Phyllium (Phyllium) hausleithneri Brock, 1999
|
publication ID |
https://doi.org/ 10.11646/zootaxa.2322.1.1 |
|
persistent identifier |
https://treatment.plazi.org/id/4C724261-6C4B-3A7C-FF39-FF733382C1DC |
|
treatment provided by |
Felipe |
|
scientific name |
Phyllium (Phyllium) hausleithneri Brock, 1999 |
| status |
|
Phyllium (Phyllium) hausleithneri Brock, 1999 View in CoL
( Figs. 45–49 View FIGURES 45–49 , 64–66 View FIGURES 52–67 , 69 View FIGURES 68–76 , 79 View FIGURES 77–84 , 92 View FIGURES 85–94 , 105–106, 118)
Phyllium hausleithneri Brock, 1999: 164 View in CoL , figs. 111a–d. HT, ♀: Holotype; Malaysia: Tapah Hills, Cameron Highlands, 1991, via Wong; Phyllium hausleithneri Brock 1999 HT View in CoL ♀; BMNH(E) #845233 (BMNH); PT, 2 ♀: Malaysia: Tapah Hills, Cameron Highlands, 1991, via Wong; Phyllium hausleithneri Brock 1999 PT View in CoL ♀ (coll. PB); PT, 2 ♀: Malaysia: Tapah Hills, Cameron Highlands, 1992, via Wong; Phyllium hausleithneri Brock 1999 PT View in CoL ♀ (coll. PB). Seow-Choen, 2000: 45, pl. 127 (♀). Grösser, 2001: 83, fig. 110. Zompro & Grösser, 2003: 136. Otte & Brock, 2005: 274. Seow-Choen, 2005: 112. Grösser, 2008: 121, fig. 144 (♀).
Phyllium (Phyllium) bilobatum, Grösser, 2008: 91 View in CoL (in part - only Malayan records).
Phyllium (Phyllium) siccifolium, Klante, 1976: 65 View in CoL (in part – only record from Selangor, Peninsular Malaysia). Ziegler, 1993: 18, figs. (♀ + coxae). Brock, 1995: 98. Brock, 1999: 158, fig. 106 (♀, egg), pl. 34 (♂). Seow-Choen, 2000: 44, pl. 122 (♀, ♂, egg). Grösser, 2001: 94, figs. 122,123 (♂, ♀). Zompro & Grösser, 2003: 136, figs. 8 (♂), 9 (♀). Otte & Brock, 2005: 274. Seow-Choen, 2005: 113. Grösser, 2008: 134 (in part - only Malayan records, e.g. fig. 160 (♂, ♀ )).
Phyllium woodi?, Klante, 1976: 67 View in CoL , figs. 11–12 (in part – only records from Peninsular Malaysia & Singapore)
[Not: Phyllium (Phyllium) siccifolium, Bragg, 2001: 198 View in CoL , figs 64 a–d – Bornean records are a distinct species]
Material examined [15 ♀, 6 ♂♂, 6 eggs]: PENINSULAR MALAYSIA: 3 ♀, 2 ♂♂, 6 eggs: West Malaysia, Perak, Tapah Hills 500–1200 m, leg. M.K.P. Yeh VIII.1993, “ Ph. (Ph.) sicifolium ” (coll. FH, No’s 0075-1, 2, 4, 5, 8 & E) ; 3 ♀: West Malaysia, Perak, Tapah Hills 500–1200 m, leg. Wong T.F. XI.1993, “ Ph. (Ph.) sicifolium ” (coll. FH, No’s 0075-3, 6 & 7) ; 1 ♀: West Malaysia , Cameron Highlands, 2.2003, “ Ph. (Ph.) sicifolium ” (coll. OC) ; 4 ♀, 1 ♂: West Malaysia , Cameron Highlands, 5.2004, “ Ph. (Ph.) sicifolium ” (coll. OC) ; 1 ♂: West Malaysia , Cameron Highlands, 5.2000, “ Ph. (Ph.) sicifolium ” (coll. OC) ; 2 ♀: West Malaysia, Perak, Tapah Hills 500–1200 m, leg. M.K.P. Yeh VIII.1993; Ph. (Ph.) hausleithneri Brock, 1999 (coll. FH, No’s 0077-1 & 2) ; 2 ♀: West Malaysia , Cameron Highlands, 4.2004, Ph. (Ph.) hausleithneri Brock, 1999 (coll. OC) ; 2 ♂♂: reared by M. Gottardo 2008; origin: Malaysia, Pahang, Cameron Highlands, Tanah Rata (coll. MG) .
Differentiation: This species shows remarkable variation concerning the shape of the abdomen in ♀ but is well characterized and distinguished from closely related species by the blue to purplish interior markings of the meso- and metacoxae. While slender ♀ resemble Ph. siccifolium Linné, 1758 and have previously been confused with this species, lobed specimens rather resemble the Javanese Ph. jacobsoni Rehn & Rehn, 1933 or the Philippine Ph. bilobatum Gray, 1843 and Ph. philippinicum n. sp.. Close relation to Ph. jacobsoni and Ph. bilobatum in particular is obvious, the first species perhaps being the adelphotaxon of Ph. hausleithneri .
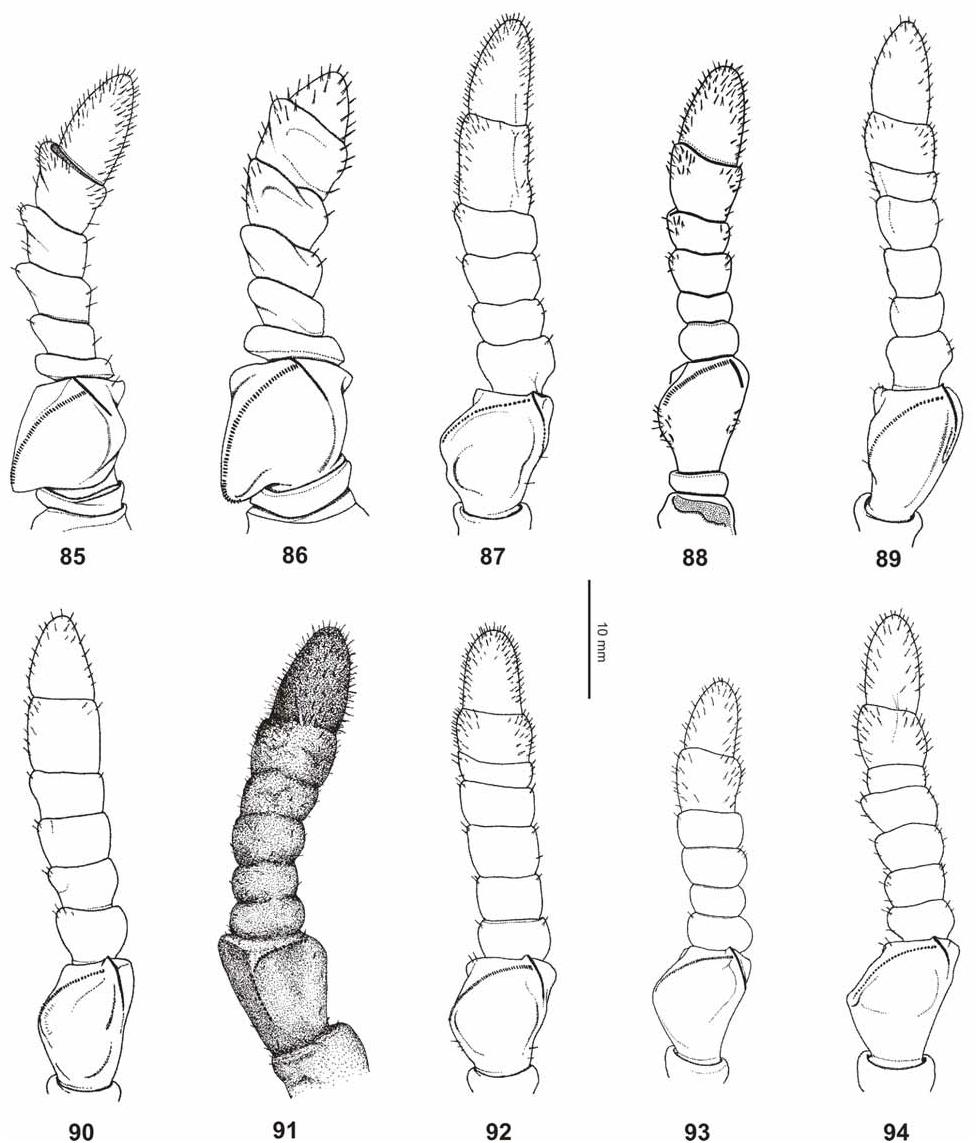
It is at once distinguished from the virtually very similar Ph. jacobsoni from Java by the larger size of both sexes. ♀ furthermore differ by: the less granulose vertex; differently structured antennae which consist of ten segments (nine in jacobsoni , Fig. 92 View FIGURES 85–94 ); only 44–48 teeth on the pars stridens of antennomere III (40 in jacobsoni ); less distinct teeth of the interior lobe of the profemora ( Figs. 64–65 View FIGURES 52–67 ) and relatively smaller cerci. ♂♂ also differ by: the shorter profemora which are only about 2x longer than the head (2.3x longer in jacobsoni ); slightly broader exterior extension and broader interior lobe of the profemora ( Fig. 66 View FIGURES 52–67 ); broader mesofemora; considerably shorter and less slender protarsi and slightly shorter antennae. The eggs differ from those of Ph. jacobsoni by the larger dimensions and relatively longer and larger micropylar plate (Figs. 105– 106).
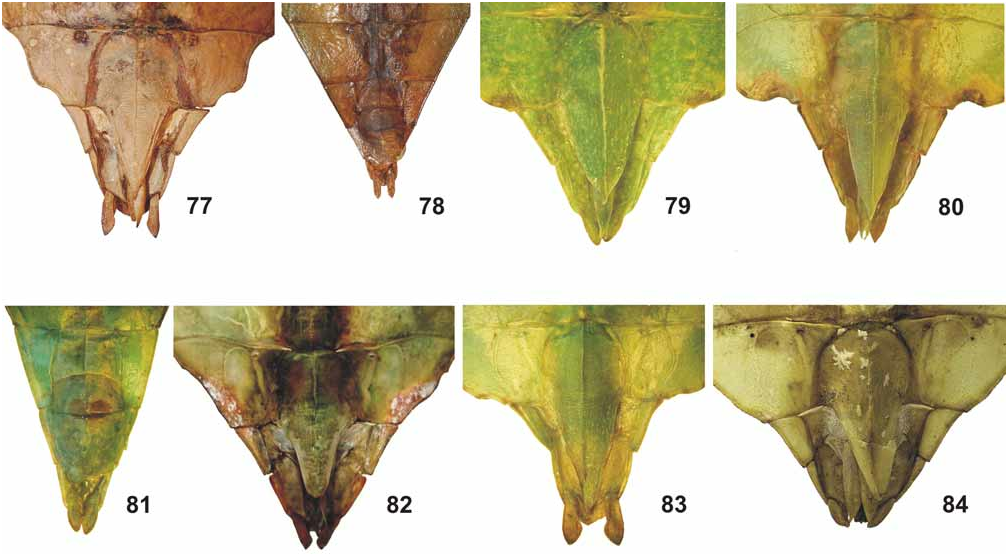
77. Ph. (Ph.) gantungense n. sp., ♀ HT [ MSNG]
78. Ph. (Ph.) gantungense n. sp., ♂ PT [ MSNG]
79. Ph. (Ph.) hausleithneri Brock, 1999 , ♀ [coll. FH]
80. Ph. (Ph.) philippinicum n. sp., ♀ PT [coll. FH]
81. Ph. (Ph.) mindorense n. sp., ♂ HT [ ZSMC]
82. Ph. (Ph.) mabantai n. sp., ♀ PT [coll. JB]
83. Ph. (Ph.) jacobsoni Rehn & Rehn, 1933 , ♀ [coll. FH, No. 0637-11]
84. Ph. (Ph.) siccifolium (Linné, 1758) , ♀ HT [ UUZM] © UUZM
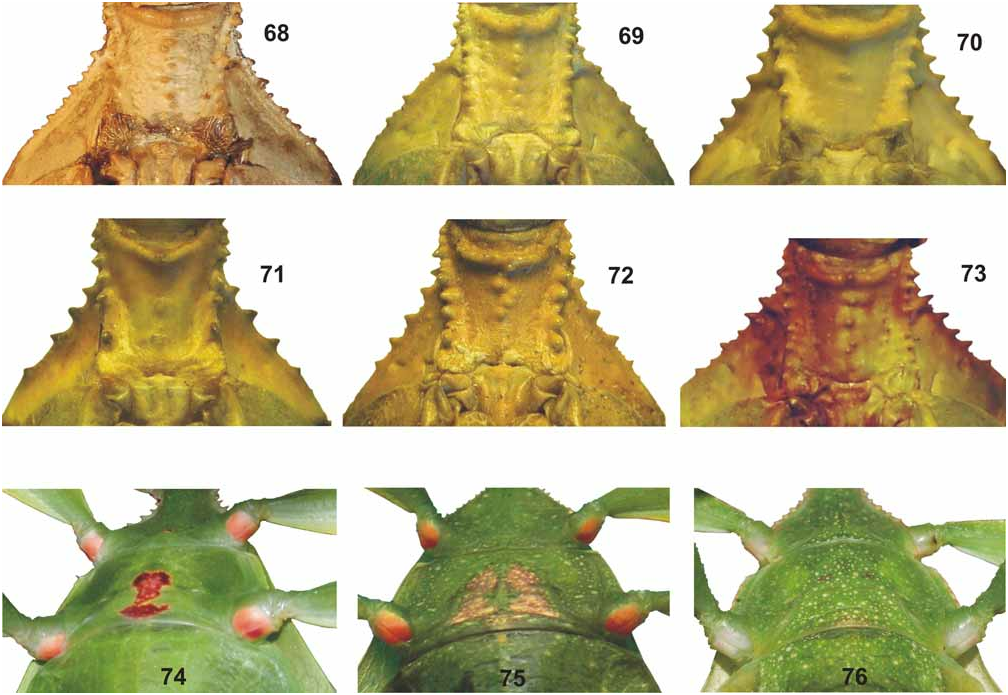
From the Philippine Ph. bilobatum ♀ differ by: the larger size; less acute and less distinct teeth of the interior lobe of the profemora ( Figs. 64–65 View FIGURES 52–67 ); more decidedly tectiform meso-praescutum which has the longitudinal median row of tubercles much more distinct; much smaller tubercles along the lateral margins of the mesopleurae ( Fig. 69 View FIGURES 68–76 ); less rounded lateral margins of the pronotum; more slender and less distinctly spinose meso- and metafemora and broader, more widely rounded lobes of abdominal segments VII and VIII (lobes much narrower and extending posteriorly in the HT of bilobatum ).
From Ph. philippinicum n. sp. it is furthermore distinguished by: the relatively shorter profemora, which are hardly 2x longer than the head; distinct longitudinal median row of tubercles on the meso-praescutum; lack of distinct spiniform tubercles along the lateral margins of the mesopleurae as well as larger, less numerous and irregularly sized teeth of the interior lobe of the profemora ( Figs. 64–65 View FIGURES 52–67 ) of both sexes. ♀ also differ by: the shorter and more robust antennae; relatively shorter antennomere IX; more numerous teeth on the pars stridens of antennomere III (44–48 compared to 40 in philippinicum , Fig. 92 View FIGURES 85–94 ); more distinctly granulose vertex; considerably shorter probasitarsus and shorter much broader subgenital plate ( Fig. 79 View FIGURES 77–84 ), and ♂♂ may also be distinguished by the much shorter antennae, which merely reach about 1/3 the way along abdominal segment III (23–24 antennomeres), and apically narrowed tegmina. The eggs clearly differ from those of Ph. philippinicum n. sp. by the much longer micropylar plate, presence of hairy structures on the ventral surface and shorter hairy structures on the later surfaces of the capsule (Figs. 105–106).
From the Moluccan Ph. siccifolium ♀ clearly differ by: the distinctly tectiform meso-praescutum which has the longitudinal median row of tubercles much more prominent; smaller tubercles along the lateral margins of the mesopleurae ( Fig. 69 View FIGURES 68–76 ); broader teeth of the interior lobe of the profemora ( Figs. 64–65 View FIGURES 52–67 ); longer subgenital plate ( Fig. 79 View FIGURES 77–84 ) and much greater number of teeth on the pars stridens of antennomere III (44–48 compared to 30–32 in siccifolium , Fig. 92 View FIGURES 85–94 ). The eggs differ considerably from the very distinctive eggs of Ph. siccifolium by being angular with longitudinal marginal rows of feather-like appendages on the capsule (Figs. 105–106).
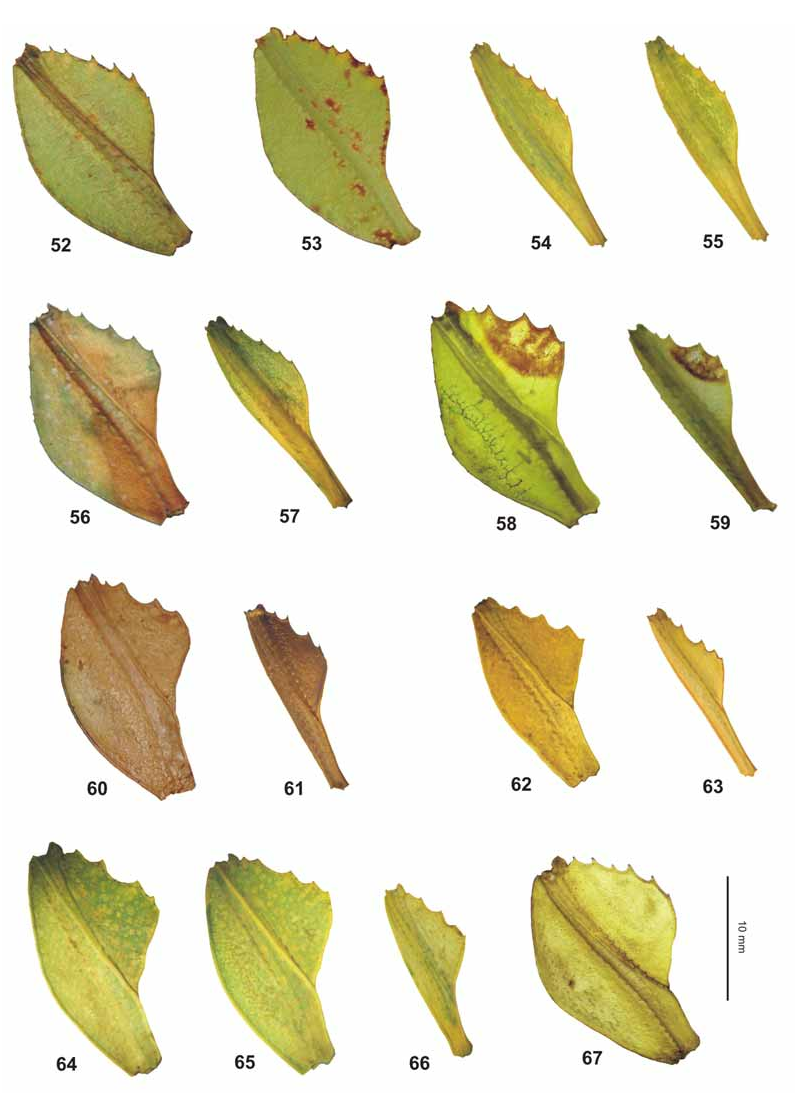
Variation: Like the closely related Ph. jacobsoni Rehn & Rehn, 1993 this species shows remarkable variation concerning to the shape of the abdomen and colouration of ♀. While slender specimens are mostly plain green, lobed ones may be prettily mottled all over with brown. The anterior angles of the mesopleurae are often marked by a white marking, which usually is more distinct in lobed specimens. The irregular brown central marking of the tegmina is generally also more decidedly developed in lobed specimens and rarely seen in slender ♀. The abdomen ranges from being gradually tapered towards the apex from segment V onwards ( Fig. 48 View FIGURES 45–49 ) or in the other extreme may have segments VII and VIII each with a prominent, rounded lobe and VI roundly angled posteriorly ( Fig. 45 View FIGURES 45–49 ). Intermediate forms have these segment just weakly lobed or rounded ( Figs. 46–47 View FIGURES 45–49 ). No such variation is seen in ♂♂ ( Fig. 49 View FIGURES 45–49 ).
Comments: Ph. hausleithneri was described upon five ♀ from the Tapah Hills, Peninsular Malaysia. Brock (1999: 164) distinguished it from the Philippine Ph. bilobatum Gray, 1843 by the larger size, different armature of the interior lobe of the profemora, distinct longitudinal median row of tubercles on the mesopraescutum, larger and more rounded lobes of abdominal segments VII and VIII and lack of conspicuous teeth along the lateral margins of the mesopleurae. Grösser (2008: 121) stated Ph. hausleithneri to be very close to Malayan specimens which he erroneously referred to as either “ Ph. bilobatum ” or “ Phyllium siccifolium ” (see above) but did not recognize the true identity and morphological range of Brock’s species. While ♀ originating from the Tapah Hills are frequently encountered and sold as dead-stock by insect-suppliers in Peninsular Malaysia, the ♂ and egg of Ph. hausleithneri have as yet remained unrecognized. However, specimens currently referred to as “ Ph. siccifolium ” or “ Ph. bilobatum ” (see e.g. Brock, 1999; Seow-Choen 2000; Grösser, 2008) are sympatric with Ph. hausleithneri and occur in the same localities (personal communication with Michael K. P. Yeh, Ipoh), a fact which arises surprise that no distinct ♂♂ or at least a different type of eggs have been observed yet.
The collections of the two first author’s (coll. FH, coll. OC) contain a good number of Malayan specimens. Based on the shape of the abdomen, the ♀ either key out as Ph. hausleithneri or “ Ph. bilobatum ” and “ Ph. siccifolium ” (sensu Grösser, 2008) , but some represent intermediate forms. All 15 ♀ at hand were carefully examined and fully agree in the structure of the antennae, shape and structure of the pronotum and mesothorax, shape and armature of the profemora ( Figs. 64–65 View FIGURES 52–67 ), shape of the subgenital plate and internal sclerite and all exhibit the same characteristic blue to purplish interior marking on the meso- and metacoxae. All of these features are remarkably constant and detailed comparison of slender and broadly lobed ♀ has, apart from the presence or absence of the lobes of abdominal segments VII and VIII, not revealed any differences that would distinguish between them on the species level. In view of the shape of the abdomen, several specimens are almost intermediate between both extremes having the lobes of abdominal segments VII and VIII just weakly or moderately developed ( Figs. 46–47 View FIGURES 45–49 ). Body lengths according to Seow-Choen (2000: 44): ♀ 77.0–92.0 mm, ♂♂ 53.0–67.0 mm.
Klante (1976: 67, fig. 12) recorded and figured a ♀ from Sumatra (Palembang) in RMNH, which he attributed to Ph. woodi Rehn & Rehn, 1933 with doubt. The specimen was not found in the RMNH collection but according to the figure presented and features discussed by Klante (1976: 68 ff) the specimen is rather unlikely to be conspecific with Ph. hausleithneri . It differs by the relatively longer and more slender pronotum and mesothorax as well as the distinctively angular interior lobe of the profemora, the latter strongly resembling the Javanese Ph. jacobsoni Rehn & Rehn, 1933 . But in addition to the same features, it differs from this species by the much larger size (body length 79.0 mm according to Klante, 1976: 70).
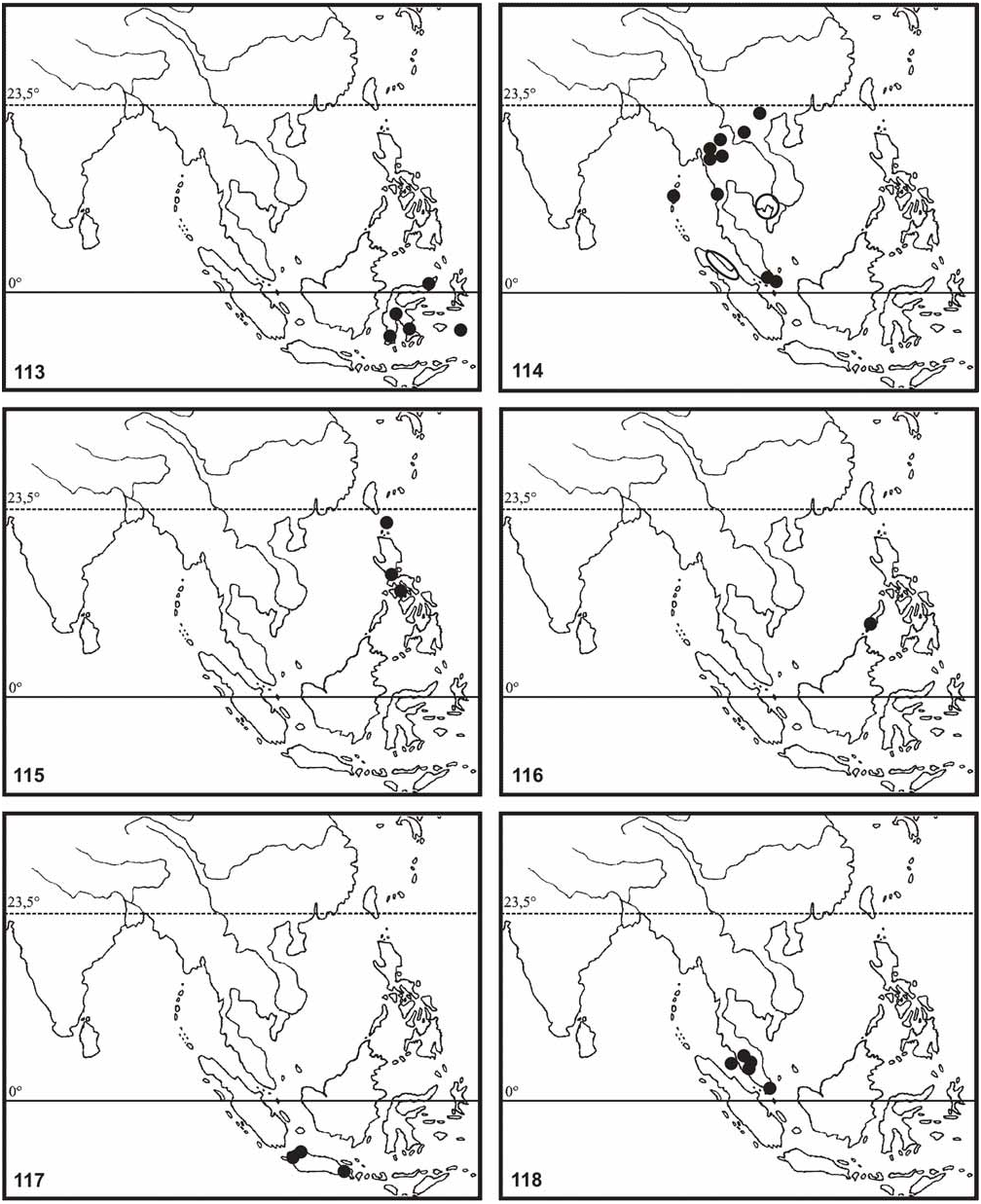
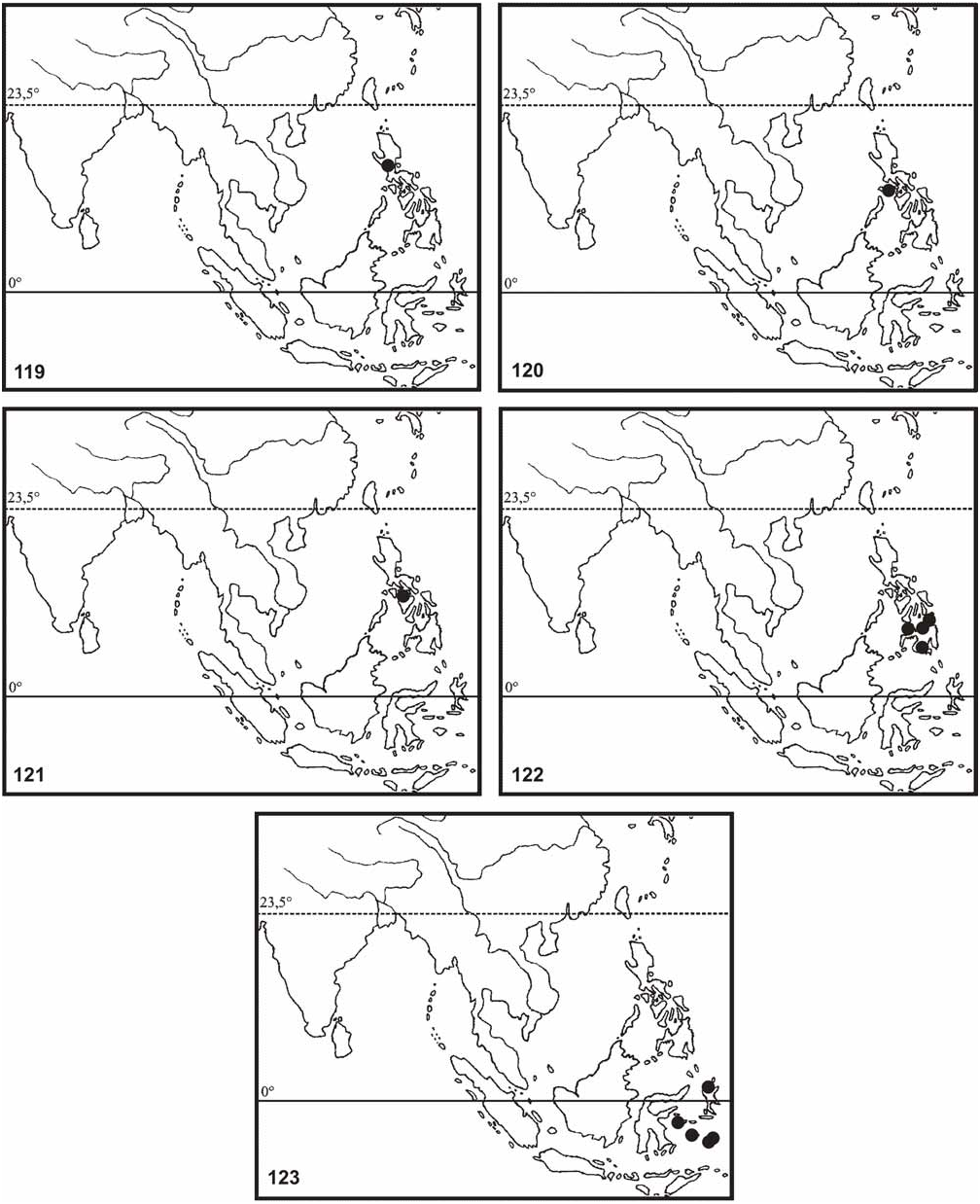
Redtenbacher (1906: 176) and Klante (1976: 65) recorded Ph. siccifolium from Borneo based on a nymph in MNHU and Bragg (2001: 198) recorded further specimens including adult insects of both sexes from Kuching ( Sarawak) and various localities throughout Sabah. These were mostly presumed to be conspecific with specimens from Peninsular Malaysia, which in fact are Ph. hausleithneri . Brief examination of the Bornean specimens in BMNH and MNHU however shows them to be distinct from either Ph. hausleithneri or the true Ph. siccifolium (Linné, 1758) here shown to be restricted to the Moluccas (see comments on this species below). ♂♂ from Sabah differ from those of Ph. hausleithneri by the generally smaller dimensions (body length 49.0–56.0 mm), much more prominently expanded abdominal segments III and IV, apically narrowed and pointed tegmina and different shape of the profemora. The large 77.0 mm ♂ from an unspecified locality traced in BMNH and recorded by Bragg (2001: 199, fig. 64b) appears to be specifically distinct yet again. However, any confirmed decisions on the identities of these Bornean specimens warrant more detailed investigation of the concerned insects and availability of additional material including ♀ and eggs from the same localities. All Bornean records are here regarded as erroneous and hence excluded from the distribution maps of both Ph. hausleithneri ( Fig. 118 View FIGURES 113–118 ) and Ph. siccifolium ( Fig. 123 View FIGURES 119–123 ).
Live-stock of Ph. hausleithneri was introduced to Europe as “ Ph. siccifolium ” on several occasions and exceptionally purchased from local insect-suppliers in the Cameron Highlands, Peninsular Malaysia. It was added to the Phasmid Study Group culture-list as PSG No. 76 but no culture was so far maintained for any longer than a few generations, this species appearing more problematic to successfully rear in captivity than other Phyllium -species. While known to feed on guava ( Psidium guajava , Myrtaceae ), mango ( Mangifera indica , Anarcadiaceae) or Nephilium lappaceum ( Sapindaceae ) in captivity in Malaysia or Singapore (Seow- Choen, 2000: 44), oak ( Quercus robur & Q. ilex , Fagaceae ) and sometimes bramble ( Rubus fruticosus , Rosaceae ) are accepted as alternative food-plants in Europe. An attempt to breed this species by the first author in 1993/1994 has failed due to the lack of availability of oak during the winter.
♂♂ spray a defensive secretion from their prothoracic exocrine glands. However, this appears almost gaseous since no noticeable liquid can be observed.
Distribution ( Fig. 118 View FIGURES 113–118 ): Peninsular Malaysia (Perak: Tapah Hills & Fraser’s Hill 4000 ft. [ Klante, 1976: 67, as Ph. woodi ]; Selangor: Bukit Kutu [ Brock, 1999: 159]; Pulau Penang [DEIC]) and Singapore (Bukit Timah Road [ Klante, 1976: 67, as Ph. woodi ]).
Southern Sumatra (Palembang [ Klante, 1976: 67, as Ph. woodi, RMNH ]) is doubtful and hence excluded from the distribution map ( Fig. 118 View FIGURES 113–118 ). Records from Borneo are most certainly based on misidentifications and deserve further evaluation.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Phyllium (Phyllium) hausleithneri Brock, 1999
| Hennemann, Frank H., Conle, Oskar V., Gottardo, Marco & Bresseel, Joachim 2009 |
Phyllium (Phyllium) bilobatum, Grösser, 2008: 91
| Grosser, D. 2008: 91 |
Phyllium hausleithneri
| Grosser, D. 2008: 121 |
| Otte, D. & Brock, P. 2005: 274 |
| Zompro, O. & Grosser, D. 2003: 136 |
| Grosser, D. 2001: 83 |
| Brock, P. D. 1999: 164 |
Phyllium (Phyllium) siccifolium, Klante, 1976: 65
| Grosser, D. 2008: 134 |
| Otte, D. & Brock, P. 2005: 274 |
| Zompro, O. & Grosser, D. 2003: 136 |
| Grosser, D. 2001: 94 |
| Brock, P. D. 1999: 158 |
| Brock, P. D. 1995: 98 |
| Ziegler, U. 1993: 18 |
| Klante, H. 1976: 65 |
Phyllium woodi?
| Klante, H. 1976: 67 |