Kuhlia rupestris, (Lacepede, 1802) (Lacepede, 1802)
|
publication ID |
https://doi.org/ 10.26028/cybium/2020-441-006 |
|
DOI |
https://doi.org/10.5281/zenodo.10886716 |
|
persistent identifier |
https://treatment.plazi.org/id/4B4A879F-FFBF-FFA2-B8D6-0937FBEB9E85 |
|
treatment provided by |
Felipe |
|
scientific name |
Kuhlia rupestris |
| status |
|
Species description View in CoL
With 12 species described in a single genus ( Kuhlia ), the Kuhliidae family (Flagtails or Aholeholes) has a tropical Indo-Pacific distribution occurring in tropical and subtropical fresh, brackish and marine waters ( Randall and Randall, 2001). Kuhlia rupestris (Lacepède, 1802) is a diadromous species and widespread in the small volcanic island streams extending from the east coast of Africa to Samoa and from northeastern Australia ( Feutry, 2011) to southern Japan along Pacific coast of Shikoku and Honshu ( Nakabo, 2013). It has a large mouth, split horizontally, and a high and compressed form with greyish blue hues on the back and silvered on the sides. Margins darkened on dorsal surface and black spots are also evident on each lobe of the caudal fin ( Lewis and Hogan, 1987; Randall and Randall, 2001). Kuhlia rupestris has a dorsal fin with 10 spiny and 11 flexible rays; 10 spiny and 10 flexible rays for the anal fin. Its lateral line presents scale rows between 39 and 41 and gill rakers number between 25 and 28 ( Randall and Randall, 2001).
Conservation challenges
Kuhlia rupestris is of interest to recreational fishermen ( Lewis and Hogan, 1987), but it is also consumed for subsistence in parts of its range (ER, Boseto, Feutry, 2015). This species is of least concern (LC) globally according to the IUCN (International Union for the Conservation of Nature), even if locally, some populations are threatened or even possibly extinct ( Mailautoka and Hoese, 2012). Its assessment as LC is mainly due to its extensive range and relative abundance in several parts of the Indo-Pacific area such as, for example, the Solomon Islands ( Boseto et al., 2007). Globally, its stocks are regarded as relatively stable ( Mailautoka and Hoese, 2012), but some populations have clearly undergone dramatic reductions in size and distribution. Queensland is a good example where localised depletion has led to the implementation of important restoration programs (Hutchinson et al., 2002, 2009a; Pusey et al., 2004; Scanlon and Marsden, 2010). This is also the case in Réunion Island, where this species has been classified since 2010 as vulnerable (VU) on the red list of threatened species ( UICN France et al., 2013) based on research on K. rupestris ecology and stock assessment in Réunion Island (2009). The particularities of K. rupestris life cycle and the important anthropogenic pressures led the scientific community to admit that the wild population is facing a high extinction risk on this island ( UICN France et al., 2013). Many potential threats to its conservation have been highlighted: i) this species is the target of a strong fishing pressure and a victim of illegal fishing; ii) the number of adults able to breed is too low, iii) many barriers to migration and loss of connectivity are disturbing this species life cycle and reducing habitat surfaces. This level of classification for K. rupestris in Réunion Island will have to be revised shortly because, since 2009, additional knowledge was provided by experts, in particular regarding its late sexual maturity ( Henderson, 2010). Furthermore, the specificities of its life cycle tend to minimize the pertinence of the listing criteria defined in a general context ( Richarson et al., 2009) and could lead to an over or under-estimation of the degree of extinction risk. This is the first listing for K. rupestris in Réunion Island and while not being perfect, it highlights several threats to the species survival and the need for additional studies locally.
KNOWLEDGE SYNTHESIS
Spatial distribution
Geographic range, larval dispersal and genetic connectivity
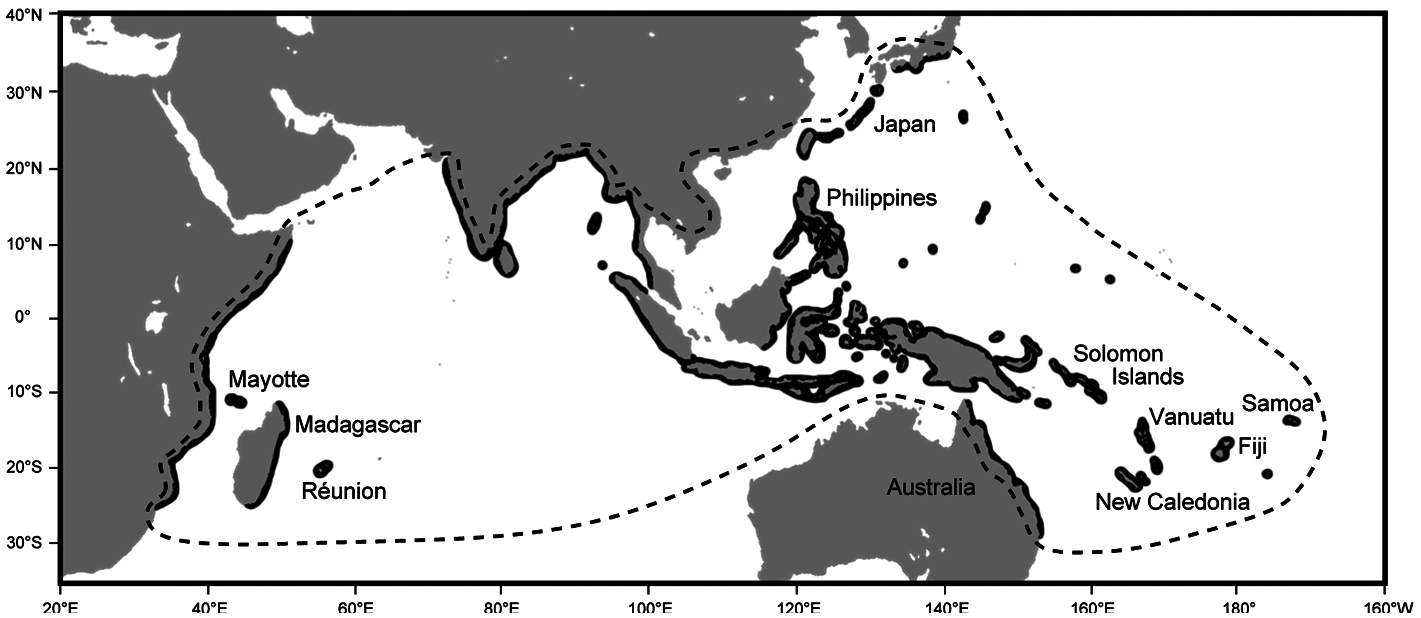
Kuhlia rupestris has a large geographic range ( Fig. 1 View Figure 1 ), from African eastwards in the Indian Ocean to Samoa Islands and southwards from Japan in Pacific Ocean ( Feutry et al., 2012a). Over the last decades, several studies have pointed out its remarkable ability to colonize such a large area. The key behind this colonisation success is the particularity of its life cycle: K. rupestris is a diadromous species ( Feutry et al., 2012b; Hamer et al., 2015). Its life cycle includes a marine larval phase, during which dispersal from one river or island to another is possible. This colonization mechanism is preponderant in freshwater taxa inhabiting tropical islands such as Gobiidae and Anguillidae species ( Keith, 2002). The larval stage constitutes a real adaptation strategy to invade vacant habitats following perturbations or disperse to new territories ( Maeda et al., 2007; Lord et al., 2010).
The analysis of otolith microstructure allows determining the pelagic larval duration (PLD), which is an important parameter defining dispersal capabilities of larvae. The PLD can generally be over 100 days for widespread diadromous species found in tropical insular systems such as the Anguillidae Anguilla marmorata ( Reveillac et al., 2008) , the Gobiidae Sicyopterus lagocephalus (Lord et al., 2010) or the Eleotridae Eleotris fusca ( Mennesson et al., 2015) . At 37.29 days ± 4.71 in the Indian Ocean and 44.11 days ± 9.37 in the Pacific Ocean ( Feutry et al., 2012c), the PLD of K. rupestris however is relatively short. It has been hypothesized that the short PLD is the major factor controlling the extent of its distribution range and connectivity between populations. Recent genetic work showed that K. rupestris consists of at least three populations (two in the Indian Ocean and one in the Pacific Ocean), possibly four (mitochondrial DNA seemed to indicate a barrier to gene flow between the north and the south Pacific). The short larval PLD is therefore not sufficient to maintain genetic homogeneity across its entire geographic range and speciation may be occurring or has already occurred. Molecular markers and estimation of larval dispersal using current modelling, however, showed that ~40 days is enough to maintain connectivity over large distances at either evolutionary or contemporary timescales ( Feutry et al., 2012a).
Until recently, Kuhlia sauvagii Regan, 1913 was believed to be endemic to Madagascar only. However, surveys of the freshwater fish fauna followed by genetic analyses revealed its presence in both Mayotte and Réunion Islands ( Feutry et al., 2012d) and it is possible that K. sauvagii inhabits other islands in the region. Kuhlia sauvagii and K. rupestris are morphologically very similar and only careful analysis allows distinguishing them one from another. In fact, during sampling campaigns occurring from 2000 and 2011 at La Réunion, the two species have been collected but all the specimens were recorded as K. rupestris with an unknown rate of misidentification. Future assessment of the status of K. rupestris in the Indian Ocean and in Réunion Island in particular needs to consider the proportion of the fish that are K. sauvagii and not K. rupestris . The main diagnostic character is the scale count along the lateral line, between 42 to 44 scales for K. sauvagii (Loiselle and Stiassyny, 2007) versus 39 up to 41 for K. rupestris . A genetic barcoding approach is a good alternative option, with the advantage of being able to identify the species with non-lethal tissue samples a posteriori in the laboratory. Samples collected in the past such as otoliths may also prove useful to assess the proportion of K. sauvagii in populations of interest.
Development and life cycle
Migration and reproduction
Multiple line of evidence seems to indicate that reproduction occurs in the brackish or marine environment. Hogan and Nicholson (1987) were the first to establish that the optimal sperm motility for salinity ranged between 25‰ and 32‰ in individuals from the Fiji Islands. Another study by Henderson (2010) indicated optimal sperm motility occurs at 36‰ salinity for males caught in Queensland. These differences could reflect either regional or experimental variability. In captivity, only individuals maintained in freshwater before receiving hormonal injections and then being moved to high salinity tanks (30‰) were capable of breeding (Hutchinson et al., 2009).
While spawning behaviour has yet to be observed in the wild, Hogan and Nicholson (1987) suggested that individuals converge to the tidal zone for breeding along coastal and offshore reefs. Additionally, breeding appears to be performed in a short-time frame ( Lewis and Hogan, 1987; Henderson, 2010).
The spawning period seems to occur during full moon and new moon phases ( Hogan and Nicholson, 1987; Hutchinson, 2009; Henderson, 2010) of the rainy season (ER, Anamparéla, Feutry, Marsden, Valade, 2015). The increase in flow velocity and a favourable moon phase could have a determinant synergic effect favouring the migration into preferential breeding habitat. Analysis of hormonal concentrations during the different gonad maturation stages have shown that the reproduction period could last for months, from October/November to March/April in Australia (Hutchinson et al., 2008; Henderson, 2010). Additionally, Hutchinson et al. (2009) reported females bearing different oocyte sizes, thus suggesting females K. rupestris perhaps have the ability to spawn multiple times each season. This is possibly an adaptation to the high variability and unpredictability of environmental conditions faced in insular systems. Kuhlia rupestris compensates for his short larval phase by having a long breading season, ensuring its survival via low and episodic recruitments staggered over time rather than one big recruitment event.
On Réunion Island, surveys of larval recruitment in the mouth of the two largest rivers indicate that the reproduction period could last up to 10 months, from November/December to August/September ( Lagarde et al., 2012).
Sexual maturity
The study of sexual maturity requires a high number of mature specimens. Thankfully, for several decades now, sacrifices are no longer required and have been replaced by non-lethal sampling. Biopsies, allowing the measure of hormonal concentrations, are done using a cannula inserted into the urogenital aperture. However, in K. rupestris , this method has only produced satisfactory results for females (Henderson, Hutchinson, 2015, pers. comm.). For the males, it is still difficult to obtain their semen (by massaging them) even if they are at the appropriate maturity stage (ER, Henderson, 2015).
Lewis and Hogan (1987) were the first to report a minimum size of 17 cm SL for “running ripe” males, whereas the minimum size for nearly ripe female was 21 cm SL. More recently, in Australia, Henderson (2010) has collected some blood samples and made ovarian biopsies on a quarterly basis in order to determinate reproduction activity. This work showed that ripe females were larger than 23.4 cm SL as opposed to 17-18 cm SL for ripe males. In Japan, however, the smallest mature male observed was 12 cm SL and more than half of the males mature at 17 cm SL (Oka and Tachihara, 2017, pers. comm.), therefore suggesting regional variability.
Furthermore, the age for sexual maturity ranges between 3 and 7 years ( Gelineau et al., 2015). In Réunion Island it has been estimated that individuals reach sexual maturity after 5 years ( Gelineau et al., 2015), whereas in Queensland, in natural environment, it is ranged between 4 and 6 years ( Henderson, 2010).
Trophic ecology
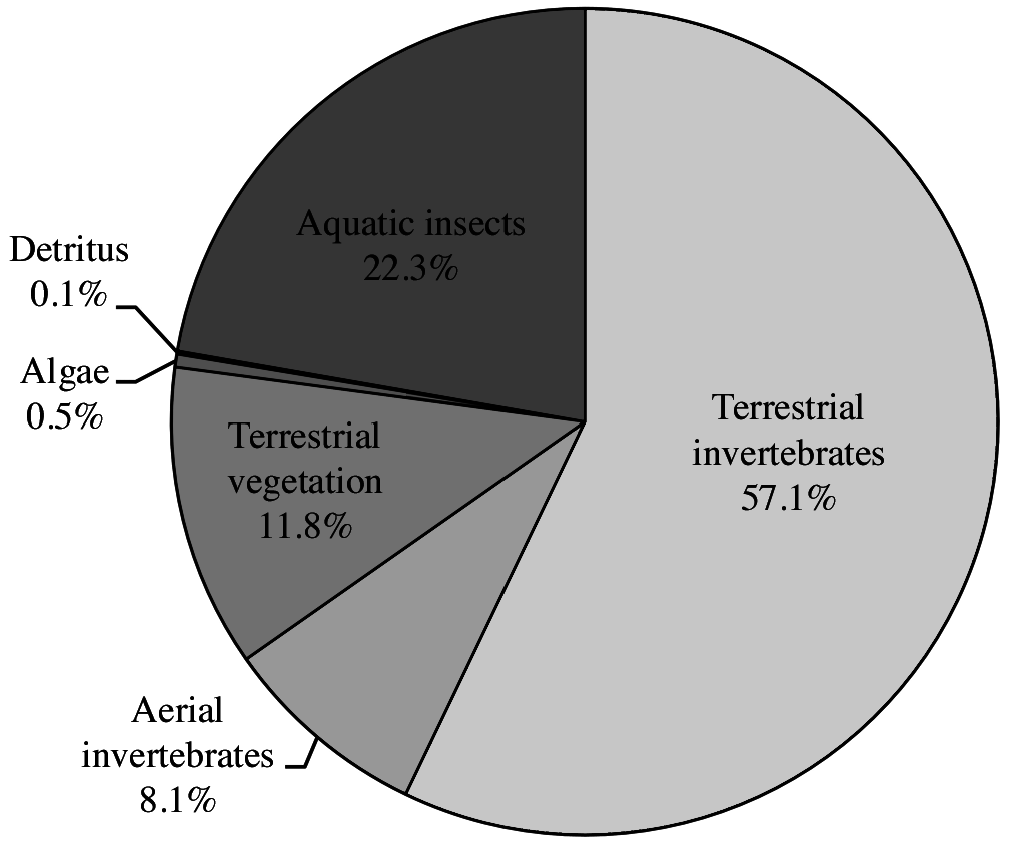
Kuhlia rupestris seems to have a fairly diverse diet. The analysis of the diet of 50 specimens from 3 different locations in northern Australia by Pusey et al. (2004) revealed that the main food source was terrestrial invertebrates, aquatic ones coming second ( Fig. 2 View Figure 2 ). Ants, spiders, grasshoppers or even frogs and smaller vertebrates were found in the stomachs of large individuals. Leave debris and fruits were also identified, but quite surprisingly, very few fish appeared in the stomach content. For young individuals, Trichopteran and Ephemeroptera larvae as well as adult stages formed the bulk of the food. Note that a breakthrough towards successful breeding in captivity was the consumption of small rotifers by K. rupestris larvae ( Hutchison et al., 2009b). Given the wide distribution of K. rupestris , additional studies with consideration of local habitat and seasonality would probably help define its diet more precisely across its range. According to experts, this species feeds on different fish species ( Sicyopterus sp. fry, young Anguilla spp. ) and macrocrustaceans ( Atyidae and Macrobrachium spp. ) (ER, Marquet, Pusey, Lagarde, 2015).
International experts consider K. rupestris an omnivorous and opportunistic species (ER, Marquet, Anamparéla, 2015). It is qualified as visual predator by some researchers (ER, Pusey, Feutry, 2015) having the ability to catch floating and deriving preys such as insects fallen into the water.
Habitat
Morphodynamical data
Kuhlia rupestris habitat is found between the lower and the middle course of the river and is most often stopped by the first significant obstacle, generally a waterfall. In some rivers, especially in steep tropical islands, this can mean only a few hundred metres of suitable habitat. Exceptional hydrological conditions may help K. rupestris reaching higher parts of the rivers than they normally would. In the absence of obstacles, K. rupestris may be encountered far inland. For example, on the Onilahy River, in Madagascar, K. rupestris occurs at up to 900 m of altitude and as far as 250 km from the sea and ( Loiselle and Stiassny, 2007). In Australia, some young individuals were found 50 km from the river mouth at an altitude of 50 m ( Pusey et al., 2004). In Réunion Island, K. rupestris is found from 0 to 450 m of altitude ( Antemi et al., 2011 -2013).
Microhabitat and preference curves
Kuhlia rupestris seems to have a distinct preference for clear water rivers with rocky substrates ( Lewis and Hogan, 1987; Hutchinson et al., 2008) presenting diverse profiles.
Two different studies, both in the Pacific region, investigated K. rupestris microhabitat preferences. It appears that K. rupestris preferentially lives in water bodies deeper than 60 cm and tends to avoid those shallower than 30 cm ( Keith et al., 2009). Its preference for deep water was also confirmed in Réunion Island where subaquatic observations were carried out ( Gelineau and Saget, 2016). These observations also revealed that K. rupestris seems to prefer to stay near the bottom or in the water column rather than close to the surface. Kuhlia rupestris occurs in a large spectrum of sediments ( Pusey et al., 2004; Keith et al., 2009) although they tend to prefer substrates dominated by hard materials such as boulders and slabs. During the adult stage, several experts report that adults prefer deep-water environments, especially those with shelter provided by boulders, dead wood or roots system under the river bank (ER, Marquet, Marsden, Hutchinson, Valade, 2015) and it is considered that this species prefers a flow velocity lower than 0.2 and 0.3 m.s –1, generally avoiding those ranged between 0.8 and 1 m. s –1 ( Keith et al., 2009).
In terms of river flow, this species is quite tolerant, occurring in rivers with flows ranging from less than 1 up to several hundred of m 3.s –1. In other words, they can be found in small creeks as well as in large rivers draining water from vast watersheds. In the tropics, this often means the potential for big flood events with very high-water flows.
Some degree of sexual segregation has been reported in Australia ( Lewis and Hogan, 1987; Henderson, 2010). Females tend to move further upstream, whereas males inhabit mid to lower reaches of the river systems. Lewis and Hogan (1987) also found that the sex ratio was approximately 10 females for 1 male and only males were found in estuaries. More recently, a sex ratio of 4 females for 1 male was reported for the Wyuna Creek population in Queensland ( Henderson, 2010).
Environmental tolerances
Kuhlia rupestris probably has an affinity for well-oxygenated waterbodies. Indeed, in the South-West Pacific Ocean it has been collected in environments, which generally exhibit mean dissolved oxygen values close to 7 mg.l –1 ( Pusey et al., 2004) and around 8 mg.l – 1 in Réunion Island ( Gelineau and Saget, 2016). It is however important to note that a study in Queensland revealed that K. rupestris could be tolerant to low dissolved oxygen concentrations (<4 mg.l –1), at least temporarily ( Hogan and Graham, 1984).
This species has wide temperature and acidity tolerances. It survives to winters in Okinawa Island, southern Japan, where the lowest temperature is about 13°C (K. Maeda, unpubl. data) and is resistant to average temperatures in excess of 25°C (ER, Boseto, Henderson, Valade, 2015). Kuhlia rupestris has been observed in acid water with a pH as low as 4.5 ( Pusey et al., 2004) and in basic water with a pH as high as 9 (ER, Raynaud, 2015).
Population dynamic
Fry and juvenile growth
Very little data available regarding the early growth of K. rupestris . Neither its embryonic development duration nor its incubation period has been studied in the wild. In Australia, during the first attempts of reproduction in captivity, fertilised eggs size were about 600 μm and larvae hatched within 12 to 15 hours after spawning. Two days old larvae measured about 2.4 mm (Hutchinson 2008).
Recent studies on the larval dispersal and migratory flows in Réunion Island revealed that K. rupestris larvae recruiting in freshwater at sizes of about 18-30 mm SL ( Richarson et al., 2010). In Japan, K. rupestris juveniles recruit into streams at about 20 mm SL mainly from October to February (Oka and Tachihara, 2017, pers. comm.). In Australia, the first size classes observed in freshwater are about 19 to 25 mm SL (Hutchinson, 2008; Henderson, 2010) and display a bimodal size distribution indicative of two major peaks of recruitment each season. In the Indian Ocean, and particularly in Réunion Island, the peak of juvenile recruitment in freshwater is observed in January-February coinciding with the new moon and the full moon phases during the rainy season ( Lagarde et al., 2012). There is also a high variability in the size of the youngest individuals, which probably reflects the extent of the breeding season. The timing of recruitment could further increase this size heterogeneity. Indeed, it was suggested that a recruitment occurring in wet and hot season, with a likely high trophic potential, could enhance the growth of fish from an early breeding, whereas the recruitment occurring during the dry season, with less favourable trophic conditions, would not allow for optimal growth ( Gelineau and Saget, 2016).
Studies of the fry growth rate in captivity provide additional data. Under controlled conditions, young individuals fed daily increased their weight from 0.57 g to 3.5 g and their length from 30-35 to 60-70 mm in less than 3 months ( Hoarau, 2009; Gelineau and Saget, 2016). During the first month, the maximal weight gain recorded was 166% versus 125% in average ( Hoarau, 2009).
Growth and longevity of subadults and adults
In the Pacific Ocean, Lewis and Hogan (1987) showed a growth of 2 cm.y –1 and a longevity reaching 14 and 20 years for males and females, respectively. In Okinawa, Japan, maximal longevity is 8 years (Oka and Tachihara, 2017, pers. comm.). In Queensland, a mark-recapture experiment associated with scale analysis on 145 individuals showed an adult growth rate of 2-4 cm.y –1 with 1 year old averaging 80 mm and the oldest specimen being 327 mm long and 13 years old. In captivity, fin ray increments suggested that a female weighing 3.54 kg was 20 years old (ER, Hutchinson, 2015). Growth can be much faster in captivity compared to the wild and some females were able to gain 1 kg.y –1.
Additionally, sexual dimorphism is well recognized for K. rupestris . Females have a higher longevity and faster growth compared to males ( Lewis and Hogan, 1987; Hutchinson et al., 2009a) although the fastest growth ever recorded was 184 mm in 200 days in a male ( Henderson, 2010). This study also showed that males generally do not exceed 7 years, whereas all individuals 10 years old and above are exclusively females.
Population size and trend
It is difficult to estimate the number of individuals present in a specific river or at a basin scale and K. rupestris is no exception with very limited data available. In Queensland, some investigations were conducted in order to assess the abundance and distribution of K. rupestris . Indeed, based on a broad data collection, collected from natural history museums, universities, fishing associations, magazines and scientific journals, Hutchison et al. (2002) were able to demonstrate that the species had declined in recent times. The increase in construction of barriers to migration was identified as the main cause for this decline.
In Réunion Island, an electrofishing survey is ongoing since 2010 on all 13 perennial rivers. Kuhlia rupestris is captured intermittently, with low abundances (up to 1- 2 specimens. 100 m –2) and variability between the rivers ( Gelineau et al., 2015). Experts have identified no particular trend there since 2000 (ER, Lagarde, Valade, 2015).
Behaviour, locomotion and moving capacity
Kuhlia species are pelagic mostly feeding from the surface ( Resh et al., 1999). Several authors identified juveniles swimming in schools (Hutchinson, 2002; Boseto et al., 2007; Feutry, 2011). Schools of juveniles can sometimes be accompanied by larger individuals assumed to be adults (ER, Henderson, 2015). Juveniles are generally more mobile and less wary than adults, which have preferentially been observed in static position near river bank overhang cavities and crevices (ER, Anamparéla, 2015).
Fish movement depends on the inherent physiological capacity of the species, the abiotic conditions of their environment (temperature, hydrology) and the presence/absence of physical barriers, which can reduce access to suitable habitats.
K. rupestris is considered a strong swimmer (ER, Marquet, Boseto, 2015), with good sprinting capacity (ER, Henderson, 2015). The optimum swimming speed for individuals of 10, 15 and 20 cm is 1 m. s –1, 1.5 m.s –1 and 2 m. s –1, respectively ( Antemi et al., 2011 -2013). However, the species is only able to maintain such speed across few meters. For larger individuals, the swimming speed is closer to 1.5 m.s –1 and is generally maintained for 5 to 20 seconds ( Kapitzke, 2010). In Australia, Veitch and Burrows (2006) observed this species overcome partial or complete submerged barrier during flood events, therefore demonstrating good upstream swimming capacities.
Although its swimming characteristics are quite clearly understood and unanimously supported by international researchers, the jump capacity is not well known. Jumping could be a particular behaviour in response to stress conditions (Valade, 2015, pers. comm.). Observations in Australia attest that they have observed some juvenile overcoming barriers of 10 to 30 cm high (Henderson, Hutchinson, 2015 pers. comm.). Individuals of about 20 cm are theoretically able to overcome barriers of 40 to 50 cm high but only in specific conditions such as: flow velocity ranged between 0.5 to 1 m.s –1 with an adequate draft (> 15 cm), presence of taking off ramp and sufficient temperature ( Antemi et al., 2011 -2013). Other factors may affect the jump ability such as number of barriers, which have been previously overcame.
THREATS AND MANAGEMENT OPTIONS
Obstacles to fish movements
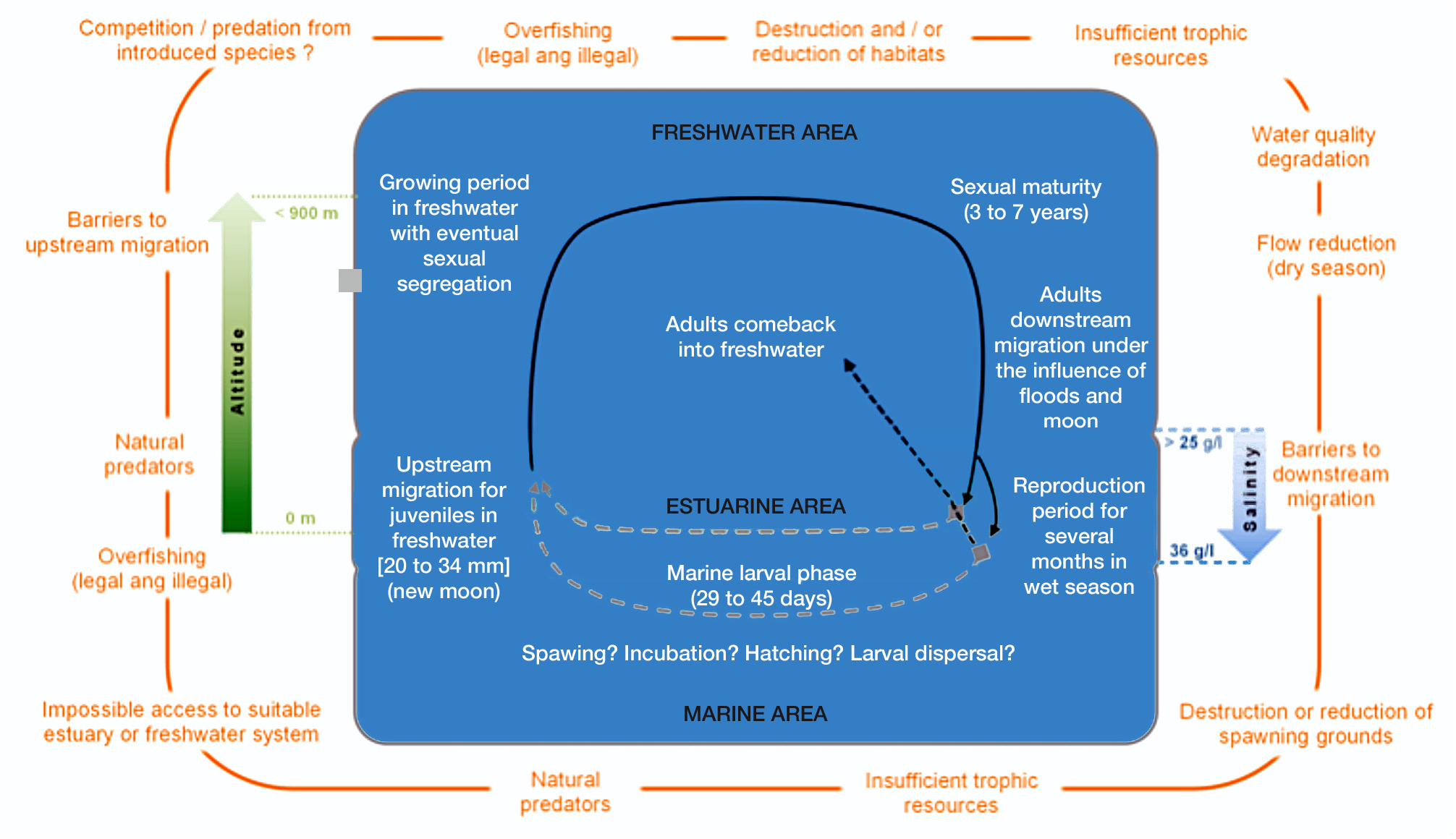
While we acknowledge that different threats may be present in different islands, some generalisations can be deducted from this compilation of information on the biology and ecology of K. rupestris . Many threats are related to the particularity of the catadromous life cycle of K. rupestris , which is summarized in Fig. 3 View Figure 3 . For example, K. rupestris needs to be able to freely move from its growing habitat to the sea as an adult, and from the sea to its growing habitat as a larva in order to complete its life cycle. In developed countries, human made obstacles to fish movements are common, and their impact is often made worse by the diversion of water for irrigation or power generation. In Australia for example, it has been shown that the species decline correlates with the construction of dams and spillways (Hutchinson et al., 2002). In recent years, some hydraulic structures in Queensland have been equipped with fishways allowing fish to overcome these otherwise insurmountable obstacles. In the absence of fishways, these structures prevent any colonization or recolonization of the impacted upper reaches, where population are doomed to extinction.
In Réunion Island, fish migrations are heavily affected in some areas. For example, in Saint-Etienne River, less than 10% of the available habitat is accessible to K. rupestris ( Antemi et al., 2011 -2013) and the implementation of fish-ways is likely to improve the health of the population.
Other human activities may affect K. rupestris adversely, for example in Réunion Island, Sicyopterus sp. fisheries in estuaries are probably affecting adult downstream migrations, or larva recruitment and post-settlement.
Water flow
Flow regime reduction or modifications, like a decrease of flood magnitude and/or an increase of low flow duration, are likely to reduce habitat suitability for K. rupestris .
In Australia, water adduction within weirs and sumps along hydraulic structures alter the average flow and involve continuity failures ( Scanlon and Marsden, 2010). Such perturbations are not exclusive to Pacific Ocean and Australia. They may occur in each river where hydraulic development occurs. In Réunion Island, the construction of hydroelectric plants along many rivers strongly impacts their flow regime. In each river, a minimum “biological flow” has to be evaluated in order to ensure the free passage and survival of aquatic organisms occurring near the barriers ( Gelineau et al., 2015).
Such perturbations are not exclusive to Australia and Réunion Island and may occur wherever humans have competing interests for water use. Kuhlia rupestris often inhabits small creek, where even small water diversion could have a dramatic impact on its survival.
Kuhlia rupestris is most certainly sensitive to insufficient flow and even more so during migration periods. For example, disconnected river mouth, even temporally, may generate high larva mortality given their relatively short marine larval phase and the maintenance of perennial water at the sea/river interface its critical to ensure the species survival (ER, Marquet, 2015).
Habitat deterioration
Habitat deterioration is another potentially important threat to K. rupestris . Australian experts (ER, Henderson, Pusey, 2015) believe that limited trophic resources, especially in small hydrosystems (creeks and small rivers), might contribute to high mortality in young stages. Moreover, studies in captivity performed by Hutchinson et al. (2008, 2009) revealed that degraded water quality, i.e. eutrophication issues, might affect larvae and young juveniles survival by reducing the zooplankton abundance, size and diversity available to them.
Also, given that a large part of the juveniles and adults diet depends on the river surroundings rather than the river itself, degradation of the river edges would have a direct impact on the diversity and quantity of food available. Kuhlia rupestris seems to particularly like riverbank overhanging cavities, roots or dead trees ( Lewis and Hogan, 1987; Boseto et al., 2007; Scanlon and Marsden, 2010). They probably provide shelter from predators, but also hides to ambush their preys. Therefore, any impact on the riparian vegetation would most likely impact negatively the survival of K. rupestris . Moreover, they are mostly visual predators, so any increase in turbidity, often associated with eutrophication or riparian vegetation loss, would affect them adversely.
Fishing
Kuhlia rupestris is fished in many countries for food supply but it is also a popular freshwater recreational sport fish. The impact of fishing is variable. For example, in Australia, it is not considered a significant threat because most fish are released alive ( Hutchison et al., 2009a). In small insular systems, where the species is consumed and appreciated for its flavour, it may be a real concern ( Lewis and Hogan, 1987; Boseto et al., 2007). The impact on these ecosystems is even more likely to become a problem given the naturally low carrying capacity of the small and short creeks, even if the habitat is perfectly preserved.
Fishing regulations for K. rupestris do not exist in most of its range, and when it exists, is not always respected. This is the case in Réunion Island (ER, Anamparéla, 2015) a phenomenon likely due to its recent implementation compared to historical use of traditional fishing technique by part of the fishermen population. In order to limit fishing mortality, while satisfying the fishers, we suggest: i) No-Kill practise established in a restricted reach of river and/or ii) creation of fishing reserve with annual banning of any fishing activity. Seasonal bans in specific areas may also be useful to protect important aspects of the life cycle like breeding and larval recruitment.
KNOWLEDGE GAPS
Regional and local connectivity
The study by Feutry et al. (2012a) was a first step towards understanding the connectivity in K. rupestris , but lots of it still remains unclear. For management purposes, it would be very useful to know if population structure occurs at a finer scale than the one identified by these authors. Indeed, a better understanding of larval dispersal and recruitment scheme (external or self-recruitment) in freshwater are one of the fundamental components to provide appropriate management plans at regional and local scales. For example, in areas like Queensland and Réunion Island, where the conservation of the species is a concern, it would be interesting to know if the population mainly relies on self-recruitment or if surrounding islands also contribute to recruitment and in which proportion. The same question also applies at an even finer scale, river drainages potentially having some degree of independence from one another within the same island. The knowledge of fine scale genetic diversity is also important if breeding programs are to be used for restocking ( Hoskin et al., 2015). The use of thousands of genetic markers now easily available ( Davey et al., 2011) and close-kin analyses have the potential to fill this knowledge gap ( Planes et al., 2009; Grewe et al., 2015; Feutry et al., 2017). The genetic data collected for these analyses could also be used to provide estimates of population size and biological parameters such as mortality and breeding success ( Bravington et al., 2016).
Reproduction, recruitment and river migrations
Size at sexual maturity is an important parameter to gather as it is often used to define a legal size for fishing. Previous studies suggest regional variability exists in this species and therefore, local studies should be undertaken if this parameter is judged important to ensure population sustainability.
Another clear knowledge gap for K. rupestris is the location of breeding and spawning grounds. It is suspected that K. rupestris spawn at sea or in the estuary plume, but this has never been observed. Despite some indications of the moon phase playing a role, exact timing also remains quite unclear. Gathering information on this critical part of the life cycle could help define temporary protected areas along the shoreline. This could be achieved thanks to acoustic, radio or RFID telemetry studies. Outcomes from telemetry could then inform targeted fishing/netting effort to recover actively spawning fish and possibly eggs and larvae.
Telemetry could also provide information about upstream and downstream fish movements, another uncertain aspect of K. rupestris biology. This includes its ability to overcome obstacles, its behaviour during extreme events such as floods or draughts, but also water flow requirements to complete its life cycle. The importance of maintaining perennial flow at the sea/river interface has been highlighted by Marquet (ER, 2015), but if better knowledge of timing for spawning migrations or recruitment was available, it might be possible, for example, to accommodate water requirements for hydroelectric power stations while having minimal impact on the species survival. Overall, the accessibility between growth and breeding habitats is one of the key parameters for diadromous population conservation. For Kuhliidae , estuaries are key habitats both as corridors between freshwater and marine habitats and as possible spawning areas. Knowledge about factors affecting the ecological status of estuaries should be improved and monitored on an interannual basis.
Another reason to study fish movements would be to test whether adults are capable of migrating from one river to another. Such capability would certainly improve survival to rapid and extreme changes in insular freshwater environments ( Feutry, 2011). Additional data on the timing of fish migration could also inform the management of human activities, such as fishing in estuaries.
Finally, except for its duration, very little is known about the pelagic larval phase. Information on larvae behaviour in the water column could help refine larval modelling approaches such as the one used by Feutry et al. (2012a) in the Coral Sea. A better understanding of the cues leading the larvae towards estuaries would also certainly help taking management decisions.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.