Calliscelio elegans (Perkins) Perkins, 2009
|
publication ID |
https://doi.org/ 10.5281/zenodo.275200 |
|
DOI |
https://doi.org/10.5281/zenodo.6214282 |
|
persistent identifier |
https://treatment.plazi.org/id/4A6287E8-AF4B-FFA1-29AB-664E1B31FBA0 |
|
treatment provided by |
Plazi |
|
scientific name |
Calliscelio elegans (Perkins) |
| status |
comb. nov. |
Calliscelio elegans (Perkins) , n.comb.
Figs. 1–5 View FIGURES 1 – 5 ; Morphbank13
urn:lsid:biosci.ohio-state.edu:osuc_concepts:245756
Caloteleia elegans Perkins, 1910: 624 . Original description.
Caenoteleia elegans: Kieffer, 1926: 550 View in CoL . Generic transfer, description.
Description. Female. General: Length: 2.0–2.4 mm (n=20); body strikingly multicolored ( Figs. 1, 4 View FIGURES 1 – 5 ): head, mesosoma, segment 1 of metasoma, legs, A1, A3-A6 deep orange yellow; anterior one-third of T2 and basal two-thirds of T6 yellow; posterior two-thirds of segment 2, segments 3-5 ebony black; apical third of segment 6 darkened; margins of ocelli darkened; A2 slightly darkened, A7 lighter in color than following clavomeres; A8-A12 nearly black; apex of mandible darkened; apex of hind femur slightly darkened; wing strikingly banded, with dark bands basally, medially and apically, separated by light bands, wing membrane beyond basal third infuscate in dark bands, hyaline in white bands, setae in dark bands black, in light bands white.
Head: head subglobose, moderately transverse; entire head uniformly with dense granular surface sculpture, lacking smooth area immediately dorsad of toruli, with dense semi-appressed golden hair; occipital carina complete medially, finely crenulate; OOL slightly <1 ocellar diameter, close to but not contiguous with inner orbit of eye; eye densely hairy, length of hairs slightly shorter than diameter of ocellus; clypeus short, anterolateral corners rounded; anteclypeus with several long erect bristles; malar sulcus largely obscured by surface sculpture; radicle distinctly elongate, one-fourth length of A1; A2 distinctly shorter than A3, subequal in length to A4; A5, A6 almost globular; clava 5-merous, claval formula A8-A12/1-2-2-2-1; clava cylindrical, clavomeres only slightly wider than long; mandible sub-tridentate, middle tooth smallest, lower tooth longer than upper.
Mesosoma: mesoscutum covered by dense granular surface sculpture, dense semi-appressed golden hair; notaulus percurrent, course considerably obscured by surface sculpture; mesoscutellum broadly transverse, width approximately 3 times length; mesoscutellum with same sculpture, pilosity as mesoscutum; metascutellar plate broad, short, weakly concave medially; dorsal propodeum deeply excavate medially, with median keels widely separated, running almost parallel (to accommodate horn on T1); side of pronotum predominantly rugose, with smooth glabrous field in epomial area; netrion finely sculptured; mesopleural depression rather narrow, deep, smooth; mesopleural carina absent; mesepisternum below mesopleural depression finely granular; mesepimeral chain of foveae not developed; metapleuron with fine longitudinal sculpture; meso-, metapleuron almost glabrous.
Wings: fore wing attenuate basally ( Fig. 4 View FIGURES 1 – 5 ), extending to middle of T5; R between tegula and costal margin with 12 semi-erect dark bristles distinctly extending beyond costal margin of fore wing; marginal vein (R1 proximal to r-rs) at least 2 times as long as wide, slightly shorter than stigmal vein; postmarginal vein (R1 13. http://www.morphbank.net/?id=476306 distal to r-rs) depigmented, slightly longer than stigmal vein; longest marginal cilia in fore wing shorter than stigmal vein; hind wing very dark basally.
Metasoma distinctly elongate, widest medially, distinctly narrowed both anteriorly and posteriorly; T1 produced into massive horn, with fine longitudinal sculpture over entire surface, base of T1 irregularly rugulose; T2 finely longitudinally striate, striae covering nearly entire surface; T3 broadly transverse, shorter than T2, delicately longitudinally aciculate, sculpture effaced medially; T2, T3 almost glabrous; T4-T5 transverse, with delicate coriaceous microsculpture, with abundant appressed golden pilosity; T6 distinctly elongate, tapering apically, apex truncate; S2 sculpture similar to T2; S3-S5 nearly smooth, with abundant appressed silvery pilosity.
Male: unknown.
Diagnosis. Within Calliscelio , C. elegans is easily identifiable on the basis of its unique color pattern with the orange-yellow head, mesosoma, T1, and base of T2; T2 (beyond its immediate base) and T3–T5 black; the banded wings with three darkened and two white bands. Additionally, the granulose sculpture of the head and mesonotum is distinctive. In contrast to most Calliscelio species, the metascutellar plate is extremely narrow and weakly concave medially to accommodate the metasomal horn ( Figs. 4, 5 View FIGURES 1 – 5 ). In most other species of Calliscelio , the plate typically extends over the apex of the horn (Fig. 6).
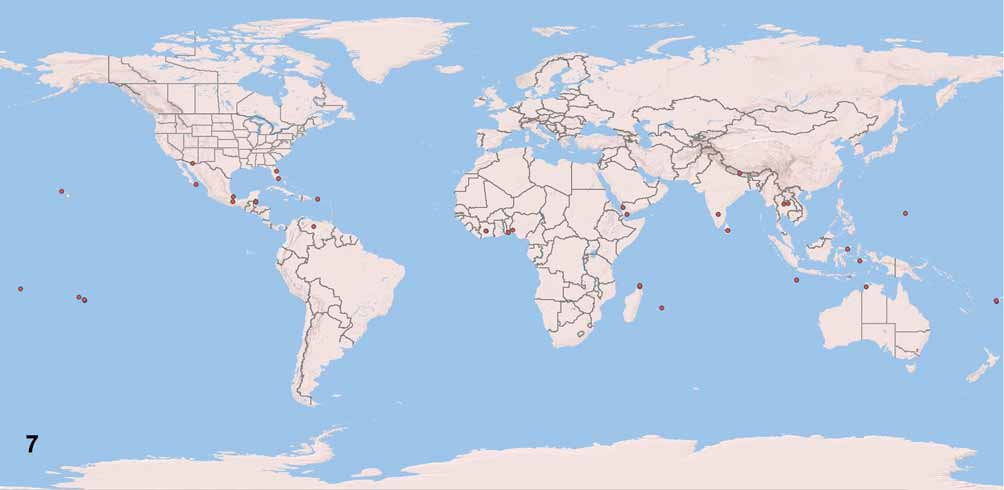
Distribution: Widespread, but rare throughout the tropics ( Fig. 7 View FIGURE 7 ), but also with additional specimens collected in northern Mexico and Florida, and also at fairly high elevation (2000 m) in Nepal. Link to distribution map: http://osuc.biosci.ohio-state.edu/HymOnline/map-large.html?id=245756.
Material examined. 60 females: AUSTRALIA: Christmas Island: 4 females, OSUC 256857–256860 ( ANIC); Northern Territory: OSUC 256856 ( CNCI). BELIZE: 4 females, OSUC 256871–256874 ( CNCI). BENIN: 3 females, OSUC 256882–256884 ( CNCI). FIJI: 2 females, FBA 015304, OSUC 256864 ( BPBM). FRENCH POLYNESIA: 8 females, OSUC 256861–256863 ( CNCI); OSUC 256866–256867 ( UCDC); UCRC ENT 111562, UCRC ENT 135651–135652 ( UCRC). GUAM: 1 female, OSUC 256854 ( CNCI). INDIA: 3 females, OSUC 256899–256901 ( CNCI). INDONESIA: 4 females, OSUC 256893–256896 ( CNCI). IVORY COAST: 2 females, OSUC 256885–256886 ( CNCI). MADAGASCAR: 2 females, OSUC 215759, CASENT 2029779 ( CASC). MAURITIUS: 1 female, MNNG 0002 ( MHNG). MEXICO: 5 females, OSUC 256868–256870 ( CNCI); OSUC 248095 ( OSUC); OSUC 256875 ( USNM). NEPAL: 4 females, OSUC 256890–256892, 256898 ( CNCI). NIGERIA: 1 female, OSUC 256881 ( CNCI). PUERTO RICO: 2 females, OSUC 256876–256877 ( CNCI). SAMOA: 1 female, OSUC 256865 ( CNCI). SRI LANKA: 2 females, OSUC 256902–256903 ( CNCI). THAILAND: 2 females, OSUC 256897, 256907 ( OSUC). UNITED STATES: Florida: 1 female, UCFC 0 079 680 ( UCFC); 1 female, OSUC 256875 ( USNM); Hawai‘i: 1 female, OSUC 256855 ( CNCI). VENEZUELA: 2 females, OSUC 256879–256880 ( CNCI). YEMEN: 3 females, OSUC 256887–256889 ( CNCI).
Biology. One specimen is recorded as being collected in roots of sugar cane in Hawai‘i.
Other less specific habitats vary from possibly natural formations such as tropical dry forest ( Madagascar), xeric oak hammock (Florida), edge of swamp ( Belize), and forest edge in Dumoga-Bone National Park ( Indonesia); to anthropogenic environments such as an agricultural field ( Mexico), agricultural experiment station ( Puerto Rico), a garden (Moorea), and “urban habitat” (Tahiti Nui).
Comments. We have now been able to accumulate over 60 specimens of C. elegans , the result of an intensive hunt among hundreds of thousands of specimens over the last 40 years. Its geographic distribution extends far beyond Hawai‘i: it is found scattered throughout the tropics and subtropics of the world, between 31°N–18°S, from sea level to over 2000 m in elevation in Nepal. For such a widespread species, it appears to be remarkably rare. The initial collecting record by Albert Koebele, for which he noted that the O‘ahu specimens were collected in the roots of sugar cane, suggests to us that C. elegans is a parasitoid of a host associated with this crop. Its wide distribution, therefore, may be the result of widespread sugar cane cultivation around the world. Even though Calliscelio is a diverse, common, and widespread genus, the only published host record for any species ( Hill, 1983) is for C. teleogrylli Hill. This species attacks the eggs of Teleogryllus commodus (Walker) ( Orthoptera : Gryllidae ), a pest in pastures that is widespread in the Pacific region. Similarly, we suspect that Calliscelio elegans attacks the eggs of one or more species of Gryllidae associated with sugar cane.
No males are among the specimens examined for this study. This may be because the male sex is chromatically different and not so conspicuous. But, in our experience, strong differences in color between sexes are not found in Calliscelio . Among the light-colored species, males are sometimes somewhat darker overall and the dark bands on the fore wings, if present in the female, may be weaker or even absent in the male. But, in general, the two sexes are similar in color. Another explanation for the lack of any male specimens may be that this species is thelytokous. This mode of reproduction is unusual among platygastrids, but certainly not unknown ( Austin et al., 2005), and would be advantageous for a species to establish itself in new parts of the world.
We note here that a number of platygastrids seem to have extraordinarily broad geographic distributions. Examples include Iphitrachelus lar Haliday , Calliscelio marlattii (Ashmead) , Aradophagus fasciatus Ashmead , Opisthacantha mellipes Ashmead and Duta virginiensis (Ashmead) . For none of these has the host been identified. Psix tunetanus (Mineo & Szabó) is found in the Mediterranean region of Africa east to Saudi Arabia, and also in both North and South America ( Johnson & Masner, 1985). This species is a parasitoid of the eggs of pentatomoids ( Hemiptera ). Trissolcus basalis (Wollaston) has been intentionally introduced around the world to control the pantropical pest Nezara viridula (Linnaeus) ( Hemiptera : Pentatomidae ). This parasitoid species was originally described from the island of Madeira (Wollaston, 1856), but was also found early on (and described as Telenomus megacephalus Ashmead ) in the West Indies in 1894. All of its closest relatives are native to Africa. These observations reinforce our conviction that a global perspective is optimal for taxonomic and systematic studies. Additionally, the number of widespread species of these small to minute parasitoids may be expanding as a result of increased globalization of commerce.
If Calliscelio elegans is, through most of its range, an introduced species, then from which part of the world did it originate? Calliscelio teleogrylli Hill is similar in the sculpture of its frons, itself a relatively unusual character for the genus. While this species has been recorded only from New Zealand, its host is widespread, and our sampling of platygastrids in the Pacific region is inadequate to confidently assert that C. teleogrylli is restricted to New Zealand. Another species with the same granular sculpture, as yet undescribed, has been collected in Yemen. Progress toward resolution of this question will require increased sampling around the world and a better understanding of the diversity and interrelationships of species in the genus Calliscelio .
| OSUC |
Oregon State University |
| ANIC |
Australian National Insect Collection |
| CNCI |
Canadian National Collection Insects |
| FIJI |
University of the South Pacific |
| FBA |
Freshwater Biological Association |
| BPBM |
Bishop Museum |
| UCDC |
R. M. Bohart Museum of Entomology |
| UCRC |
University of California, Riverside |
| ENT |
Ministry of Natural Resources |
| GUAM |
University of Guam |
| MHNG |
Museum d'Histoire Naturelle |
| USNM |
Smithsonian Institution, National Museum of Natural History |
| SRI |
Serengetti Research Institute |
| UCFC |
University of Central Florida |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Calliscelio elegans (Perkins)
| Masner, Lubomír, Johnson, Norman F. & Musetti, Luciana 2009 |
Caenoteleia elegans:
| Kieffer 1926: 550 |