Viridotheres asaphis, Ahyong, 2020
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4763.3.7 |
|
publication LSID |
urn:lsid:zoobank.org:pub:2B674B7A-83AA-42E1-8393-42A99CE320A9 |
|
DOI |
https://doi.org/10.5281/zenodo.3805301 |
|
persistent identifier |
https://treatment.plazi.org/id/4A17CB65-405D-FF96-FF3B-FCF19BDD82BB |
|
treatment provided by |
Carolina |
|
scientific name |
Viridotheres asaphis |
| status |
sp. nov. |
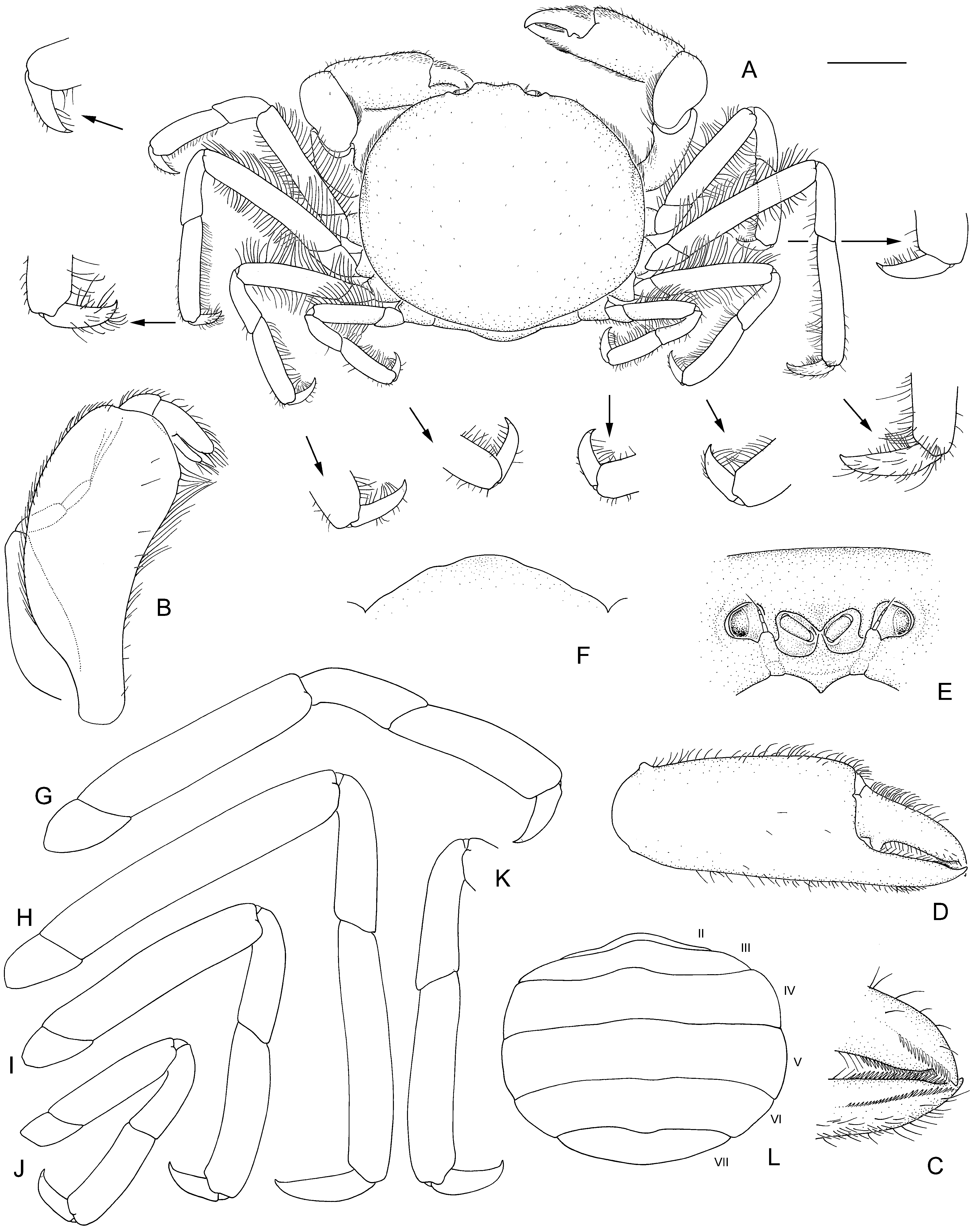
Viridotheres asaphis View in CoL sp. nov.
( Fig. 1B, C View FIGURE 1 , 2 View FIGURE 2 )
Type material. HOLOTYPE: AM P104025 , spent female (cl 6.1 mm, cw 7.2 mm), Palm Island , Queensland, Australia, 18°40’S, 146°33’E, from Asaphis dichotoma ( Anton, 1838), coll. E.H. Rainford, 1923 GoogleMaps . PARATYPE: MNHN IU- 2019-5158, ovigerous female (cl 5.6 mm, cw 6.6 mm), Ohland Bay, near Paagoumene, New Caledonia, 20°28.335’S, 164°10.5917’E, intertidal, coralline blocks on sand, fringing reef flat, Koumac 2 KM 704, from Asaphis violascens ( Forskål, 1775) ( MNHN IM- 2019-3122), coll. P. Lozouet et al., 31 Oct 2019.
Description. Carapace subcircular, slightly wider than long, widest at midlength, weakly arched axially or transversely; pterygostomial surface strongly setose, setae visible in dorsal view. Front weakly produced, anterior margin concave in dorsal view, width about 0.2× cw. Dorsum smooth, with minute, sparsely scattered setae. Epistome interantennular septum narrow; median buccal margin with broad, triangular median point. Antennular sinus of similar size to orbit; antennules folded obliquely. Antennal articles 1 and 2 fused to epistome. Eyes visible in dorsal view, filling orbit, cornea pigmented.
Maxilliped 3 ischiomerus length exceeding twice width; outer margin convex; inner margin concave, distal margin rounded, without distinct angle. Carpus about half propodus length. Propodus spatulate, gently tapering in distal half, apex rounded, dorsally and distally setose, length almost twice dactylus length. Dactylus digitiform, distally setose, inserted near propodal midlength, apex reaching to end of propodus. Exopod inner margin gently convex, outer margin strongly convex; flagellum with 2 articles, distally setose.
Chelipeds symmetrical from left to right, surfaces setose. Dactylus and pollex relatively straight, crossing distally, with slight gape, neither expanded distomesially. Dactylus occlusal margin gently convex to straight, with blunt triangular tooth proximal to midlength, with row of minute finely graded denticles on distal half of occlusal margin and on distomesial surface; proximal margin setose, most prominent on proximal half, extending to dorsal margin palm. Pollex occlusal margin gently convex to straight, sparsely setose, with row of slender finely graded denticles on distal half of occlusal margin and on distomesial surface; with fringe of setae on inner ventral margin. Propodus palm dorsal margin 1.7× height, 1.6× length of dactylus; ventral margin straight, setose. Carpus unarmed, inner margin with setal fringe, short, scattered setae distally.
Pereopods 2, 4, 5 (walking legs 1, 3, 4) symmetrical from left to right, unarmed, setose on flexor and extensor margins, longest and densest on merus. Pereopod 3 (walking leg 2) length symmetrical from left to right, with combined length of dactylus-propodus-carpus on right side (0.7 × cw) longer than left (0.6 × cw); merus, when folded anteriorly, reaching beyond frontal margin of carapace. Relative lengths: pereopod 3> pereopod 2> pereopod 4> pereopod 5. Pereopod 2 basis anterior surface setose, otherwise smooth. Dactyli similar, simple, evenly arcuate, apex sharp, flexor margin setose, unarmed; pereopod 2–4 dactyli 0.4× propodus length, of pereopod 5, 0.5× propodus length. Relative dactylus lengths: pereopod 3> pereopod 2> pereopod 4> pereopod 5.
Thoracic sternum anterior margin broadly rounded; sternites 1–3 indistinguishably fused, setose. Abdomen with 6 free somites and telson, covering bases of walking legs.
Colour in life. Overall translucent white; eyes dark maroon. Ovigerous female paratype with maroon egg masses visible through carapace.
Host. Asaphis violascens ( Forskål, 1775) ( Psammobiidae ). The host of the Australian specimen was recorded as Asaphis dichotoma ( Anton, 1838), which is currently regarded as a junior synonym of A. violascens (see Willan 1993).
Etymology. Named after the generic name of host bivalve; used as a noun in apposition.
Remarks. Among species currently assigned to Viridotheres , V. asaphis , together with V. gracilis ( Bürger, 1895) ( Philippines) , V. lillyae ( Manning, 1993) (West Africa) and V. marionae Manning, 1996 ( Cape Verde Islands), differ from congeners in having densely setose rather than almost glabrous or sparsely setose walking legs. The new species is readily distinguished from V. gracilis , V. lillyae and V. marionae by the proportionally much longer pereopod 3 in which the merus, when folded anteriorly, reaches beyond the anterior margin of the carapace rather than distinctly falling short. Viridotheres asaphis , however, most closely resembles a species from southern India currently placed in Nepinnotheres , N. sanguinolariae ( Pillai, 1951) (see Ng & Kumar 2015), sharing a slender maxilliped 3 ischiomerus with length exceeding twice width and with a rounded rather than angular distomesial margin in addition to the strongly setose pereopods. Given the correspondence in diagnostic generic features between females of V. asaphis and P. sanguinolariae , including the pereopod 3 dactylus being the longest of the walking leg dactyli, the two species should be considered congeneric, so the latter is transferred to Viridotheres as V. sanguinolariae comb. nov. Females of V. asaphis differ from V. sanguinolariae in having proportionally longer walking legs with the carpus-propodus about 0.6–0.7 cw (versus half) and with the distal end of the merus, when folded anteriorly, reaching beyond the anterior margin of the carapace (rather than falling short). Slight length asymmetry evident in some other species of Viridotheres (e.g., V. marionae and V. takedai ) in which the dactylus-carpus-propodus is slightly longer on one side (usually the right), is also present in V. asaphis , but apparently not V. sanguinolariae . The pereopod length differences identified between V. asaphis and V. sanguinolariae assume that Pillai’s (1951: fig. 4) figures are correctly rendered. As described by Pillai (1951), however, a further distinction is that the cheliped propodus of V. sanguinolariae is distomesially hollowed, whereas that of V. asaphis is simple and unexpanded. Viridotheres asaphis and V. sanguinolariae otherwise agree closely, and this strong morphological similarity is paralleled by host choice. Both species are associated with bivalves of the family Psammobiidae , but of different genera: Asaphis violacens Forskål, 1775 in the case of the new species, and Soletellina diphos ( Linnaeus, 1771) (as Sanguinolaria diphos in Pillai 1951 ) in the case of V. sanguinolariae . Significantly, host choice specificity is becoming increasingly recognized to differ between closely related pinnotherid species ( Ng & Kumar 2015; Ng 2018; Ahyong 2018; Ng & Ahyong unpubl.). Such specificity is apparently evident here too, with two closely related species of Viridotheres , V. asaphis and V. sanguinolariae , selecting bivalve hosts from the same family but of different genera.
Males of V. asaphis are not currently known, but those of V. sanguinolariae have a transversely ovate carapace ( Pillai 1951: fig. 4b), unlike the subcircular form of known males of other species of the genus (i.e., V. kupang Ahyong, 2019 ; V. lillyae Manning, 1993 ; V. takedai Ahyong, Komai & Watanabe, 2012 ). Given the commonalities in female morphology and host bivalves of V. asaphis and V. sanguinolariae , males of the new species probably resemble those of the latter in carapace shape.
Viridotheres asaphis sp. nov. is the first species of the genus to be recorded from Australia and New Caledonia, extending the known range of the genus into the southwestern Pacific. With the discovery of V. asaphis sp. nov. and capture of D. camposi during Koumac 2, 15 species of pinnotherid crabs are now known from Australia ( Ahyong & Brown 2003; Ahyong 2018) and five from New Caledonia ( Ng & Richer de Forges 2007; Ahyong 2018).
Distribution. Northeastern Australia and New Caledonia; intertidal.
| MNHN |
Museum National d'Histoire Naturelle |
| KM |
Kotel'nich Museum |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |