Moribaetis Waltz & McCafferty 1985
|
publication ID |
https://doi.org/10.11646/zootaxa.4521.2.5 |
|
publication LSID |
lsid:zoobank.org:pub:F992EA3F-2AD0-49F8-9230-266B4D4C3ACE |
|
DOI |
https://doi.org/10.5281/zenodo.5952424 |
|
persistent identifier |
https://treatment.plazi.org/id/4A1E8797-FF60-AC70-FF48-87D6FC439174 |
|
treatment provided by |
Plazi |
|
scientific name |
Moribaetis Waltz & McCafferty 1985 |
| status |
|
Moribaetis Waltz & McCafferty 1985 View in CoL View at ENA
( Figs 1–105 View FIGURES 1–15 View FIGURES 16–21 View FIGURES 22–24 View FIGURES 25–33 View FIGURES 34–43 View FIGURES 44–47 View FIGURES 48–52 View FIGURES 53–55 View FIGURES 56–61 View FIGURES 62–70 View FIGURES 71–76 View FIGURES 77–84 View FIGURES 85–88 View FIGURES 89–95 View FIGURES 96–101 View FIGURES 102–105 )
Subgenus Moribaetis Waltz & McCafferty 1985: 243 (imago, larva).
Genus Moribaetis: Lugo-Ortiz & McCafferty 1996: 368 View in CoL (larva).
Type species: Baetis maculipennis Flowers 1979 .
Systematic position. In the rank-free classification Moribaetis belongs to the following subsequently subordinated taxa: Tetramerotarsata Kluge 1997 > Liberevenata Kluge 1997 (i.e. Baetidae s.l.)> Turbanoculata Kluge 1997 (i.e. Baetidae s.str.)> Anteropatellata Kluge 1997 > Baetovectata Kluge & Novikova 2011 > Baetungulata Kluge & Novikova 2011. In male genitals, gonovectes are bent in the manner characteristic for Baetovectata ( Fig. 47 View FIGURES 44–47 ); fore wings have two marginal intercalaries per space, that is also characteristic for Baetovectata ( Fig. 53 View FIGURES 53–55 ); in mature larva ready to moult to subimago, subimaginal gonostyli are folded under larval cuticle in such a manner that 2nd segment of gonostylus as a whole is directed caudally-medially ( Fig. 44 View FIGURES 44–47 ), but not laterally, in contrast to baetids other than Baetovectata [see character (17) below]. Claw of mature larva has one asymmetric row of denticles, characteristic for most Baetungulata ( Figs 23–24 View FIGURES 22–24 ); as in other Baetungulata, tergalii are unable to make respiratory movements; maxillary palp is two-segmented ( Flowers 1979: fig. 9; Waltz & McCafferty 1985: figs 28, 37); on fore leg of male subimago tarsomeres 2–4 are covered by blunt microlepides [see character (10)].
All characters testify that Moribaetis belongs to Baetovectata-Baetungulata, that means that it is a member of the higher taxon Anteropatellata; therefore, the lack of the character peculiar for Anteropatellata [see character (9)], is here assumed as a secondary loss.
Being a member of Baetungulata, Moribaetis does not belong to a large taxon Baetofemorata Kluge & Novikova 2011 subordinated to Baetungulata: in contrast to Baetofemorata, larval femur of Moribaetis lacks femoral patch (a compact group of minute setae on inner side of femur near its base).
Annotated diagnosis of Moribaetis . This diagnosis is based on two species, M. maculipennis and M. latipennis sp. n., which are known as larvae, subimagoes and imagoes; the third species, M. salvini , is known as male imago only (see below), and the fourth species, M. brachiostrinus , is known as larvae only.
(1) Labrum is widened in distal part, thickened in proximal part; ventrally with sclerotized lateral margins ( Fig. 74 View FIGURES 71–76 ); on its dorsal surface, number of long setae in the pair of antero-lateral rows is increased, these rows nearly meet the submedian pair of long setae, so that all these long setae form nearly integral transverse row ( Fig. 71 View FIGURES 71–76 ). Such a labral structure is found in some other taxa.
(2) Incisor of each mandible is blade-like, i.e. greatly elongated (several times longer than kinetodontium), straight, with denticles diminished or lost ( Figs 18–21 View FIGURES 16–21 , 79–82 View FIGURES 77–84 ). Incisors are often broken ( Figs 16–17 View FIGURES 16–21 , 77–78 View FIGURES 77–84 ), so their initial shape should be observed either on freshly-moulted specimens, or inside old mandible just before moulting. Besides Moribaetis , similar blade-like mandibular incisors independently evolved in some other distantly related baetid taxa.
(3) Prostheca of right mandible is slender, straight, directed parallel to incisor, pointed or terminated by few slender processes ( Figs 21 View FIGURES 16–21 , 82 View FIGURES 77–84 ); this is one of the types of right prostheca, which are repeated among Baetidae . Prostheca of left mandible varies from the form usual for Baetidae (in M. latipennis sp. n. – Fig. 80 View FIGURES 77–84 ) to similar to right prostheca (in M. maculipennis – Fig. 19 View FIGURES 16–21 ).
(4) Maxilla of the « Baetis - type », i.e. with 1st dentiseta canine-like, heavily sclerotized and bent toward canines ( Fig. 83 View FIGURES 77–84 ; Flowers 1979: fig. 9, Waltz & McCafferty 1985: figs 28, 37). The same in many other non-related baetid taxa.
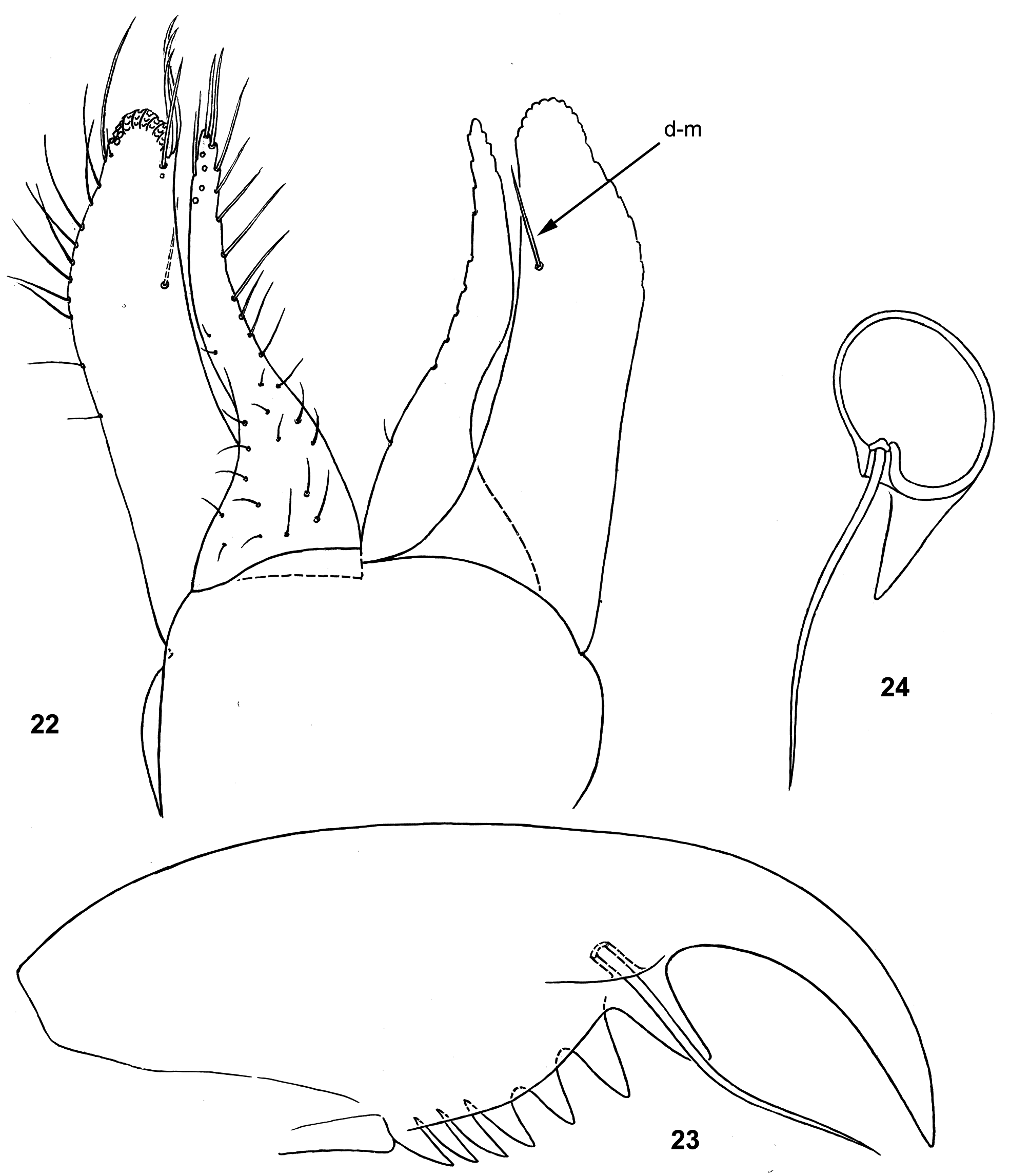
(5) Labium has peculiar structure and differs from other taxa by shape of paraglossa and one seta instead of the dorso-median row ( Fig. 22 View FIGURES 22–24 , 76 View FIGURES 71–76 ). Paraglossa is sharply narrowing toward apex; its long latero-apical setae are sparse on lateral margin and compactly concentrated on extreme apex of paraglossa, forming here 3 rows; ventro-median setal row is completely absent; dorso-median row is reduced to a single long seta ( Fig. 22 View FIGURES 22–24 : d-m). Glossa is long and narrow, its dorso-lateral row is reduced to a few setae on lateral margin near apex.
Formerly paraglossae of M. maculipennis and the larva ascribed to M. salvini were erroneously described as having only 2 rows of apical setae ( Flowers 1979: 190, fig. 10; Waltz & McCafferty 1985: 246, 247, 250); actually they have 3 rows ( Figs 22 View FIGURES 22–24 , 84 View FIGURES 77–84 ).
Romero & Esquivel (2018) reported 2 rows for M. brachiostrinus . Judging by the shape of paraglossae ( Romero & Esquivel 2018: fig. 6), which are the same as in the two species mentioned above, we assume that paraglossae of M. brachiostrinus also have 3 rows of apical setae.
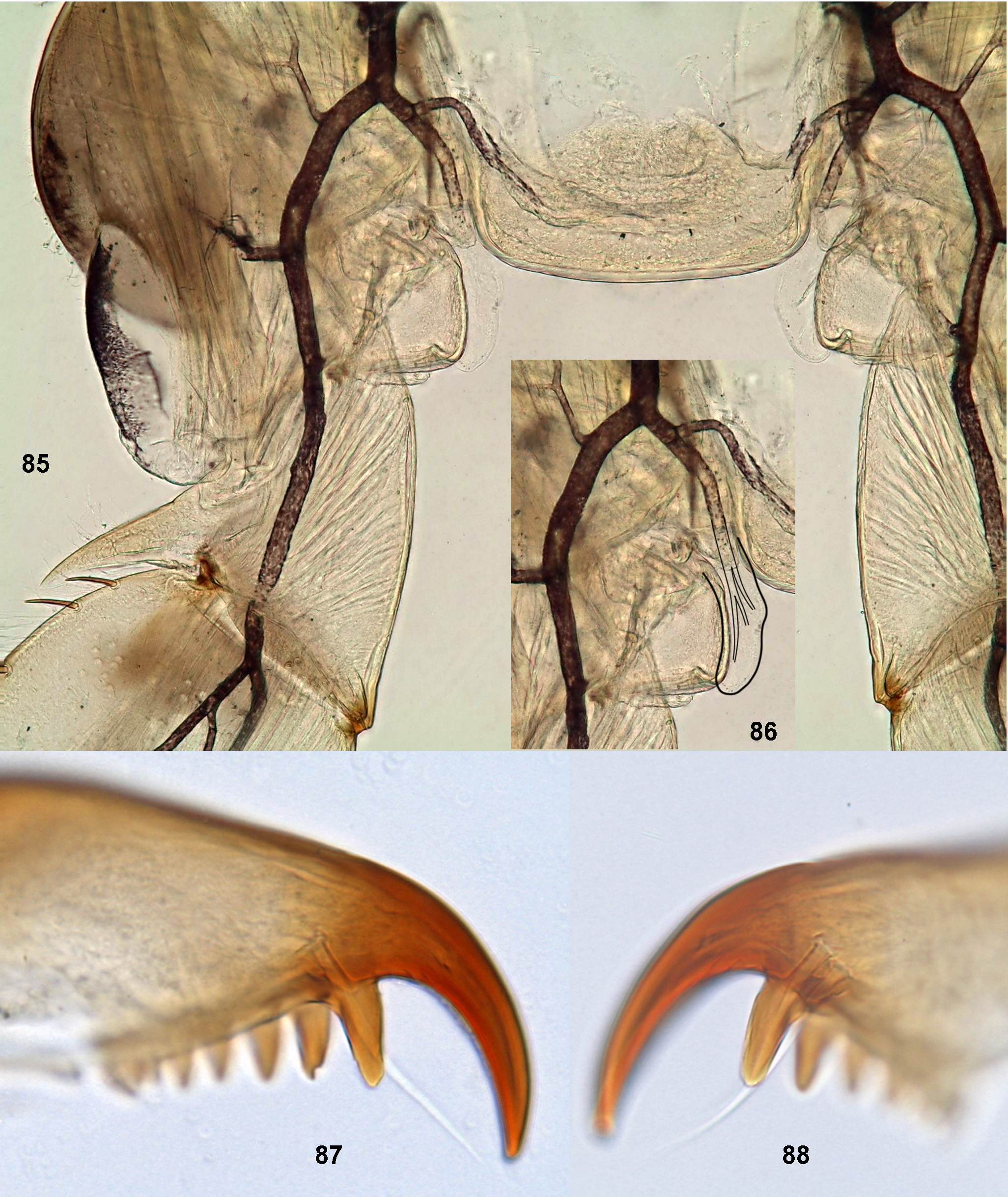
(6) Larval prothorax with a pair of tracheal gills, each representing a short and simple (non-branched) outgrowth arising from the membrane connecting prosternum with coxal bases ( Waltz & McCafferty 1985: fig. 31). These outgrows were termed «osmobranchia» ( Waltz & McCafferty 1985), but actually they represent true tracheal gills, because they are supplied with special tracheae: just proximad of coxal base, the leg trachea has a thick branch which passes into the gill ( Figs 85–86 View FIGURES 85–88 ). In contrast to prothorax, mesothorax and metathorax have no gills, and their leg tracheae have no such branches. While the trachea passing into the gill is rather thick, it is unclear if these gills can play any role in respiration because they are very small in relation to the body size and in relation to tergalii, which are large, well-tracheated ( Figs 5–11 View FIGURES 1–15 ) and also serve as tracheal gills. Larvae of Moribaetis inhabit places with the fastest water current, where the role of any gills is likely minimal.
(7) Larval legs are differentiated so that fore, middle and hind legs have the following differences in proportions and setation ( Figs 25–27 View FIGURES 25–33 , 89–91 View FIGURES 89–95 ):
Fore, middle and hind femora slightly differ in length, with fore femur being the shortest and hind femur being the longest; fore and middle tibia are of equal length, while hind tibia is 1.2 times shorter; fore, middle and hind tarsi slightly differ in length, with fore tarsus being the longest and hind tarsus being the shortest. Thus, on fore leg tibia and tarsus combined are 1.3 times longer than femur, while on hind leg tibia and tarsus combined are subequal to femur or only 1.1 times longer. In course of development to subimago and to imago, legs become significantly longer, and their proportions are changed, but hind tibia and tarsus continue to be shorter than middle ones.
Fore femur is the thickest; short spine-like setae on inner side of femur ( Figs. 33 View FIGURES 25–33 , 95 View FIGURES 89–95 ) occupy widest area on fore femur, narrower area on middle femur and narrowest area on hind femur. Apices of fore and middle femora with dense bunch of 4–9 spine-like setae of the same structure as spine-like setae of the longitudinal row on outer side [see (8)] ( Figs 92–93 View FIGURES 89–95 ); apex of hind femur usually with two such setae ( Fig. 94 View FIGURES 89–95 ) (as on all femora of many other Baetidae ).
(8) Larval legs with stout pointed spine-like setae, fine setae and scales in angulated bases [similar to scales of abdomen—see (13)]. Each femur [different on different leg pairs – see (7)] on outer side bears a regular sparse row of stout pointed spine-like setae; between them stretches an interrupted irregular row of fine setae ( Fig. 92 View FIGURES 89–95 ). Apex of each femur with smaller spine-like setae ( Figs 92–94 View FIGURES 89–95 ). Each tibia with row of small pointed spine-like setae on outer side ( Fig. 92 View FIGURES 89–95 ) and with irregular spine-like setae on inner side.
(9) Patella-tibial suture is modified as the following: on each larval leg it stretches longitudinally and gradually disappears not reaching inner (ventral) side of tibia ( Figs 25–27 View FIGURES 25–33 , 89–91 View FIGURES 89–95 ). On middle and hind legs of subimago and imago patella-tibial suture reaches inner side of tibia ( Fig. 30, 32 View FIGURES 25–33 ), but on fore leg of female subimago and imago (known only for M. maculipennis ) patella-tibial suture stretches longitudinally and does not reach inner side of tibia ( Fig. 28 View FIGURES 25–33 ), sometimes being shortened and vestigial ( Figs 29, 31 View FIGURES 25–33 ) (male subimago and imago have no patellatibial suture on fore legs, as in all other Baetidae ). In this respect Moribaetis somewhat resembles the basal plesiomorphon Protopatellata, which in all stages of both sexes have patella-tibial suture only on middle and hind legs, but not on fore legs.
(10) In subimagoes of both sexes, all tarsal segments on all legs are entirely covered with blunt microlepides only. The same in some other Baetungulata-non-Baetofemorata and in all Baetofemorata.
(11) Larval claw bears a large seta on posterior side; this seta located equidistantly with the most distal denticle of the claw; base of this seta is inserted into a cylindrical canal ( Figs 23–24 View FIGURES 22–24 , 87 View FIGURES 85–88 ). Individual claws of M. latipennis sp. n. have two such setae ( Fig. 88 View FIGURES 85–88 ) (sometimes three, according to Waltz & McCafferty 1985).
(12) Hind wing is relatively wide, with short, slightly hooked costal projection and 3 longitudinal veins, among which 2nd vein is symmetrically bifurcated, with intercalary between its branches; sometimes additional intercalary(es) and cross vein(s) are present ( Fig. 55 View FIGURES 53–55 ). Larval protopteron has corresponding form and venation ( Fig. 67 View FIGURES 62–70 ).
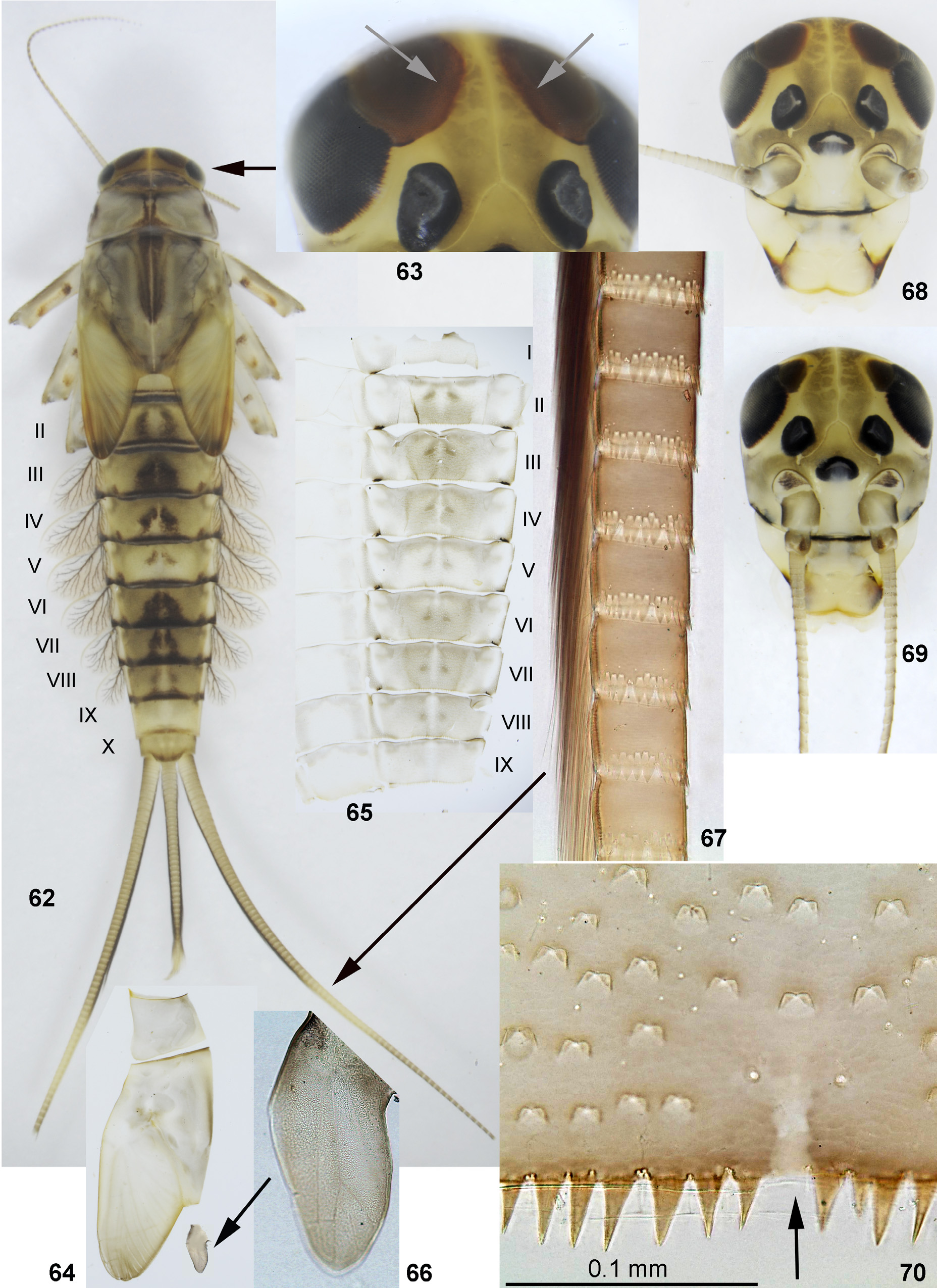
(13) Larval abdominal terga and sterna with numerous short scales in operculate sockets ( Fig. 70 View FIGURES 62–70 ). Similar scales are present on thoracic pleura and on legs. Such kind of scale sockets with two operculae is found in many baetid taxa, and probably is initial for Baetidae [ Kluge 2004: Turbanoculata (12)].
(14) Larval abdominal terga with long and pointed denticles on posterior margins; in both examined species denticles are absent on tergum I and present on terga II–X; on tergum IX row of denticles is interrupted behind pair of submedian setae ( Fig. 70 View FIGURES 62–70 ). Abdominal sterna with smaller pointed denticles, which are present either on sterna V–IX (in M. maculipennis ), or only on sterna VIII–IX (in M. latipennis sp. n.).
(15) Tergalii (unable to make respiratory movements, as in other Baetungulata) have the following structure. Tergalii I more or less diminished, with costal and anal ribs very short. Tergalii II–VII with costal rib welldeveloped and anal rib rather short, so that a long part of hind margin is free of rib; costal rib with serration ( Figs 5– 11 View FIGURES 1–15 ).
(16) Larval paracercus is well-developed, cerci greatly elongated, longer than body ( Figs 1–2 View FIGURES 1–15 , 62 View FIGURES 62–70 ); both cerci and paracercus bear very dense primary swimming setae, which are present on most their segments; bases of these setae are pressed together and regularly alternate as shifted dorsally and ventrally from the common line ( Fig. 14 View FIGURES 1–15 ). Segments of cerci are cylindrical, with boundaries not inclined and with denticles on posterior margin not enlarged on any side of cerci or paracercus; secondary swimming setae are absent ( Fig. 63 View FIGURES 62–70 ).
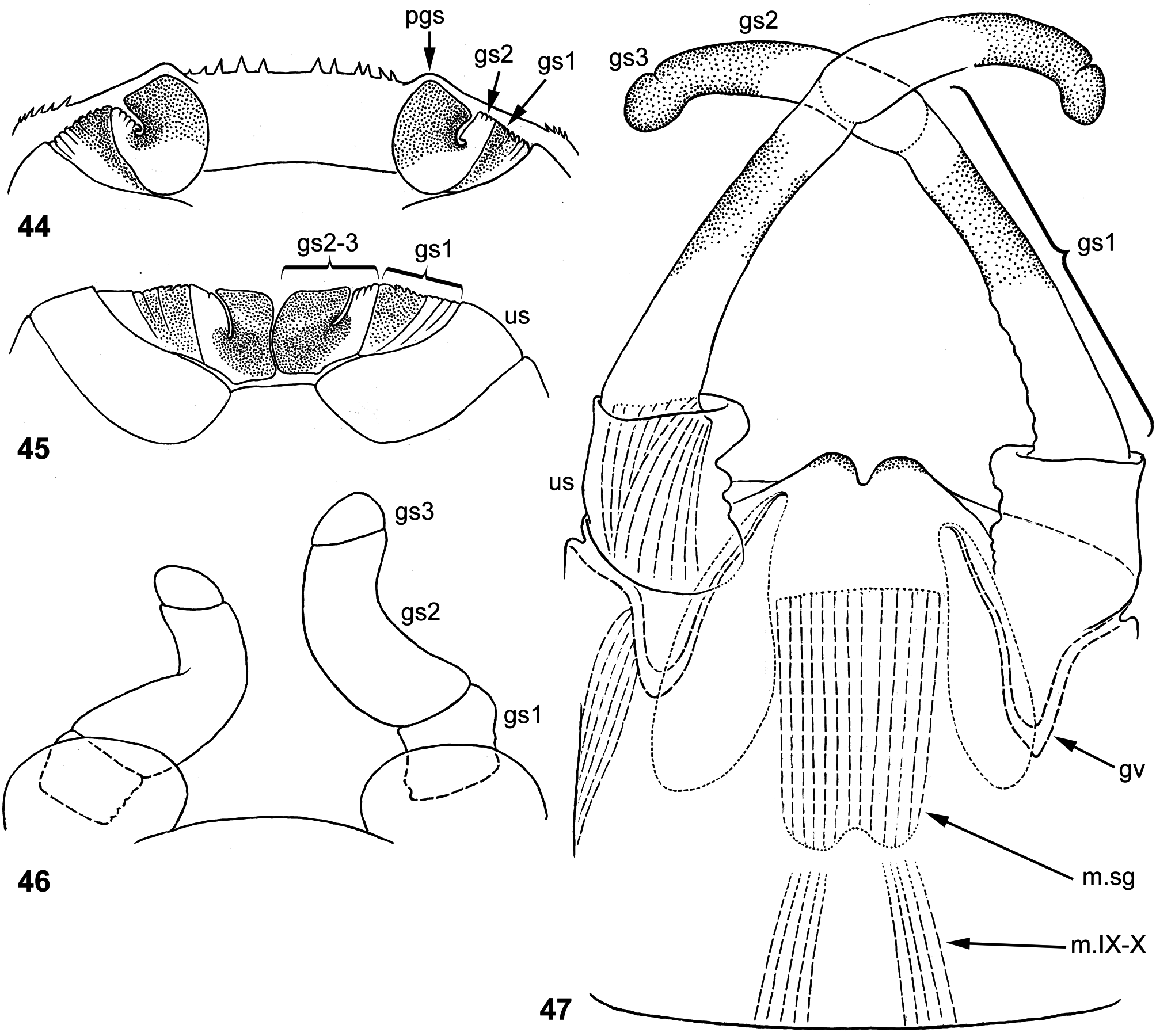
(17) Gonostyli have unusual shape and paradoxical development: in definitive condition 1st segment is longer than 2nd segment ( Figs 39 View FIGURES 34–43 , 47 View FIGURES 44–47 ); in course of development, 2nd segment grows earlier, so in subimago 1st segment is much shorter than 2nd ( Figs 37 View FIGURES 34–43 , 46 View FIGURES 44–47 ); in mature larva developing subimaginal gonostyli are folded under larval cuticle in a peculiar manner, which can be termed « Moribaetis - type ». When subimaginal wings are already fully developed and crumpled under cuticle of protopteron, developing subimaginal gonostylus has 1st segment extremely short, and 2nd segment forming a loop (3rd segment indistinguishable at this stage); apex of gonostylus locates inside apex of larval protogonostylus ( Figs 36 View FIGURES 34–43 , 44 View FIGURES 44–47 ). Just before moult, gonostyli become longer and meet medially by their loops of 2nd segments ( Fig. 45 View FIGURES 44–47 ). This « Moribaetis - type » of gonostyli folding proceed from the « Nigrobaetis - type » (at which 2nd segment is directed caudally and bent with convexity directed medially): in Moribaetis 2nd segment is longer and its bent is transformed to a loop.
Other peculiar features of genitals are the following: sterno-styligeral muscle is unusually short, so that originates far from fore margin of abdominal sternum IX; a pair of protuberances is present between unistyligers; 3rd segment of gonostylus is very short ( Fig. 47 View FIGURES 44–47 ).
Gonostyli have colorless cuticle and brown hypodermal coloration; their hypodermal coloration varies individually, being developed first of all in distal part of 1st segment, in distal part of 2nd segment and in small 3rd segment. This hypodermal coloration appears in last larval instar ( Figs 36 View FIGURES 34–43 , 44 View FIGURES 44–47 ), that helps to reveal homology of segments at different stages of development ( Figs 36–39 View FIGURES 34–43 , 44–47 View FIGURES 44–47 ).
All these features of genital structure in larval, subimaginal and imaginal stages are examined for M. maculipennis , while genitals of M. latipennis were examined for a single specimen of imago, which have not shed subimaginal cuticle. Genitals of this specimen ( Fig. 101 View FIGURES 96–101 ) have following features the same as in M. maculipennis : pair of protuberances between unistyligers; subimaginal gonostyli with sharply curved 2nd segments and very short 3rd segment; inside them colored imaginal gonostyli with 1st segments strongly crumpled; the same shape of gonovectes; the same unusually short sterno-styligeral muscle.
(18) Coloration is similar in both examined species and has the following features:
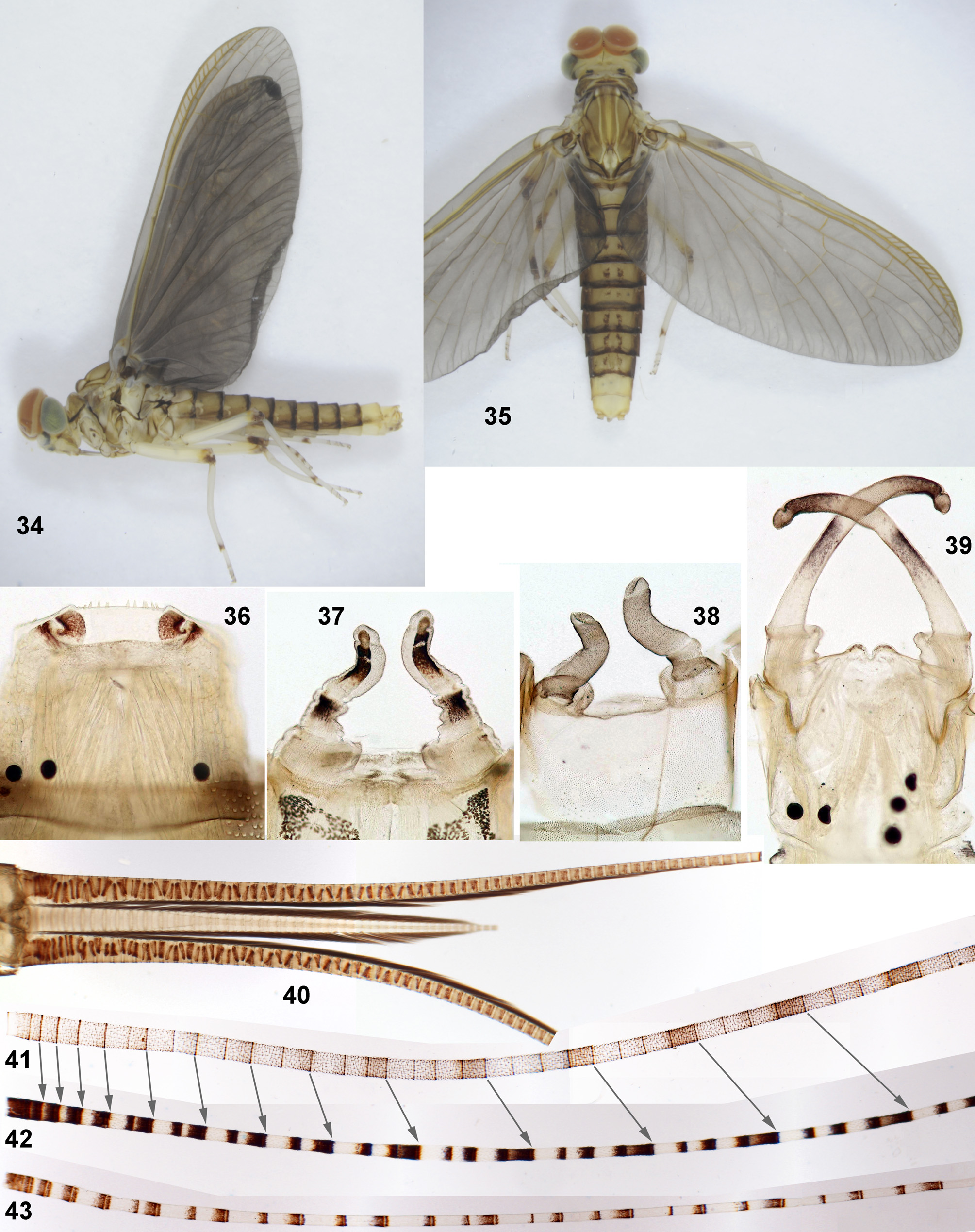
Hypodermal coloration (which is present independently of moults and passes from larva to subimago and to imago) consists of peculiar contrasting brown markings on thorax ( Fig. 3 View FIGURES 1–15 ), abdomen and legs. On abdominal terga it includes paired submedian stripes, usually well developed on uromeres III–IV and VI–VIII; shape of these stripes is somewhat different in M. maculipennis and M. latipennis sp. n. (see species characteristics below). Hypodermal coloration of legs consists of two brown or reddish spots on posterior side of femur, one at middle and another near apex ( Figs 50 View FIGURES 48–52 , 54 View FIGURES 53–55 , 62 View FIGURES 62–70 ). In contrast to hypodermal coloration of trunk and legs, hypodermal coloration of imaginal cerci appears only at the end of last larval instar, when subimaginal cerci grow under larval cuticle ( Fig. 40 View FIGURES 34–43 ).
Cuticular pigmentation of larva (present only in larval stage and becoming most intensive at the end of each larval instar) consists of diffusive brown and colorless areas on head, thorax, legs and abdomen ( Figs 4 View FIGURES 1–15 , 64–66 View FIGURES 62–70 ). Cuticle of larval frons has the following difference between species: in M. maculipennis it becomes pigmented only during the last larval instar ( Fig. 12 View FIGURES 1–15 ), but remains to be colorless during previous instars ( Fig. 13 View FIGURES 1–15 ); in M. latipennis sp. n. it becomes pigmented at previous instars also ( Fig. 69 View FIGURES 62–70 ). Cuticle of larval cerci is nearly unicolor in M. maculipennis ( Fig. 1 View FIGURES 1–15 ) and has darker and lighter areas in M. latipennis sp. n. ( Fig. 62–63 View FIGURES 62–70 ).
General coloration of larva is determined by combination of these cuticular and hypodermal pigments. Abdominal segments IX and X usually have both cuticular and hypodermal coloration poorly developed or absent, so tip of abdomen is often contrastingly light ( Figs 1–2 View FIGURES 1–15 , 62 View FIGURES 62–70 ).
Subimaginal cuticular coloration is the following: pronotum is light brown; mesonotum is light brown with certain sutures darker brown ( Fig. 52 View FIGURES 48–52 ); thoracic pleura have light and dark brown areas ( Fig. 51 View FIGURES 48–52 ); wings are dark brown due to dark brown ring at base of each microtrichion, veins lack this coloration ( Fig. 98 View FIGURES 96–101 ); legs are unicolorous, nearly colorless, with dark brown marks at base of tibia ( Figs 31–32 View FIGURES 25–33 ); abdominal terga are uniformly light brown, sterna much lighter.
Imaginal wings have membrane with contrasting brown marking on colorless background; in all three known species these markings border cross veins and are present also in proximal parts of costal and subcostal fields, where cross veins are absent ( Figs 48–50 View FIGURES 48–52 , 53–54 View FIGURES 53–55 , 100 View FIGURES 96–101 , 102 View FIGURES 102–105 ). At least in M. maculipennis and M. latipennis sp. n., this wing pigmentation appears in course of subimaginal development: just after moult from larva to subimago, wings are uniformly grey due to coloration of subimaginal cuticle ( Figs 34–35 View FIGURES 34–43 ); before moult from subimago to imago, wings get imaginal markings visible through dark subimaginal cuticle ( Figs 96–97 View FIGURES 96–101 ).
Distribution. Central America (central Mexico, Guatemala, Costa Rica, Panama). Species from South America and from non-tropical areas of North America, which were formerly attributed to Moribaetis , do not belong to this genus (see «Composition»).
Adaptation. Larvae of Moribaetis maculipennis and M. latipennis sp. n. keep themselves at places with strongest current and stony bottom. In contrast to larvae of Mayobaetis (which inhabit the same biotopes), larvae of Moribaetis do not crawl out of the water.
Composition. Moribaetis includes two species known as larvae and imagoes, Moribaetis maculipennis ( Flowers 1979) and Moribaetis latipennis sp. n. (see below). The third known species, Moribaetis salvini (Eaton 1885) , is known only as male imago; structure of its gonostyli, shape and venation of hind wing and coloration of fore wing testify about its belonging to Moribaetis (see below). The fourth species, Moribaetis brachiostrinus Romero & Esquivel 2018 , is described as larvae; it is closely related to M. latipennis sp. n. Six other species, attributed by some authors to Moribaetis , do not belong to it.
Originally the genus Moribaetis included, besides the nominative subgenus, the subgenus Mayobaetis Waltz & McCafferty 1985 with a single species Moribaetis ( Mayobaetis) ellenae ( Mayo 1973) ; subsequently the taxon Mayobaetis got a generic rank ( Lugo-Ortiz & McCafferty 1996). In contrast to Moribaetis , in Mayobaetis paraglossa has usual shape and bears three setae of the dorso-median row ( Waltz & McCafferty 1985: fig. 10); patella-tibial suture is fully developed and crosses inner side of tibia.
Three species known as winged stages only, aneto Traver 1971 [ Baetis ], comes Navás 1912 [ Baetis ] and socius Needham & Murphy 1924 [ Baetis ], were placed in the genus Moribaetis by Lugo-Ortiz & McCafferty (1999). Their placement in Moribaetis is regarded to be groundless ( McCafferty 2000, Cruz & Salles 2014).
In contrast to Moribaetis , the species aneto [ Baetis ] has unforked 2nd vein of hind wing and usual shape of gonostyli with 1st segment much shorter and thicker than 2nd, and with 3rd segment elongate ( Traver 1971: figs 1, 4; Cruz & Salles 2014: figs 10–11).
The species comes [ Baetis ] was said to be «similis salvini Etn. » and having hind wing with forked 2nd vein ( Navás 1912: 194, fig. 1a); however, these characters are not enough to determine its systematic position ( Cruz & Salles 2014).
The species socius [ Baetis ] was described as female subimago; in contrast to Moribaetis , its hind wing is narrow, curved, with fork of 2nd vein asymmetric and without 3rd vein ( Needham & Murphy 1924: pl. XII: fig. 153A). Judging by this structure of hind wing and relatively large size (body length 8 mm), it may belong to Mayobaetis .
The species originally named Moribaetis macaferti Waltz (in Waltz & McCafferty) 1985 was described as larvae from Costa Rica and Guatemala. Male imago, ascribed to this species by McCafferty & Lugo-Ortiz (1998), actually belongs to the new species Moribaetis latipennis sp. n. (see below), so true imago of macaferti [ Moribaetis ] is unknown. In contrast to true Moribaetis , larva of macaferti [ Moribaetis ] has labrum not widened and bearing usual «1+5–6 stout submarginal setae»; mandibular incisors are not blade-like; paraglossae are not narrowed apically and bear 3 setae in the dorso-median row; patella-tibial suture is fully developed and crosses inner side of tibia ( Waltz & McCafferty 1985: 243–245, figs 15–21). This species was attributed to Moribaetis based on presence of unpaired subapical seta on larval claw [see character of Moribaetis (11) above] and presence of minute procoxal «osmobranchia» [see character of Moribaetis (6)]. Combination of its larval characters (absence of frontal keel; non-widened labrum with few submarginal setae; non-blade-like mandibles; nonnarrowed paraglossae with several setae in the dorso-median row; fully-developed patella-tibial suture; unpaired subapical seta on claw; presence of hind protopteron) is in agreement with the characteristics of the taxon Caribaetis Kluge 1992 , which is recently accepted as a genus ( Kluge & Novikova 2014). So this species can be presumably treated as Caribaetis macaferti comb. n.
The species described under the name Moribaetis mimbresaurus McCafferty 2007 is known only as imagoes. The only arguments to attribute it to Moribaetis were «large size, membrane pigmentation in the forewing, the basally margined, distally oriented, sharp costal process of the hindwing, short stalk of the turbinate eye, and small terminal segment of the genital forceps» ( McCafferty 2007). However, all these characters are found in other taxa, including Rhodobaetis Jacob 2003. Size of some species of Rhodobaetis varies in great range, reaching one centimeter of fore wing length in individuals developed in cold water. Pigmentation of distal parts of costal and subcostal fields of fore wings is found in many taxa, including Rhodobaetis. Among Rhodobaetis, costal process varies from directed anteriorly to hooked distally. Stalk of turbinate eye of mimbresaurus [ Moribaetis ] has moderate height, as in many baetid taxa. Small terminal segment of the gonostylus is usual for Rhodobaetis. In contrast to Moribaetis , this species has fore wing without dark spots around cross veins ( McCafferty 2007: fig. 3), hind wing without furcation on 2nd vein ( McCafferty 2007: fig. 4) and a usual shape of gonostyli, with 1st segment much shorter and thicker than 2nd segment ( McCafferty 2007: fig. 5). Until larva of this species is known, it should be preliminary treated as Baetis ( Rhodobaetis) mimbresaurus comb. n.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
Moribaetis Waltz & McCafferty 1985
| Kluge, Nikita J. & Bernal Vega, Juan A. 2018 |
Moribaetis : Lugo-Ortiz & McCafferty 1996 : 368
| Lugo-Ortiz, C. R. & McCafferty, W. P. 1996: 368 |
Moribaetis
| Waltz, R. D. & McCafferty, W. P. 1985: 243 |