype BMNH 1912.2 .1.65, Red Sea, Coll. C. Crossland, 1904-1905. Slide of the holotype BMNH 1912.2 .1.65, housed at the Queensland Museum. QM G339446 , Hurghada, Egypt, Red Sea, 27 o 17.80’ N, 33 o 46.26’ E, 5-7 m. SCUBA Coll. M. A. L. H. Ezz El-Arab, October 2018, NIOF GRRF8000 . QM G339445 , same collection details as QM G339446 . GoogleMaps
Distribution: Known only from the fringing reefs of the Red Sea.
Description: Large tree-like sponge; long irregular branches, which themselves branch irregularly, arising from small base. The branches which form the sponge are irregular in shape, but usually more or less cylindrical ( Fig. 1 View FIGURE 1 ). Numerous prominences and swellings occur on the branches and short processes arise from these which are most likely new branches. The branches vary in their length, the longest is up to 100 cm, but are typically 50 cm. The frequent branching, the junctions which occasionally occur between contiguous branches, and the way the branches are tangled, tend to diminish the height of the actual individual. The branches vary in diameter from 5 to 10 mm.
The actual surface of the sponge is quite smooth, being covered by very delicate dermal membrane, but it is covered with slight prominences and low ridges, owing to the pushing up of the dermal membrane by skeletal fibres laying just below. However, there are no actual projections of the fibres from the surface, but the spicules project very slightly and render it minutely hispid.
The oscula are being distinguishable, the distance between the oscules vary from 2.5 to 3 cm, and the diameter of the oscula about 2 to 3 mm.
The color is brick red during life, while color in alcohol is dark brown black. The alcohol in which the sponge has been preserved is colored bright orange red. The texture is compressible and resilient, whilst the sponge does not cut nor tear easily.
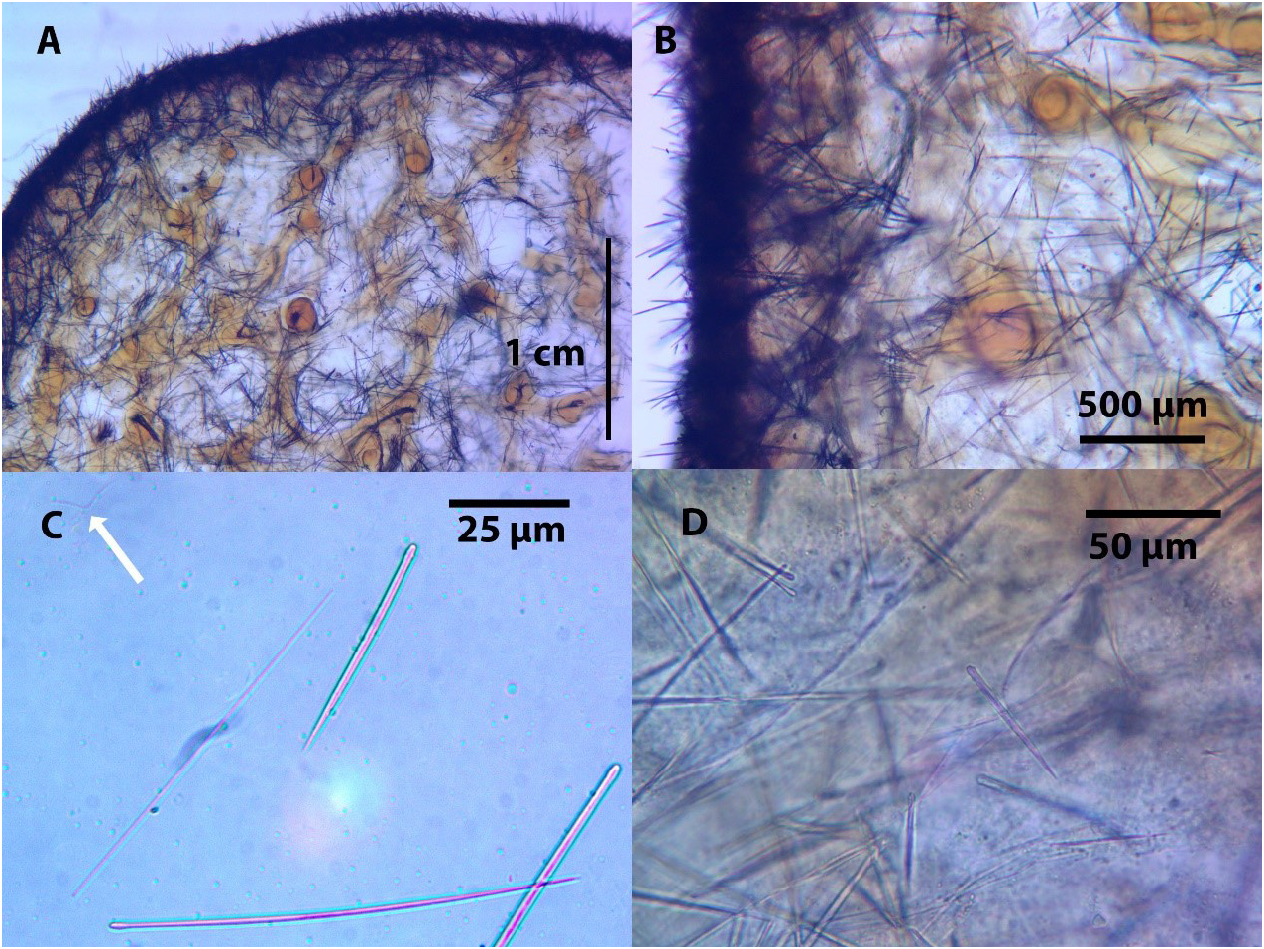
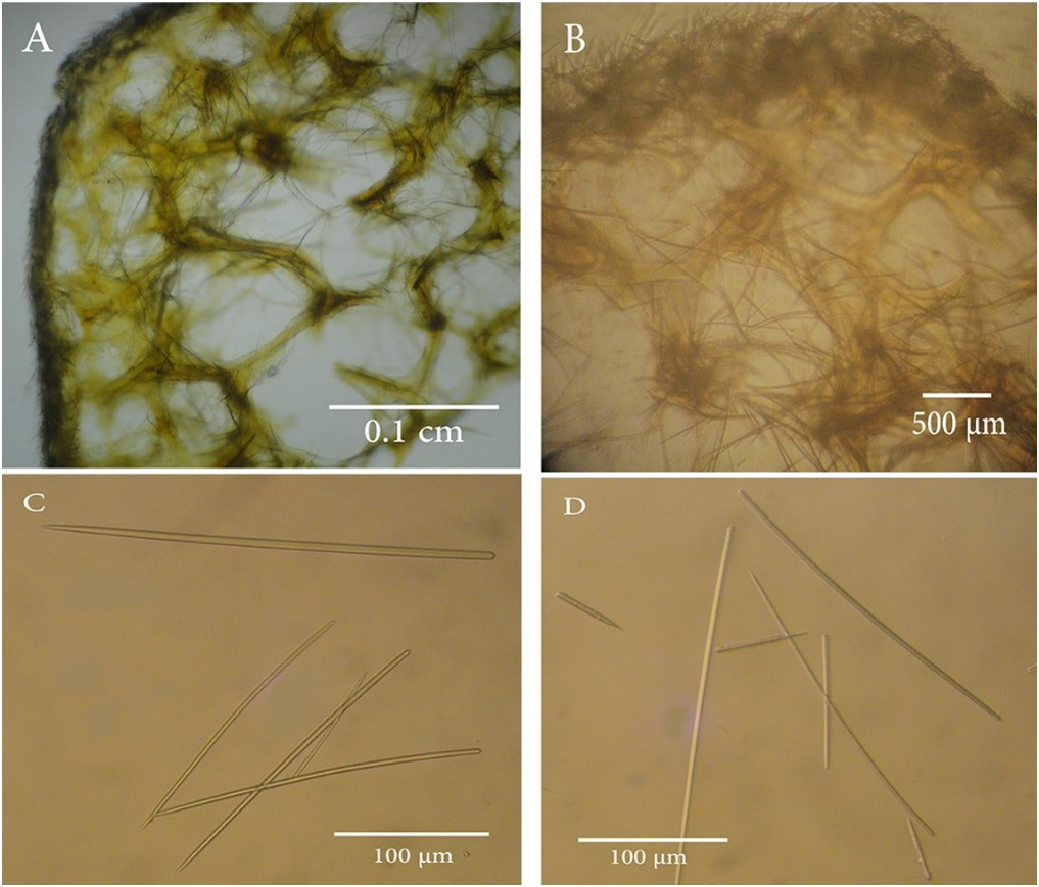
Skeleton: The choanosomal skeleton consists of a dense reticulation of (subtylo)styles and styles-cored fibres, and spicules scattered irregularly about throughout the sponge. The choanosomal (subtylo)styles or styles are usually thicker and often curved; as the predominant spicule type. The fibres are strongly coated with spongin and the reticulation is very close. There cannot be made a distinction into primary or secondly fibres, no separation of the fibres into groups being possible either in direction or size, for the reticulation, although the fibres are not orientated, they are regular, and of equal size. The fibres average 0.08 mm in diameter, but at a junction of two or more fibres, there is a slight swelling into a knob, which usually measures 0.12 mm in diameter ( Fig. 2A, B View FIGURE 2 ) ( Fig. 3A, B View FIGURE 3 ). The fibres at the sub-ectosome are condensed and irregularly oriented. The ectosomal skeleton consists mainly of densely matted brushes of tangentially orientated auxiliary (subtylo)styles which are usually straight and thin. The ends of the (subtylo)styles project from the surface, resulting in the ectosomal skeleton forming a dense felting over the surface of the sponge ( Fig. 2A, B View FIGURE 2 ).
Spicules:
Megascleres: The spicules consist predominately of (subtylo)styles, which are frequently curved, with slight necks ( Fig. 2C View FIGURE 2 ) ( Fig. 3C, D View FIGURE 3 ). They range in length from 79 to 439 µm in the fresh material and 85 to 300 µm in the holotype, while the original description ( Row, 1911) ranged between 300–330 µm in the length ( Table 1). They are slender in width between 1 and 8 µm in the fresh material and between 1 and 4 µm in the holotype, while the original description ( Row, 1911) recorded 2 µm width ( Table 1). The (subtylo)styles form a slender core in most of the fibres, where they are frequently arranged in a slightly plumose manner, but they never project outside the fibre. A few spicules occur with oxeote or stylote ends.
(Acantho)styles are present in small numbers. They are weakly spined, with a length of 22 to 69 µm, and a width of between 2.4–6.5 µm ( Table 1). These were also observed during examination of the holotype during this study with a range of length from 38 to 60 µm, and a range of width between 2 and 5 µm. The (acantho)styles were not recorded in the original description ( Row 1911), ( Table 1). In the sample examined in Egypt (NIOF GRRF8000) (acantho)styles were also observed, with a length of 70 µm, and a width of between 4–6 µm ( Table 1) ( Fig 3. D View FIGURE 3 ).
Microscleres: The microscleres consist of toxas that are rare in most samples ( Table 1). In the fresh material, toxas range in length from 15 to 300 um, ( Table 1). Whilst in the holotype the toxas ranged in length from 25 to 160 µm which encompasses the 60 µm from the original description ( Row, 1911).
Very rare sigmas were observed twice in the holotype, with a length of 20 µm. also, very rare sigmas were observed in the sample examined in Egypt ( NIOF GRRF8000 ), with a length of 15 to 30 µm. It is unclear if these are native as they were not observed in the original description and other fresh material .
Palmate isochelas (from 20 to 30 µm in length), were observed in some of the material NIOF GRRF8000 , but as they were not observed in the type material or any of the other material examined ( Table 1), they are regarded as non-native .
Remarks: A re-examination of the holotype and the fresh material revealed the presence of rare (acantho)styles, which were not mentioned in the original text by Row (1911). However, the measurements of the styles and toxas in this study match those of the original description. The styles, whilst predominantly (subtylo)styles, also include oxeote or stylote ends as mentioned by Row (1911). The styles on their length could potentially be split into two overlapping groups. However, due to the variable ends of the styles which extends over both potential groups indicate this is more likely to just be an example of variation. The styles are not specific for fibers of ectosomal regions, however the styles located in the fibers do tend to be the larger styles, and the small styles only seem to occur in the ectosome and the choanosome. Because of the variation in size and shape of the styles it is not specific for the tissue. There is some confusion with the labelling of the type specimens at the British Museum of Natural History, as specimen BMNH 1912.1.65 is clearly O. arbuscula , as the tree like specimen matches the description on p347 of Row (1911) and is clearly illustrated in the photograph on Plate 39, Figure 22, although the name on the jar incorrectly indicates it is the type of O. horrida . Similarly, the specimen BMNH 1912.2.1.63 is the specimen described as O. horrida on p349 of Row (1911), but labelled in the jar as O. arbuscula . As these specimens are of orders of magnitude of different sizes, it’s unlikely the specimens have been replaced in the wrong jars, and the labels were incorrectly swapped over. It must have occurred before or during the placement of the specimens in the ground glass jars from their previous housings. No doubt this mislabeling has added to the confusion about whether they are two different species or just one. It is even possible that this mistake was made by Row in 1911 as examination of the sections of the holotype of Ophlitaspongia arbuscula i.e. BMNH 1912.2.1.65, here in Figures 2A and 2B View FIGURE 2 show the distinctive knobbly connections of the fibers mentioned from O. arbuscula on page 348 of Row (1911). Unfortunately, a fragment of holotype of Ophlitaspongia arbuscula i.e. BMNH 1912.2.1.65 was not available due to covid restrictions in order to do a SEM analysis of the spicules. This labelling mistake was already present when examined by John Hooper in 1996, but not noted. This mislabeling was also not noticed by de Laubenfels, (1954)
| QM |
Queensland Museum |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |