Monstrilla albatrossi, Suárez-Morales, 2022
|
publication ID |
https://doi.org/ 10.1080/00222933.2022.2032444 |
|
DOI |
https://doi.org/10.5281/zenodo.6458326 |
|
persistent identifier |
https://treatment.plazi.org/id/4766B618-E87C-FFC3-75C5-FBDFF79EFCB7 |
|
treatment provided by |
Plazi |
|
scientific name |
Monstrilla albatrossi |
| status |
sp. nov. |
Monstrilla albatrossi sp. nov.
http:// urn:lsid:zoobank.org:pub:E9677EB2-23A1-4005-92AC-0DACAACC3B29
( Figures 1–4 View Figure 1 View Figure 2 View Figure 3 View Figure 4 )
Material examined
Holotype. One adult male, vial deposited at the National Museum of Natural History, Smithsonian Institution, Washington, DC ( USNM–74005 ) .
Type locality
Panabutan Pt., west coast of Mindanao, Philippines, plankton trawl, surface waters. R/V Albatross cruise sta. 5133 (~ 7° 35 ʹ 06.39 ″ N, 122° 08 ʹ 05.31 ″ E; coll. 6 February 1908 )).
Etymology
The species is named in reference to the illustrious steamer R/V Albatross of the United States Commission of Fish and Fisheries, operating in the western Pacific in 1887–1909 and sampling the rich pelagic copepod fauna of this region.
Diagnosis
Male Monstrilla with distinct cuticular ornamentation of cephalothorax represented by wide, complete belt-like striation pattern covering 1/3 of post-oral cephalothoracic surface plus intercalate fields of small papillae on dorsal, ventral, and lateral surfaces, fifth antennulary segment with rounded proximal protuberance. Genital complex type II with corrugate margin connecting both lappets and acute apical opercular processes. Fifth legs represented by pair of rounded processes on ventral surface of fifth pedigerous somite.
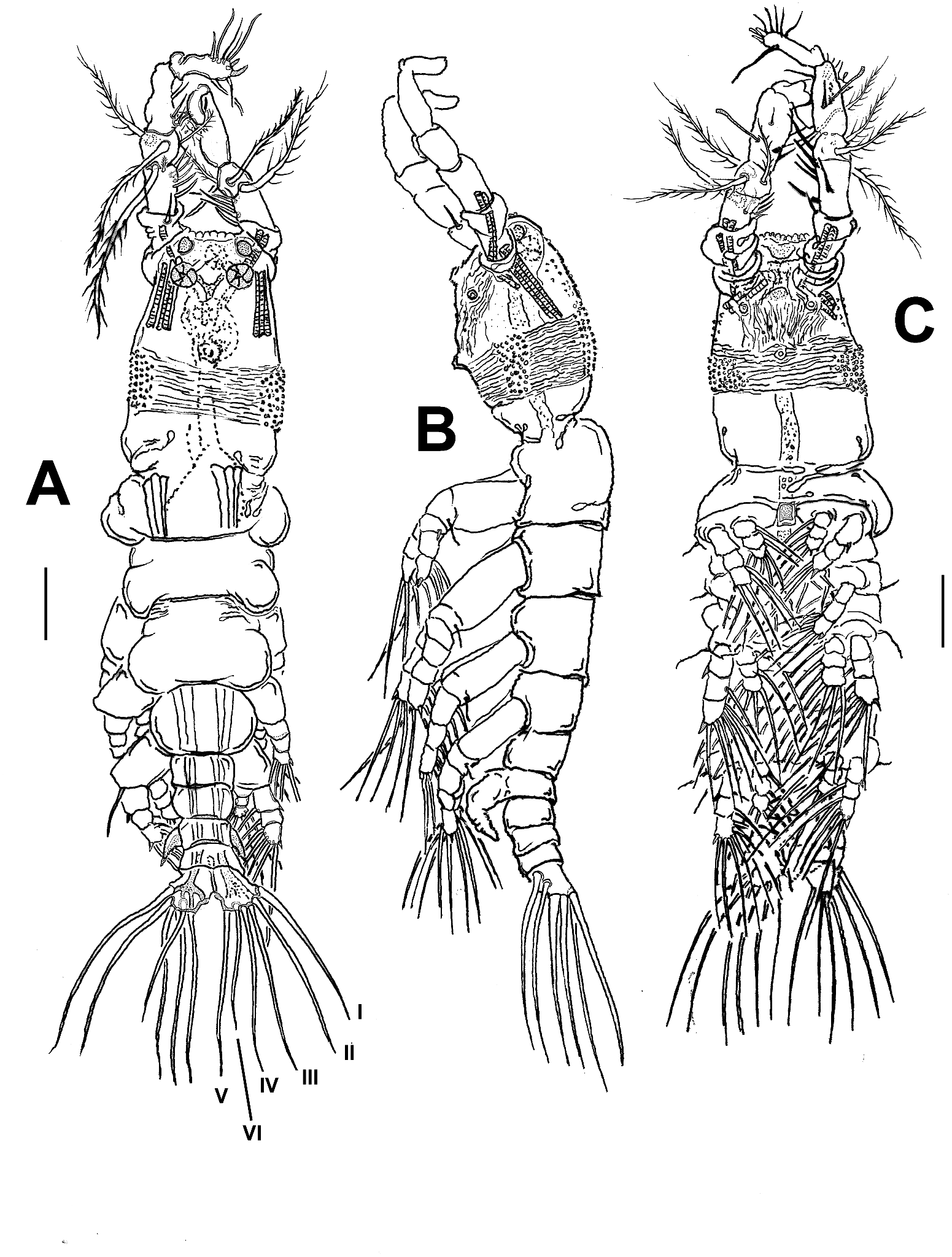
Description of adult male
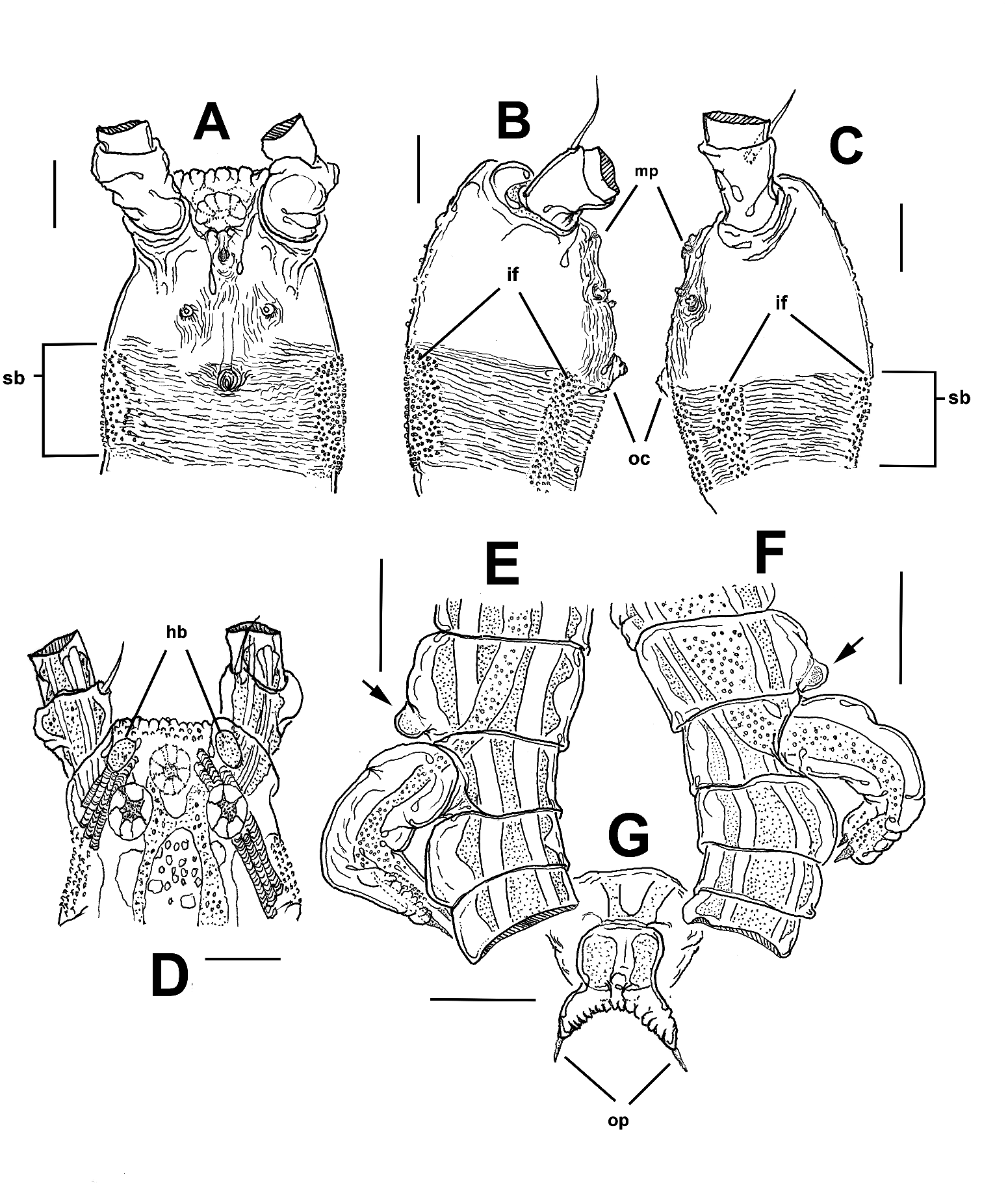
Body shape and tagmosis as usual in male Monstrilla (Suárez-Morales 2000; Suárez-Morales and Castellanos-Osorio 2019). Total body length (measured in dorsal view from anterior end of cephalothorax to posterior margin of anal somite): 1.25 mm. First pedigerous thoracic somite incorporated into cephalothorax. Cephalothorax short, relatively robust, representing slightly more than 47% of total body length ( Figure 1 View Figure 1 (a–c)). Oral cone located 45% of the way back along ventral surface of cephalothorax (oc, Figures 1 View Figure 1 (a, b) and 2(b,c)). Cephalic region anteriorly wide, forehead flat. Ocelli present, pigment cups small, round in dorsal view, separated by one eye diameter, poorly developed, almost unpigmented. Small oval hyaline bodies (hb in Figure 2 View Figure 2 (d)) (see Suárez-Morales 2018) adjacent to rounded ocelli. Cuticular ornamentation of cephalothorax including field of transverse papillae on dorsal forehead surface ( Figure 2 View Figure 2 (d)). In addition, usual pair of nipple-like processes on ventral surface between oral cone and antennule bases; processes small, with adjacent field of faint cuticular striae ( Figure 2 View Figure 2 (a–c)). Cephalothorax with continuous belt of shallow transverse cuticular striae covering nearly 1/3 of its post-oral half (sb in Figure 2 View Figure 2 (a–c)); cephalothoracic striated belt with intercalate fields of small scattered papillae on ventral, dorsal and lateral surfaces (if in Figure 2 View Figure 2 (a–c)) Oral cone weakly produced, with adjacent field of faint, transverse striae (oc in Figure 2 View Figure 2 (b, c)). Rounded medial protuberance present on ventral surface between nipple-like processes and antennule bases; protuberance moderately produced, with notch, visible in lateral view (mp in Figure 2 View Figure 2 (b, c)).
Urosome consisting of five somites: fifth pedigerous somite (largest of urosome), genital somite (with genital complex), two free somites and short anal somite. Ratio of lengths of urosomites (from proximal to distal): 26.8:23.3:25:17.7:7.2 = 100 ( Figure 2 View Figure 2 (e, f)).
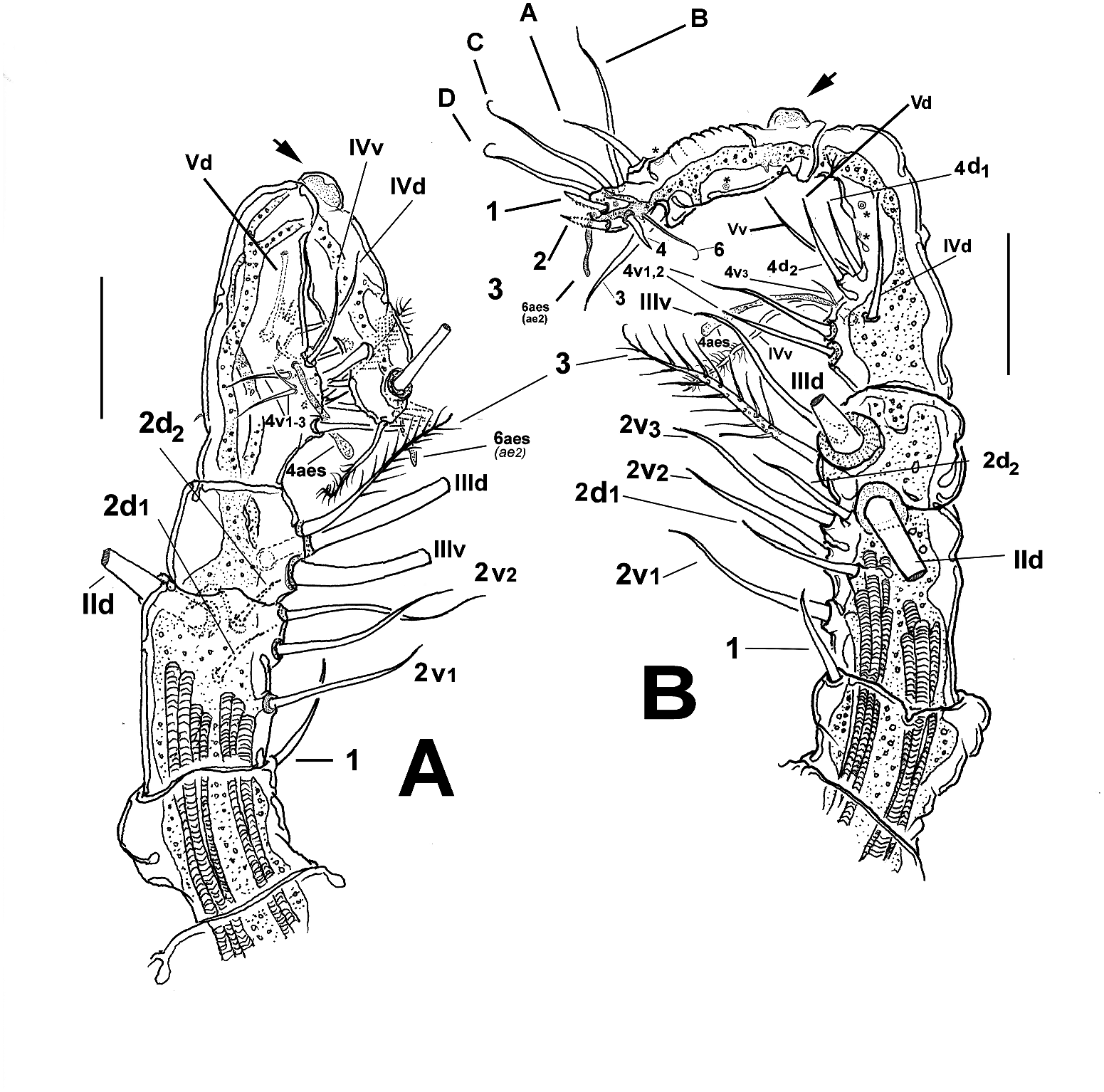
Antennules representing about 35% of total body length, and almost 72% of cephalothorax length. As usual in male Monstrilla , antennules five-segmented, geniculation between segments 4 and 5 ( Figure 3 View Figure 3 (a, b)). Following setal nomenclature proposed by Grygier and Ohtsuka (1995) for female monstrilloid copepod antennules, seta 1 moderately long, almost reaching half length of succeeding second segment. Elements 2v1–3 and 2d1,2 setiform, long, element IId long, thick, with protuberant distal socket. Third segment short, subquadrate, with setiform, flexible medial element 3 reaching about proximal 1/3 of fourth segment. Setae IIId, IIIv present, long. Fourth segment armed with nine elements: 4d1,2, 4aes, 4v1–3, IVd, Vv, Vm. Elements 4v1,2 twice as long as element 4v3. Distal segment lacking modified setae, with conspicuous rounded proximal protuberance (arrows in Figure 3 View Figure 3 (a, b)) and armed with 11 setal elements (sensu Huys et al. 2007) including setae 1–6 and unmodified elements A–C plus short, slender aesthetasc 6aes (sensu Grygier and Ohtsuka 1995), or ae2 (sensu Huys et al. 2007) in apical position. Sockets on middle and distal surfaces of fourth and fifth antennulary segments (indicated by asterisks in Figure 3 View Figure 3 (b)) suggest loss of some (likely four) setal elements, particularly of the ‘b’ group (sensu Grygier and Ohtsuka 1995) in the holotype specimen. Length ratio of antennular segments (proximal to distal): 11.4:19.8:11.4:27.8:29.6 = 100 ( Figure 2 View Figure 2 (a)).
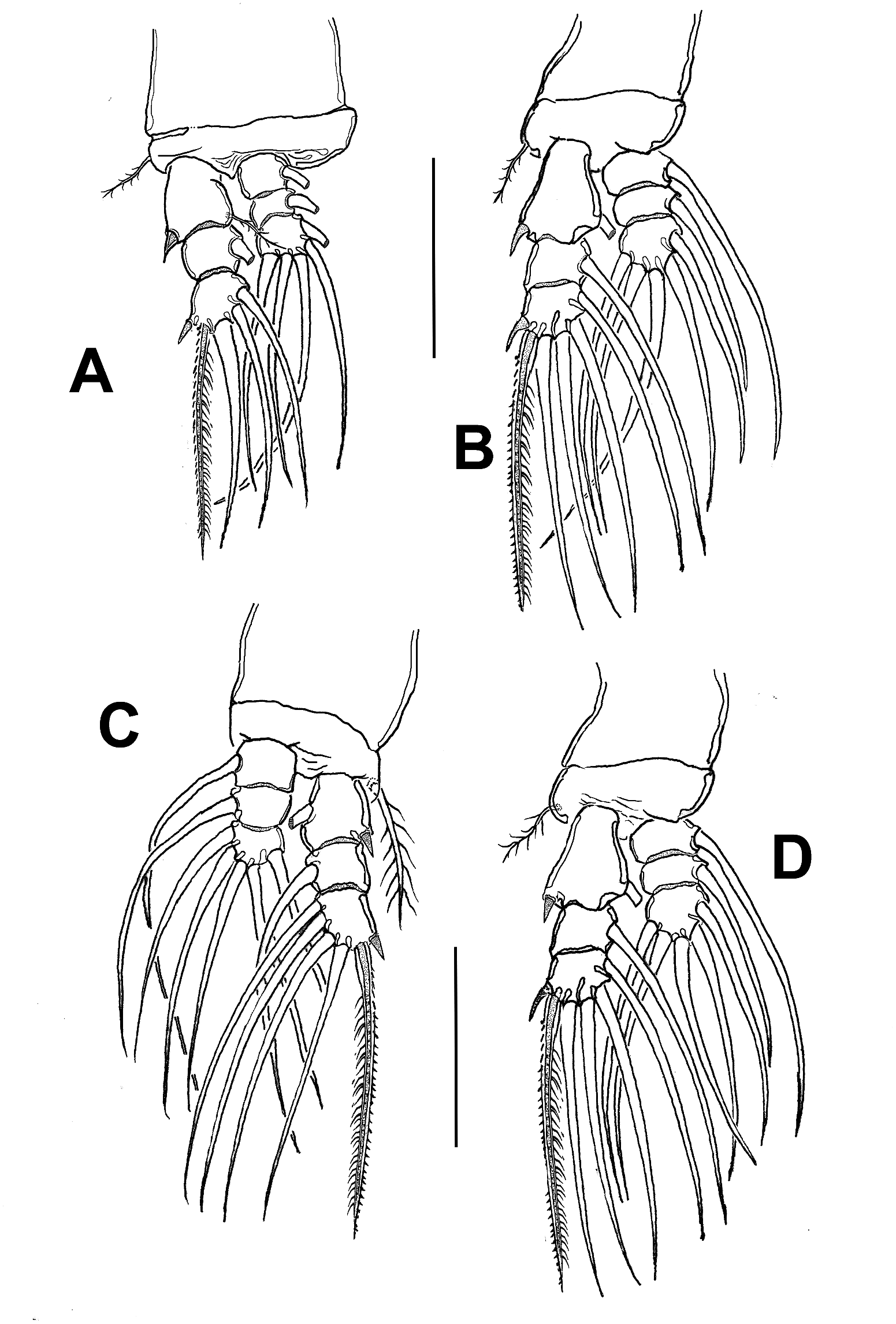
First incorporated pedigerous thoracic somite and succeeding three pedigers each bearing well-developed biramous swimming legs ( Figure 1 View Figure 1 (b, c)). Swimming legs 1–4 with triarticulate endopodites and exopodites and same armament pattern, except for leg 1 exopodite, with one seta fewer on distal segment than in other legs ( Figure 4 View Figure 4 (a–d)). Exopods longer than endopods. Distal spiniform seta of third exopod with denticles along outer margin, inner margin weakly setulose in all legs. Coxae joined by smooth rectangular coupler ( Figure 1 View Figure 1 (c)).
Basis separated from coxa by diagonal articulation. Basis of swimming legs 1, 2 and 4 with short basipodal seta; seta on leg 3 being thicker and 2.5–3 times as long as in other legs ( Figure 4 View Figure 4 (a–d)). All natatory setae lightly and biserially plumose.
Armament formula of legs 1–4: basis exopod endopod Leg 1 0-1 I-1; 0-1; I, 2, 2 0-1; 0-1; 1, 2, 2 Legs 2–4 0-1 I-1; 0-1; I, 2, 3 0-1; 0-1;1,2,2
Fifth legs absent, but represented by pair of low rounded protuberances on ventral surface of fifth pedigerous somite (arrows in Figure 2 View Figure 2 (e, f)). Genital somite carrying genital complex consisting of short, thick medially curved shaft bearing paired distal genital lappets reaching distal margin of succeeding urosomite ( Figure 2 View Figure 2 (e, f)). Lappets divergent, digitiform, tapering distally into apical acute opercular processes (op in Figure 2 View Figure 2 (g)); margin connecting both lappets curved, coarsely and evenly rugose ( Figure 2 View Figure 2 (g)).
Caudal rami subrectangular, with distal margin slightly wider than proximal, ramus approximately 1.3 times as long as wide. Each ramus bearing six well-developed caudal setae (setae I–VI sensu Huys and Boxshall 1991), seta VI being shortest. Caudal setae II and III about as long as medial seta IV ( Figure 1 View Figure 1 (a)).
Female
Unknown.
Remarks
The holotype specimen is still in good condition for taxonomic study considering the long time elapsed (113 years) since it was collected. However, the cephalothorax appears damaged (depressed), probably by forceps during handling, and several antennular setal elements are missing, particularly on the last segment. This is why only the structures on antennule segments 1–4 were homologised with the pattern proposed by Grygier and Ohtsuka (1995) for female monstrilloids. But mixing of the two patterns (i.e. using the female notation for male antennules) should be avoided. The specimen was not dissected during examination and returned whole to its vial.
Prior to this record of M. albatrossi sp. nov. only six other species of Monstrilla had been reported from the Philippines area (Suárez-Morales 2000): M. cymbula A. Scott, 1909 , M. elongata Suárez-Morales, 1994 , M. grygieri Suárez-Morales, 2000 , Caromiobenella helgolandica (Claus, 1863) (as M. helgolandica ), M. leucopis (Scott, 1909) and M. viridis Dana, 1849 . Monstrilla cymbula was described from a female specimen collected from the Sulu Islands (Scott, 1909). Monstrilla grygieri was recorded from a female found in Panabutan, Sulu Sea (see Suárez-Morales 2000). Far East records of M. leucopis ( Wilson, 1950; McAlice 1985) are unlikely to represent this species because it appears to be restricted to Norway (SuárezMorales 2010). The record of the Caribbean M. elongata from the Philippines area (see Suárez-Morales 2000) is highly dubious, and the two adult females deposited at the National Museum of Natural History (USNM 73856) most probably represent an undescribed species. The Philippines record of Caromiobenella helgolandica (as M. helgolandica ), also from the Sulu Islands (Scott 1909), is unsurprising because of the presumed wide distribution of this nominal species, currently deemed to represent a species complex ( Grygier and Ohtsuka 1995; Suárez-Morales 2010, 2011; Jeon et al. 2018).
McAlice (1985) reidentified four male specimens of M. serricornis (Sars, 1921) (now in Caromiobenella Jeon, Soh and Lee, 2018 , collected from the Buritari Lagoon, Gilbert Islands, now Kiribati) during the ‘Albatross’ cruise as M. canadensis (= C. helgolandica ). Sekiguchi (1982) recorded several males of M. serricornis in central Japan; this was probably the first confirmed record of the species in the area, since the male reported by Wilson (1950) as M. serricornis in the Philippines was probably a misidentification of either M. canadensis or the species described here (see Grygier 1995). Furthermore, McAlice (1985) stated that neither specimen from Caldera Bay, Mindanao, identified as M. serricornis by Wilson (1950) bears the slightest resemblance to that species. In M. serricornis , as in all other species of Caromiobenella , the inner distal margin of the terminal antennular segment bears a set of distinctive, conspicuous serrate processes ( Jeon et al. 2018), absent in M. albatrossi sp. nov., but the general resemblance of these specimens in body shape and size may have led to Wilson’s misidentification of the specimen.
The male specimen studied here can be included in the genus Monstrilla by virtue of the combined presence of two urosomites between the genital somite and the anal somite, the structure of its genital complex, the presence of well-developed or reduced fifth legs, geniculate antennules lacking discernible modifications, the possession of six caudal setae in both sexes, and five-branched antennulary setae sometimes present. This combination of characters does not occur in any other known genus of monstrilloids ( Jeon et al. 2018; Suárez-Morales and Castellanos-Osorio 2019).
The new species can be easily recognised by its unique cephalothorax cuticular ornamentation. There are only a few known monstrillids with a complete or incomplete belt-like cephalothorax striation pattern; the clearest example is Cymbasoma striatum from south-eastern Britain, which shows a wide striation field covering half the length of its cephalothorax and running around the cephalothorax (see Suárez-Morales 2000b, figs 1, 6, 7). Also, the widespread nominal species Cymbasoma reticulatus Giesbrecht, 1893 , the Caribbean C. bowmani Suárez-Morales and Gasca, 1998 , the Pacific C. concepcionae Suárez-Morales and Morales-Ramírez, 2003 and C. guerrerense SuárezMorales, 2009, and the Floridian C. davisi Suárez-Morales and Pilz, 2008 show partial striation patterns covering either the lateral or ventral cephalothorax surfaces, but not completing a cuticular belt ( Suárez-Morales and Gasca 1998; Suárez-Morales and Morales-Ramírez 2003; Suárez-Morales and Pilz 2008; Suárez-Morales 2009). Among the known species of Monstrilla , comparable cephalothoracic striation patterns are present only in the Caribbean M. marioi Suárez-Morales, 2003 and M. chetumalensis Suárez-Morales and Castellanos-Osorio, 2019 ; a well-developed cuticular ornamentation has been found in the male of Caromiobenella brasiliensis ( Dias and Suárez-Morales 2000) , with a wide band of transverse striae on both the lateral and ventral surfaces of the cephalothorax (see Cruz Lopes da Rosa et al. 2021. In M. marioi the striation is relatively sparse and limited to the perioral ventral surface (Suárez-Morales 2003). In M. chetumalensis the striation pattern is denser and concentrated on the anterior cephalic area ( Suárez-Morales and Castellanos-Osorio 2019). In all these species of monstrilloids the striation comprises only simple transverse striae, thus diverging from the pattern shown by the new species M. albatrossi , with a more complex striation pattern including axial patches of papillae ( Figure 2 View Figure 2 (a–c)). This is the first time this type of cuticular ornamentation is reported in male monstrilloids.
The structure of the genital complex is among the most important taxonomic characters for male monstrilloids ( Suárez-Morales 2000c, 2003). Two types of male genitalia have been reported to occur in Monstrilla ( McAlice 1985; Suárez-Morales 2000, 2003): one (type I) having a relatively long, slender genital shaft with short, globular distal, nondivergent lappets, as in M. bahiana Suárez-Morales and Dias, 2001 , M. reidae SuárezMorales, 1993 and M. globosa Suárez-Morales, 2003 ; and a second one (type II) showing a short, robust shaft with non-globose, separate distal lappets, as in the widespread M. grandis Giesbrecht, 1891 (see Suárez-Morales et al. 2013), M. elongata SuárezMorales, 1994, M. longiremis Giesbrecht, 1892 and M. marioi Suárez-Morales, 2003 .
Overall, the new species M. albatrossi most closely resembles M. chetumalensis and M. marioi ; both congeners share with the new species several relevant characters: (1) the same type of genital complex (type II) and genital lappet structure, (2) a well-developed cephalothoracic cuticular ornamentation, (3) similar body proportions, (4) the presence of paired protuberances representing the fifth legs on the fifth pedigerous somite, and (5) a corrugate forehead. However, the new species shows a unique combination of characters, the most distinctive being: (1) cephalothorax with a belt-like striation pattern covering 1/3 of the post-oral cephalothoracic surface, (2) cuticular striation pattern including intercalate fields of small papillae, (3) type II genital complex with corrugate margin connecting the two lappets, (4) fifth antennulary segment with rounded proximal protuberance. The cephalic cuticular striation of both M. marioi and M. chetumalensis differs widely from the pattern observed in the new species. In both species, the cephalic striation is well developed but limited to the ventral perioral area and forehead. Also, in M. chetumalensis the fifth pedigerous somite has (1) a pair of posterolateral expansions and (2) a dorsal field of striae ( Suárez-Morales and Castellanos-Osorio 2019; Figure 2 View Figure 2 (d)). In this species the margin connecting the genital lappets is straight. Furthermore, both M. marioi and M. chetumalensis have four caudal setae (Suárez-Morales 2003; Suárez-Morales and Castellanos-Osorio 2018), thus diverging from the six caudal setae present in the new species M. albatrossi . Considering this finding, the Philippine diversity of Monstrilla now includes seven nominal species (see Suárez-Morales 2000a).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.