Liphistius endau Sedgwick & Platnick, 1987
|
publication ID |
https://doi.org/10.5281/zenodo.893555 |
|
DOI |
https://doi.org/10.5281/zenodo.6042351 |
|
persistent identifier |
https://treatment.plazi.org/id/4C30A452-FFEE-FFF9-B919-FEF0392BF8DD |
|
treatment provided by |
Plazi (2017-10-18 21:12:42, last updated 2024-11-26 07:45:54) |
|
scientific name |
Liphistius endau Sedgwick & Platnick, 1987 |
| status |
|
Liphistius endau Sedgwick & Platnick, 1987 View in CoL
Figs 2A View Fig. 2 , 6-7 View Fig. 6 View Fig. 7
Liphistius endau Sedgwick & Platnick, 1987: 361 View in CoL -363 (description of female). – Foelix & Erb, 2010 (study on venom gland openings on cheliceral fangs). – Foelix et al., 2010 (study on scopula hairs of male).
Type material: AMNH; juvenile female holotype (not examined; see paragraph “ Variation ” below); Malaysia, Johore [sic], Ulu Endau area, from the banks of Sungai Jasin ; 10.XI.1985; leg. W.C. Sedgwick.
Material examined: MHNG, sample SIM-01/10; 2 males (matured 1.II.2002, 27.II.2003), 2 females (moulted 27.IV.2002, 28.IV.2002), 2 juvenile males, 3 juvenile females; Johor, Endau-Rompin National Park, between Kuala Jasin and Kuala Marong (2°31’44’’N, 103°22’02’’E), 40 m (rain forest along stream); 3.-5. VII.2001 GoogleMaps ; leg. P.J. Schwendinger. – MHNG; left palp of mature male; Johor, near Gunung Belumut ; leg. C. Sainsbury, don. R. Foelix. – MHNG (sample Sum-00/02); 2 females; Johor, Gunung Muntahak, Kota Tinggi Waterfalls (1°49’51”N, 103°49’56”E), 170 m (rainforest near stream); 5.II.2000 GoogleMaps ; leg. P.J. Schwendinger. – MHNG (sample SIM-01/07); 2 penultimate males, 4 females, 1 juvenile; same locality, 170 m; 24.-26.VI.2001 GoogleMaps ; leg. P.J. Schwendinger. – MHNG (sample MAL-04/05); 2 penultimate males, 2 females, 1 juvenile; same locality, 170 m; 26.-27.V.2004 GoogleMaps ; leg. P.J. Schwendinger. – MHNG (sample TM-14/02); 3 males (matured 20.VIII., 22.X., 6.XI.2014), 2 females; same locality, 120 m; 21.-22.VI.2014 GoogleMaps ; leg. P.J. Schwendinger. – MHNG, SMF; 2 males (one killed 30. IX.2010, the other died 22.III.2012), 1 female; Malaysia, locality and collector unknown; don J. Kral.
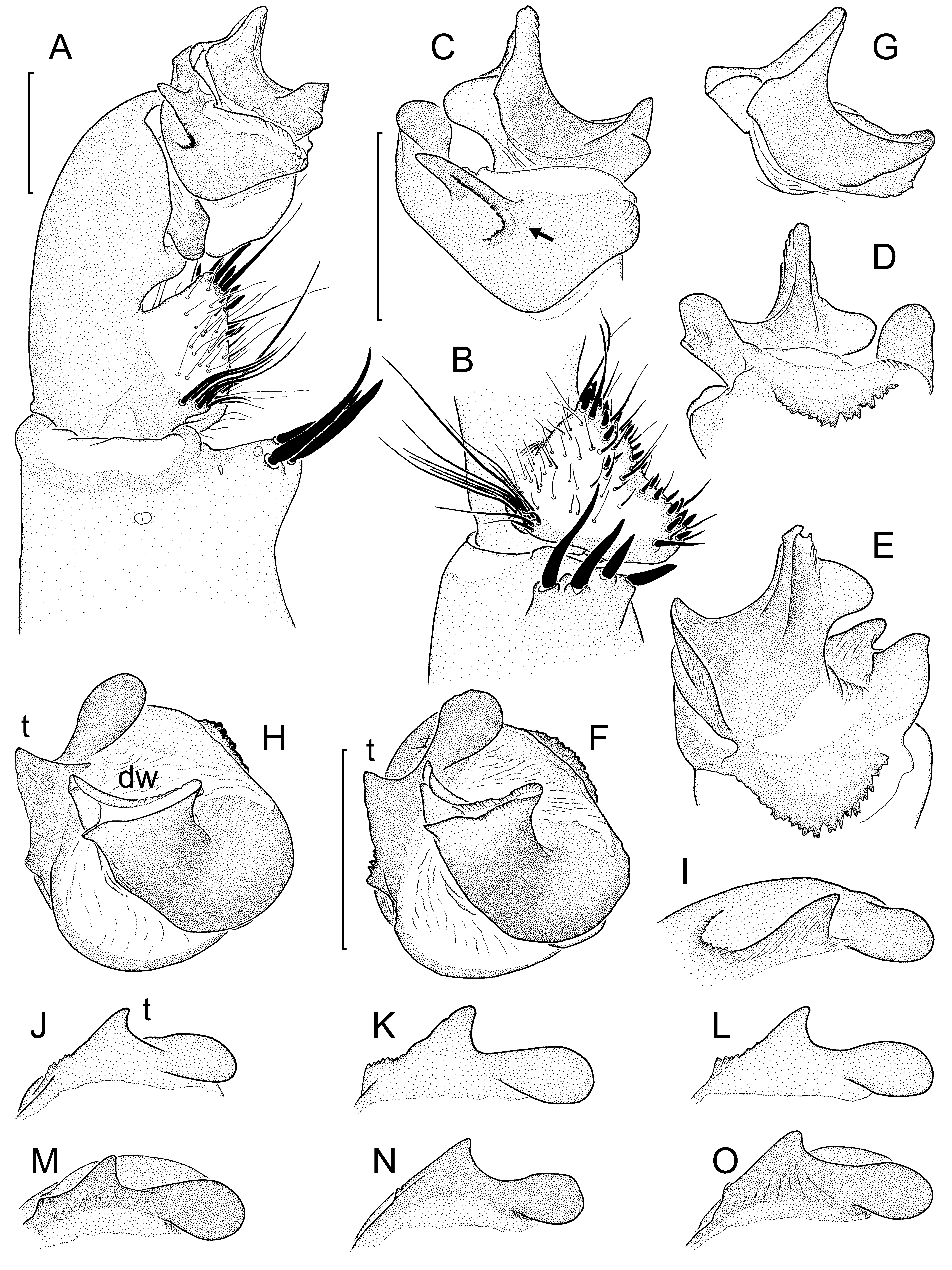
Diagnosis: Large, dark-coloured species. Males distinguished from those of the similar L. malayanus by tibial apophysis of male palp situated more distally ( Fig. 6A View Fig. 6 cf. Fig. 4B View Fig. 4 ); paracymbium much deeper ( Fig. 6B View Fig. 6 cf. Fig. 4A View Fig. 4 ); tegulum with more teeth on proximal margin ( Fig. 6 View Fig. 6 D-E cf. Fig. 4 View Fig. 4 D-F); distal edge of contrategulum with only one very large, triangular tooth prolaterally; dorsal apex of contrategulum more symmetrical, both lateral margins almost equally arched ( Fig. 6F View Fig. 6 , H-O cf. Fig. 4 View Fig. 4 G-L); dorsal wall of sclerotized part of embolus proper much wider than ventral wall and curved prodorsad ( Fig. 6F, H View Fig. 6 cf. Fig. 4 View Fig. 4 G-H), with a large lobate extension prodorsally ( Fig. 6 View Fig. 6 C-E cf. Fig. 4 View Fig. 4 D-F); adpressed membranous part of embolus proper distally much wider than proximally ( Fig. 6C View Fig. 6 cf. Fig. 4C View Fig. 4 ); opening of spermophore a long and narrow slot ( Fig. 6 View Fig. 6 F-H cf. Fig. 4 View Fig. 4 G-H). Females distinguished from those of L. malayanus by median portion of genital atrium clearly sunk below lateral portions and usually carrying more hairs (mostly no hairs in L. malayanus ); posterior stalk usually (except in small females) fused with pigmented lateral patches in genital atrium ( Fig. 7A View Fig. 7 , C-D, F, H-I cf. Fig. 5A View Fig. 5 , C-I); receptacular cluster not or only slightly protruding beyond anterior margin of poreplate, divided into three more or less distinct subclusters ( Fig. 7B, E, G View Fig. 7 cf. Fig. 5B, J View Fig. 5 ).
Description of male (matured 27.II.2003): Colour in alcohol (much darker in life; colouration as in female, Fig. 2A View Fig. 2 ): All sclerotised parts uniformly brown, except for cream-coloured proximal portion of chelicerae, cream-coloured membranes of prosoma and light brown opisthosomal membranes.
Bristles on carapace: Short bristles along all margins (strongest on posterior margin, longest behind, on and in front of eye mound); none on coxal elevations; five short bristles anterior to fovea.
Scopula: Tarsus I with thin scopula in distal half of ventral side, divided for its entire length by narrow, pale, glabrous longitudinal median stripe and by some bristles; tarsus II with slightly denser scopula in distal three-quarters, only distally divided by median stripe; tarsi III-IV with dense scopula covering distal four-fifths, only distally divided by median stripe.
Cheliceral teeth: Eleven small ones on promargin of cheliceral groove on both sides.
Palp: Tibial apophysis situated distally, not clearly set back from distal margin of tibia, carrying four moderately long (dorsal ones shorter than ventral ones) megaspines ( Fig. 6 View Fig. 6 A-B). Both apical lobes of cymbium very short and equally rounded. Paracymbium basally very deep ( Fig. 6B View Fig. 6 ), its cumulus only slightly elevated, carrying stiff bristles reaching base of contrategulum (looking shorter in Fig. 6A View Fig. 6 because pointing ventrad rather than distad). Subtegulum without apophysis. Tegulum short and wide, coarsely dentate along entire proximal margin ( Fig. 6 View Fig. 6 D-E). Contrategulum with indistinct, widely arched proventral process ( Fig. 6F View Fig. 6 ); distal edge with denticles at proventral end distinctly elevated on a U-shaped ridge ( Fig. 6A, C View Fig. 6 ), with a single large triangular tooth prolaterally and with spatulate, quite symmetrical dorsal apex ( Fig. 6F View Fig. 6 ). Para-embolic plate only little elevated ( Fig. 6A View Fig. 6 , C-E); sclerotised part of embolus proper strongly compressed dorso-ventrally, its dorsal wall distinctly wider than its ventral wall, curved prodorsad ( Fig. 6F View Fig. 6 ) and ending in a pronounced, prodorsad-directed, rounded lobe ( Fig. 6 View Fig. 6 C- E); membranous part of embolus proper distally much wider than proximally ( Fig. 6C View Fig. 6 ), its proximal portion slightly pigmented.
Measurements: Total length 22.30; carapace 9.26 long, 8.40 wide; opisthosoma 10.33 long, 7.62 wide; eye mound 1.34 long, 1.50 wide; palpal coxa 2.97 long, 1.98 wide; labium 0.69 long, 1.58 wide; sternum 4.06 long, 2.48 wide (1.29 on ventral surface); palp 15.83 long (4.43 + 2.79 + 5.74 + 2.87); leg I 27.21 long (7.21 + 3.61 + 5.90 + 7.05 + 3.44); leg II 28.11 long (7.21 + 3.61 + 6.15 + 7.62 + 3.52); leg III 30.99 long (7.54 + 3.77 + 6.56 + 9.02 + 4.10); leg IV 38.27 long (9.34 + 3.93 + 7.54 + 12.21 + 5.25).
Additions to description of female: Posterior margin of genital sternite more or less distinctly invaginated ( Fig. 7A View Fig. 7 , C-H; 7I is a very small juvenile). Vulval plate ( Fig. 7 View Fig. 7 ) strongly sclerotised and pigmented, roughly as long as wide, with a more or less distinct lateral constriction in posterior third. Genital atrium with many lateral hairs and in many cases also with additional median hairs; median zone of genital atrium clearly sunken below lateral zones (indistinct in small females). Posterior stalk wide, completely fused with poreplate and with strongly pigmented, buldging lateral parts of genital atrium ( Fig. 7A View Fig. 7 , C-H). Poreplate entirely and strongly pigmented, its anterior margin slightly invaginated, with a pair of pronounced anterolateral lobes. CDO large to very large, its posterior margin not sunken, giving the opening the shape of a horseshoe ( Fig. 7 View Fig. 7 C-D, F) or of an open quadrangle ( Fig. 7A, H View Fig. 7 ); enlarged pores inside CDO leading to receptacular cluster ( Fig. 7A View Fig. 7 , C-D, F); the latter large and complex but not or only slightly protruding beyond anterior margin of poreplate, divided into three more or less distinct subclusters ( Fig. 7B, E, G View Fig. 7 ).
Variation: Carapace lengths in males (n=7) 9.26-12.04, in the largest females (n=4) 13.33-14.94; carapace widths 8.09-10.99 and 10.74-12.84, respectively. The three males from Kota Tinggi have a shorter scopula on their anterior legs (I: very thin, medially divided, covering only distal quarter; II: slightly denser, only apically divided, covering distal half; III: dense, undivided, covering three-quarters; IV: dense, undivided, covering distal four-fifths) than males from the type locality and from an unknown locality (I: thin, divided, distal half; II: slightly denser, apically divided, distal tree-quarters; III-IV: dense, undivided, distal fourfifths).
Variation in the shape of the distal edge of the contrategulum is shown in Fig. 6F View Fig. 6 , H-O, variation in the shape of the vulval plate in Fig. 7 View Fig. 7 . With age (and body size) the number of hairs in the genital atrium of females increases.
All females from Kota Tinggi have fewer hairs in the genital atrium than females from other localities. Among six medium to large-sized females from that locality only one large female has two hairs in a median position ( Fig. 7C View Fig. 7 ), all others (including another large female) have none.
The female holotype (in AMNH, not examined) has a carapace length of 7.5 and a width of 6.6, and is thus only about half the size of the three largest females examined. The illustrations of the holotype vulva ( Sedgwick & Platnick, 1987: figs 1-2) differ from the vulvae examined by lacking distinct anterolateral lobes on the poreplate (also absent in the smallest juvenile female examined; Fig. 7I View Fig. 7 ). The posterior stalk of the holotype vulva is not yet fused with the pigmented lateral zones of the genital atrium (as it is also the case in the two juvenile females examined; Fig. 7 View Fig. 7 H-I) and it appears to lack lateral hairs (as it is the case in the smallest female examined; Fig. 7I View Fig. 7 ). The holotype is therefore a juvenile female with a not fully developed vulval plate. As (according to the original description) its vulval plate corresponds quite well with vulval plates of juveniles examined from the same area (possibly even from the same locality), and as no other Liphistius species is known from that area, there is no doubt that these specimens are conspecific.
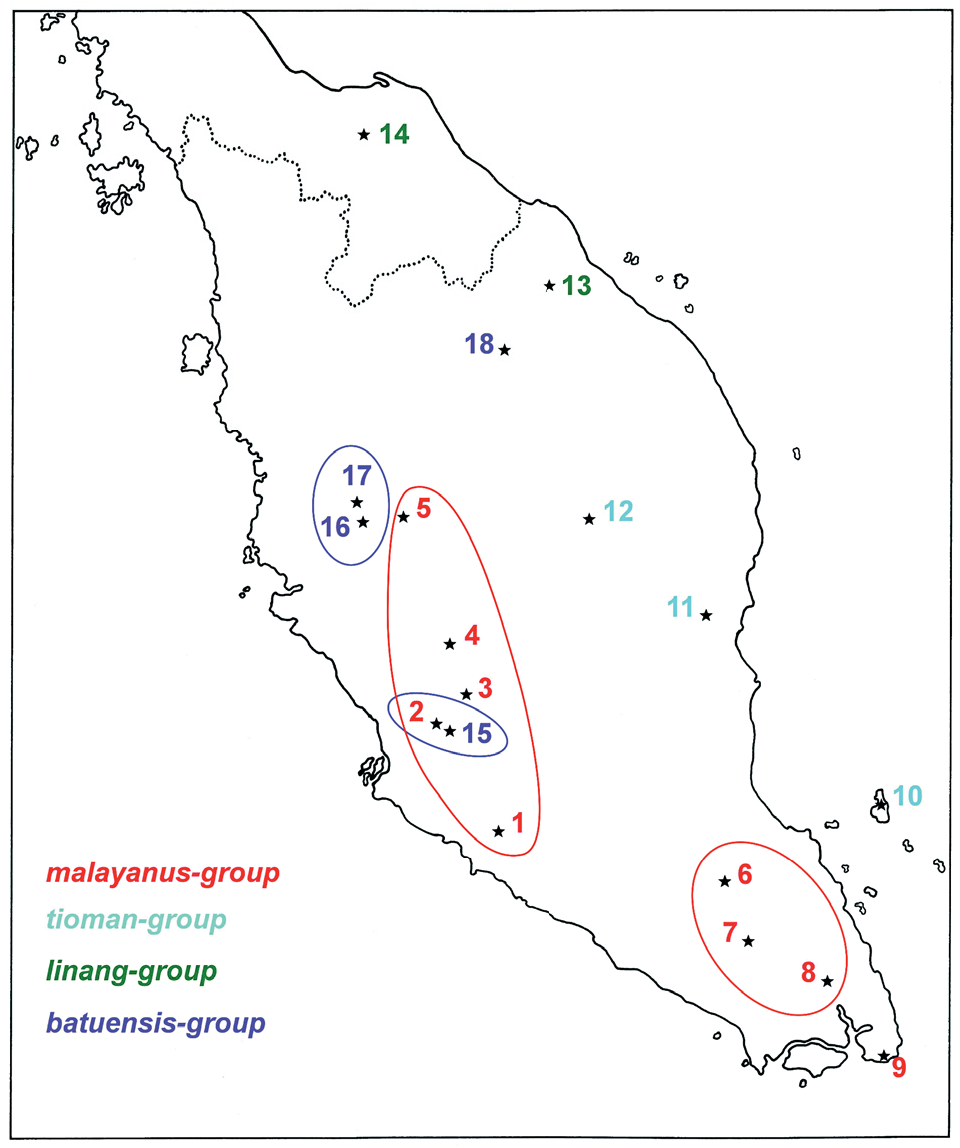
Distribution: Known from three localities in the northern and western part of Johor State ( Fig. 1 View Fig. 1 , localities 6-8).
Relationships: Large size, details of the male palp (e. g. shape of distal edge of contrategulum and its dorsal apex; shape of tibial apophysis) and details of the vulval plate (bulging lateral and posterolateral margins on ventral side of poreplate forming a distinct boundary between poreplate and posterior stalk) indicate that L. endau and L. malayanus are more closely related to each other than to L. johore and L. gracilis sp. nov.
Biology: The new specimens from the type locality or from very close to it (in the Endau-Rompin N.P.) were found on the banks of a stream in a rain forest; specimens from the Kota Tinggi Waterfall were collected from the sloping forest floor and soil banks on both sides of rain forest stream running over a series of falls. Most burrows were in the soil, but at the Kota Tinggi Waterfall two medium-sized burrows were constructed in the rotten wood of a fallen tree, with the signal lines spread over the wood surface. At the type locality the burrow entrance of a large female had nine signal threads, at the Kota Tinggi Waterfall four burrows (of three large females and one penultimate male) were equipped with nine signal lines; all other burrows had a maximum of eight lines running over rock, soil and tree roots. The longest signal line (of the largest female) was 34 m long, those of other burrows examined were not more than 21 cm long. Trapdoors of four penultimate males were 2.6-3.2 cm long and 4.0- 4.7 cm wide; that of the largest female 3.3 and 5.9, respectively. The latter spider lived in a 35 cm long burrow. Males became mature in August, October and November (after 2-5 months in captivity) and in February (after one and a half years in captivity, therefore probably not corresponding to conditions in nature). One male (from the Kota Tinggi Waterfall) ate a half-dead cricket a few days after its last moult but not again later. I also observed this in newly matured males of L. dangrek Schwendinger, 1996 , and it had been reported for males of L. desultor by Murphy & Platnick (1981: 46), but it occurs only rarely and probably only in large species. Usually Liphistius males cease feeding when becoming adult.
Egg cases were constructed (all of them in captivity or during transport) in June and July. One egg case built in Geneva by a female from the type locality was 5.0 cm long, 4.7 cm wide, 2.8 cm high and contained 453 eggs. Another female from the same locality built during the transport an egg case of tissue paper, with a 3 cm diameter, containing 331 eggs. A third, old and empty egg case at the Kota Tinggi Waterfall was 4.0 cm long, 54.6 cm wide, 1.8 cm high. A fourth female from the same locality built a tissue paper egg case of 4.2 cm diameter and 2.0 cm height, containing 395 light orangecoloured eggs.
Foelix R., Erb B. 2010. Mesothelae have venom glands. The Journal of Arachnology 38: 596 - 598.
Murphy J. A., Platnick N. I. 1981. On Liphistius desultor Schiodte (Araneae, Liphistiidae). Bulletin of the American Museum of Natural History 170: 46 - 56.
Schwendinger P. J. 1996. New Liphistius species (Araneae, Mesothelae) from western and eastern Thailand. Zoologica Scripta 25 (2): 123 - 141.
Sedgwick W. C., Platnick N. I. 1987. A new species of Liphistius (Araneae, Mesothelae) from Johore, Malaysia. Malayan Nature Journal 41: 361 - 363.
Fig. 1. Localities of Liphistius species of the malayanus - group, tioman - group, linang - group and batuenis - group in peninsular Malaysia and southern Thailand (coast of Sumatra omitted): 1 - Gunung Angsi (type locality of L. malayanus); 2 - Templer Park and Gua Anak Takun (L. malayanus, L. batuensis); 3 - Genting Highlands (L. malayanus); 4 - Fraser’s Hill (L. malayanus); 5 - Cameron Highlands (type locality of L. malayanus cameroni); 6 - Sungai Jasin in Endau Rompin National Park (type locality of L. endau); 7 - Gunung Belumut (L. endau); 8 - Gunung Muntahak (L. endau; type locality of L. gracilis sp. nov.); 9 - Sungai Rengit (type locality of L. johore); 10 - Gunung Kajang on Tioman Island (type locality of L. tioman); 11 - Gua Charas in Bukit Charas (type locality of L. panching); 12 - Nusa Camp in Taman Negara (type locality of L. negara sp. nov.); 13 - Jeram Linang Waterfall (type locality of L. linang sp. nov.); 14 - Sankalakhierie Mountains (type locality of L. indra); 15 - Batu Caves (type locality of L. batuensis); 16 - Gua Tempurung (type locality of L. tempurung); 17 - Gua Cicak (L. tempurung); 18 - Gua Keris (type locality of L. priceae sp. nov). Localities with conspecific populations are encircled. Colours distinguish species groups.
Fig. 2. Habitus of two Liphistius species from peninsular Malaysia and southern Thailand. (A) Liphistius endau, female from Kota Tinggi (Malaysia), dorsal view. (B) Liphistius indra sp. nov., male paratype from the Sankalakhierie Mountains (Thailand), same view.
Fig. 4. Liphistius malayanus, details of palp of four males: Templer Park (A, C-D, H-I); holotype of L. malayanus cameroni (B, E, G); Cameron Highlands, ZRC (F, J), Fraser’s Hill, MHNG (K-L). (A) Paracymbium and tibial apophysis of left palp, retroventral view. (B) Tibial apophysis of right palp, ventral view. (C) Distal part of left palpal organ, proventral view (arrow indicating V-shaped row of denticles at proventral end of distal edge of contrategulum). (D, F) Same, retrodorsal and slightly proximal view. (E) Same, retrodorsal view. (G-H) Left palpal organ, distal view (dorsal side up). (I, K) Distal edge of contrategulum of right palp, distal view (dorsal side to the left). (J) Same, distal and slighly prolateral view. (L) Same of left palp, distal and slighly prolateral view (dorsal side to the right). Abbreviations: a - dorsal apex of contrategulum; de - distal edge of contrategulum. Scale lines: 1.0 mm (A; B; C-D; E, G; F; H-L).
Fig. 5. Liphistius malayanus, vulval plate of eight females: paratype of L. m. cameroni from Berinchang, Cameron Highlands (A- B); exuvia of female from Ringlet, Cameron Highlands (C); female (SMF 64093) from Fraser’s Hill (D); female (moulted 24. VI. 2002) from Fraser’s Hill; poreplate slightly deformed by brief immersion in cold KOH (E); female (SMF 56206) from Fraser’s Hill (F); female (SMF 60037) from Genting Highlands (G); female (moulted 8. XII. 2005) from Genting Highlands (H); exuvia of female from Templer Park (I-J). (A, C-I) Dorsal view. (B, J) Ventral view. Scale lines: 1.0 mm (A-B; C, F; D; E, G; H; I-J).
Fig. 6. Liphistius endau, details of left palp of eight males: from type locality, matured 27. II. 2002 (A-F); from type locality, matured 1. II. 2002 (G-I); from unknown locality, died 22. III. 2012 (J); from unknown locality, killed 30. IX. 2010 (K); from Gunung Belumut (L); from Kota Tinggi, matured 22. X. 2014 (M); same place, matured 20. VIII. 2014 (N); same place, matured 6. XI. 2014 (O). (A) Distal part of palp, ventral view. (B) Paracymbium and tibial apophysis, retroventral view. (C) Palpal organ, proventral view (arrow indicating U-shaped row of denticles at proventral end of distal edge of contrategulum). (D) Same, retrodorsal and slightly proximal view. (E) Same, retrodorsal view. (F, H) Same, distal view (dorsal side up). (G) Embolus complex, proventral and slightly distal view. (I) Distal edge of contrategulum, distal and slighly prolateral view (dorsal side to the right). (J-O) Same, distal view. Abbreviations: dw - dorsal wall of sclerotized part of embolus proper; t - tooth on distal edge of contrategulum. Scale lines: 1.0 mm (A-B; C-E, G-H; F, I-O).
Fig. 7. Liphistius endau, vulval plate of six females: adult from unknown locality (A-B); largest female from Kota Tinggi (C); exuvia of reproductive female (moulted 27. IV. 2002) from type locality (D-E); exuvia of reproductive female (moulted 28. IV. 2002) from type locality (F-G); medium-sized juvenile from type locality (H); small juvenile from type locality (I). (A, C-D, F, H-I) Entire vulval plate, dorsal view. (B) Poreplate, ventral view. (E, G) Entire vulval plate, ventral view. Scale lines: 1.0 mm (A-B; C; D-E; F-G; H-I).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Liphistius endau Sedgwick & Platnick, 1987
| Peter J. Schwendinger 2017 |
Liphistius endau
| Sedgwick W. C. & Platnick N. I. 1987: 361 |