Phyllium (Phyllium) siccifolium (Linné, 1758)
|
publication ID |
https://doi.org/ 10.11646/zootaxa.2322.1.1 |
|
persistent identifier |
https://treatment.plazi.org/id/4C724261-6C4E-3A71-FF39-FB1D3104C057 |
|
treatment provided by |
Felipe |
|
scientific name |
Phyllium (Phyllium) siccifolium (Linné, 1758) |
| status |
|
Phyllium (Phyllium) siccifolium (Linné, 1758) View in CoL
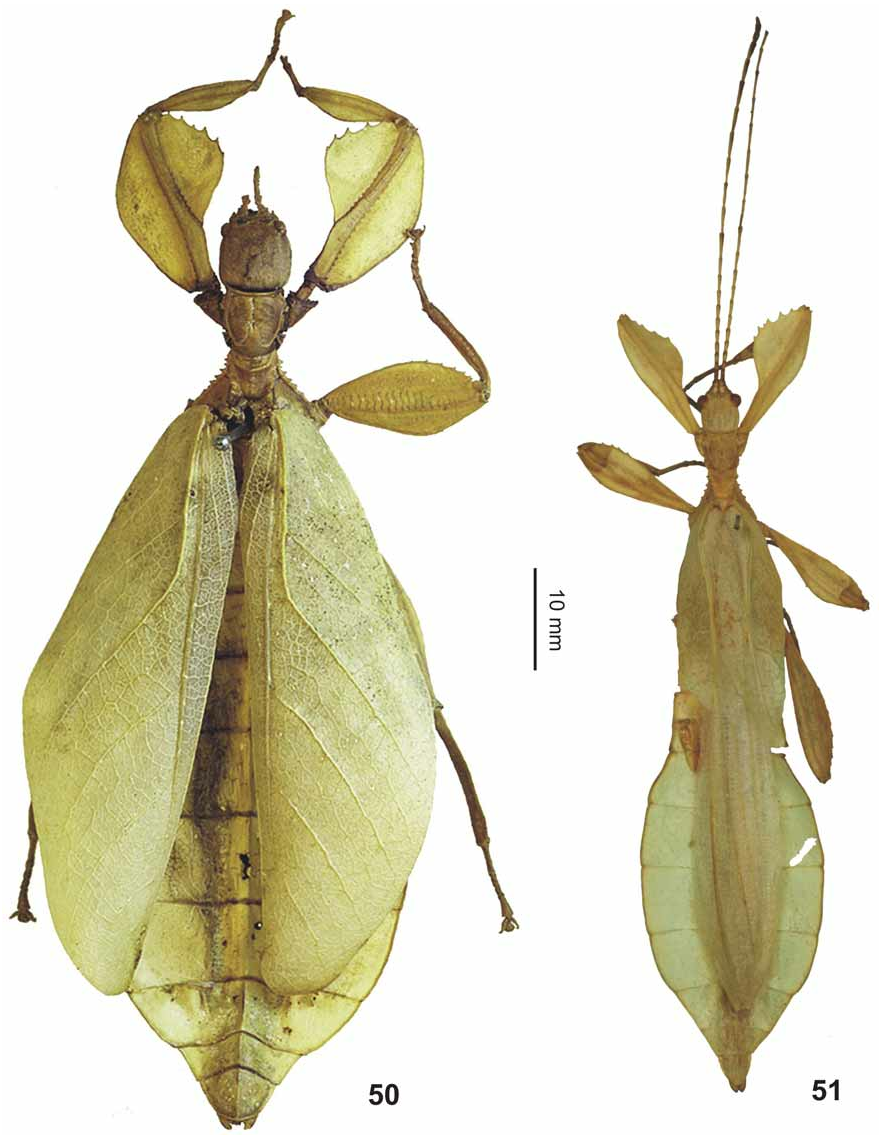
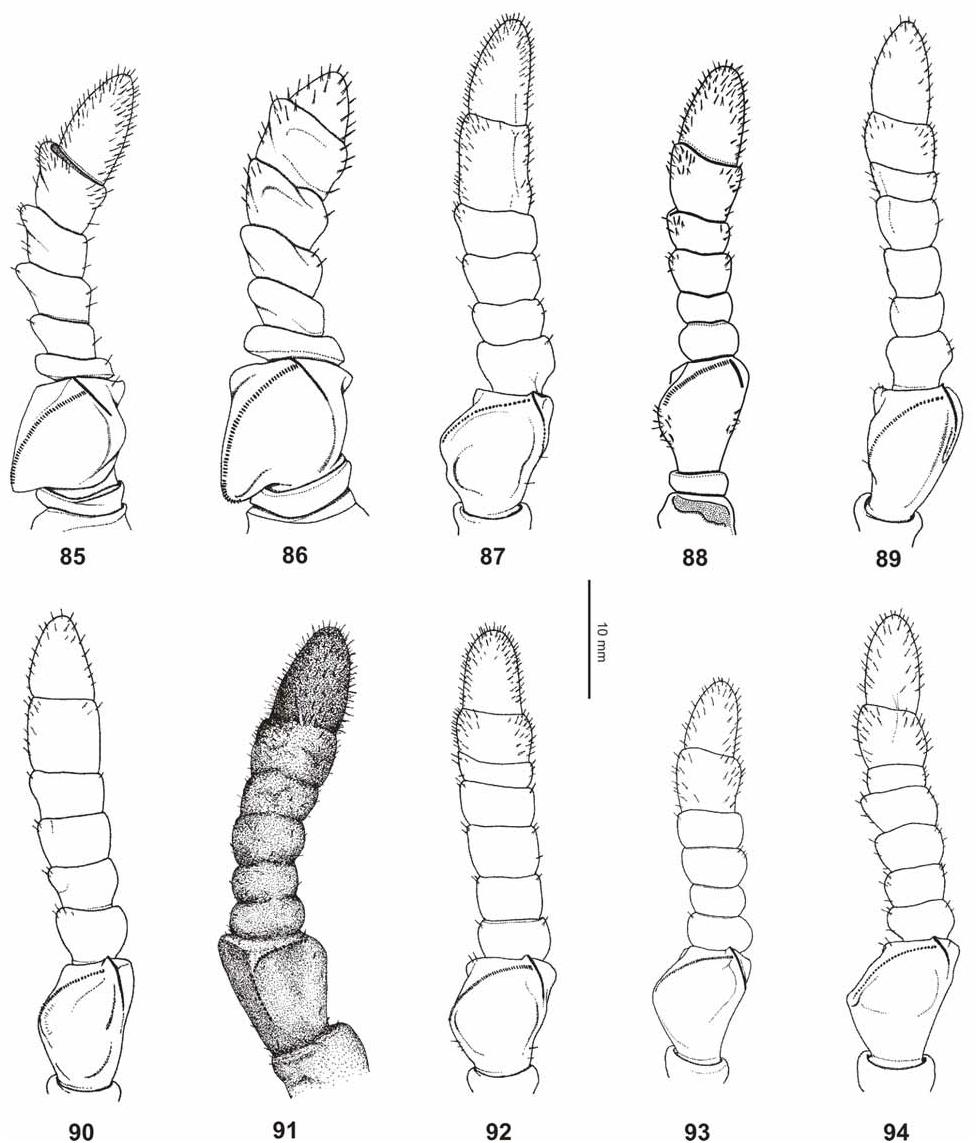
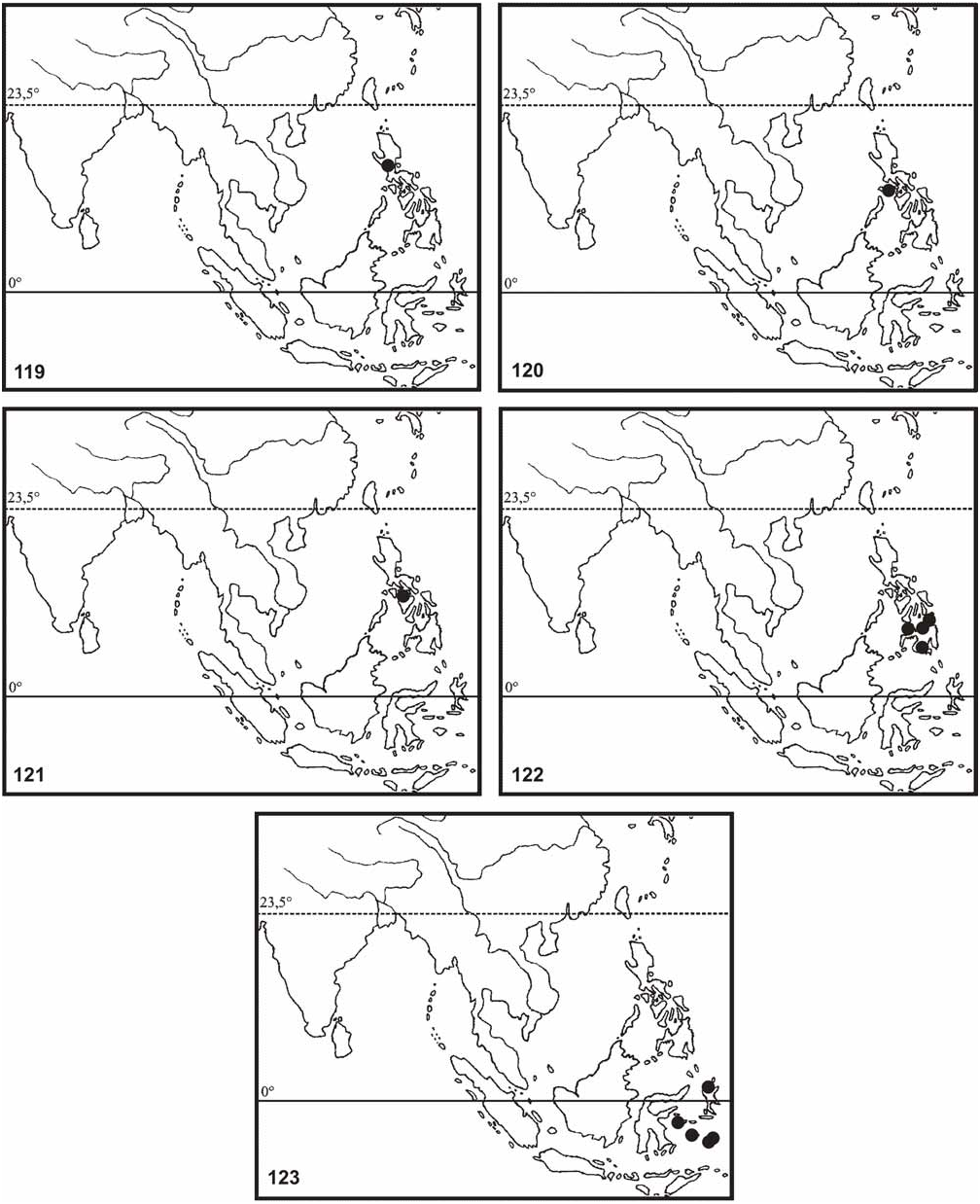
( Figs. 50–51 View FIGURES 50–51 , 67 View FIGURES 52–67 , 70 View FIGURES 68–76 , 84 View FIGURES 77–84 , 94 View FIGURES 85–94 , 123 View FIGURES 119–123 )
Gryllus (Mantis) siccifolium Linné, 1758: 425 , No. 3. HT, ♀: Indes, Gustav IV Adolph; Gryllus (Mantis) siccifolius type; Coll. n° 1818 (UUZM).
Linné, 1764: 111.
Mantis siccifolia, Wallin, 1997: 28 .
Phasma siccifolia, Stoll, 1813: 21 , pl. 7:24 (♂), 7:25a–c (nymphs), 7: 26 (♀). [Later described as Phasma citrifolium Lichtenstein, 1796 – see below]
Phyllium siccifolium, Redtenbacher, 1906: 176 View in CoL , pl. 6: 19 (in part – only records from Moluccas).
Phyllium (Phyllium) siccifolium, Klante, 1976: 65 View in CoL (in part – only records from Moluccas).
Bragg, 2001: 198: figs. 64a–e (in part – only the illustrated ♀ from Ceram in BMNH) .
Brock, 2002: 3, fig. 4 (♀, HT).
Otte & Brock, 2005: 274 (in part).
Phyllium tobeloense Grösser, 2007: 15 View in CoL , figs. 1–4. HT, ♀: Indonesien, Nord Molukken, Halmahera, Tobelo, VIII. 1998 (DEIC). n. syn.
Grösser, 2008: 140, figs. 174–175.
[Not: Phyllium (Ph.) siccifolium, Brock, 1999: 158 , fig. 106 = Ph. (Ph.) hausleithneri Brock, 1999 ]
[Not: Phyllium (Ph.) siccifolium, Seow-Choen, 2000: 44 , pl. 122 = Ph. (Ph.) hausleithneri Brock, 1999 ]
[Not: Phyllium (Ph.) siccifolium, Grösser, 2001: 94 , figs. 121–123 – Malayan records relate to Ph. (Ph.) hausleithneri Brock, 1999 , Philippine records relate to Ph. (Ph.) philippinicum n. sp. and records from China and India deserve clarification]
[Not: Phyllium (Ph.) siccifolium, Grösser, 2008: 134 , figs. 158–160 – Malayan records relate to Ph. (Ph.) hausleithneri Brock, 1999 , Philippine records relate to Ph. (Ph.) philippinicum n. sp. and records from China and India are doubtful]
The numerous citations and references of Ph. siccifolium may at this point be looked up in the Phasmid Species File online http://phasmida.speciesfile.org (author: Paul D. Brock). Only selected references are presented above.
The following seven species were synonymised with Ph. siccifolium (Linné, 1758) , most of which are without exact localities or definite type-specimens:
Phyllium brevicorne Latreille, 1806: 272 View in CoL . HT, ♀: Moluccas (location of type not known). [Synonymised by Gray, 1835: 30]
Phasma chlorophylla Stoll, 1813: 69 , pl. 23: 89 (♂). HT, ♂: no locality ( Jardin de Plantes Paris – MNHN, not traced) [Synonymised by Redtenbacher, 1906: 176].
Comment: Westwood (1859: 173) stated the ♂ type to be in the Jardin de Plantes , Paris hence it is likely to have been transferred to the MNHN collection at some time. Extensive research in MNHN could however not trace the specimen, which must be regarded lost. In aspect of the shape of the profemora as illustrated by Stoll (1813, pl. 23: 89) it is likely to be a ♂ Ph. siccifolium (Linné, 1758) .
Phasma citrifolium Lichtenstein, 1796: 78 . ST, ♂ (♂), ♀ (♀): Amboina, coll. L.F. Holthuisen (location of types not known). [Synonymised by Gray, 1835: 30]
Comment: Name for “La fueille de Citron” Stoll, 1813: 21 (pl. 7: 24–26). The ♂ (pl. 7: 24) quite certainly is the opposite sex of the ♀ (pl. 7: 26) and nymphs (pl. 7: 25a–c) illustrated by Stoll (1813), all of which are from “Amboina”, the presumed type-locality of Ph. siccifolium (Linné, 1758) .
Phyllium donovani Gray, 1835: 31 View in CoL . HT, ♂ (nymph): One of the islands of the Indian seas (location of type not known). [Synonymised by Redtenbacher, 1906: 176]
Comment: Nomen novum for the specimen illustrated as Phyllium siccifolium by Donovan (1800: pl. 11: 3).
Mantis foliatus Perry, 1810 : plate 24. HT, ♀ (nymph): no locality (not traced) [Synonymised by Westwood, 1859: 172]
Phyllium gorgon Gray, 1835: 31 View in CoL . HT, ♀ (nymph): no locality (Originally in Bullock’s collection – not traced). [Synonymised by Westwood, 1859: 172]
Comment: Nomen novum for the specimen illustrated as Mantis foliatus by Perry (1810: pl. 24).
Phyllium stollii Le Peletier de Saint Fargeau & Serville, 1827: 115 .
Comment: Unnecessary replacement name for Phasma chlorophylla Stoll, 1813 (pl. 23: 89) – junior objective homonym.
Material examined [ 14 ♀, 2 ♂♂]: AMBON : 1 ♂: Amboina, 11–17.xi.1923. C.J. Brooks; coll. No. 18010; C. J. Brooks B.M. 1936-681 ( BMNH) ; 1 ♀: Amboina, Felder; Phyllium siccifolium L. ♀ Brunner det. (4). ( MNHU) ; 3 ♀: Dr. Doleschal, 1859, Amboine; Mus. Caes. Vindobona; det. Redtenb. Phyllium siccifolium ; 1637 ( NHMW, No. 296) ; 1 ♀: 7386; Coll. Br. v. W., Amboina, Deyrolle; det. Redtenb. Phyllium siccifolium ( NHMW, No. 296) .
CERAM: 1 ♀: Ceram, 55-8 ( BMNH) .
BANGGAI: 1 ♀: Bangkei, H. Kühn 1885; Phyllium siccifolium L. ♀ Brunner det. (2). ( MNHU) .
BURU: 1 ♀: 24.651; Coll. Br. v. W., Ins. Buru, H. Kühne; det. Redtenb. Phyllium siccifolium ( NHMW, No. 296) .
HALMAHERA: 2 ♀: Indonesien, Molukken, Halmahera Island , Tobelo, VI.2006; Phyllium (Ph.) tobeloense Grösser, 2007 (coll. FH, No. 0657-1 to 2); 1 ♀: Nord-Molukken, Halmahera, Tobelo, 1999 (coll. OC) .
MOLUCCAS: 1 ♂: Coll. Br. v. W., Molukken, Depuset ded.; det. Redtenb. Phyllium siccifolium ; 5009 ( NHMW, No. 296) ; 1 ♀: Coll. Br. v. W., Molukken, Depuiset ded.; det. Redtenb. Phyllium siccifolium ( NHMW, No. 296) ; 1 ♀: Coll. Br. v. W., Tydor (Moluk.), Fruhst.; det. Redtenb. Phyllium siccifolium , 19.951 ( NHMW, No. 296) .
NO DATA: 1 ♀: no locality ( NHMW, No. 296) .
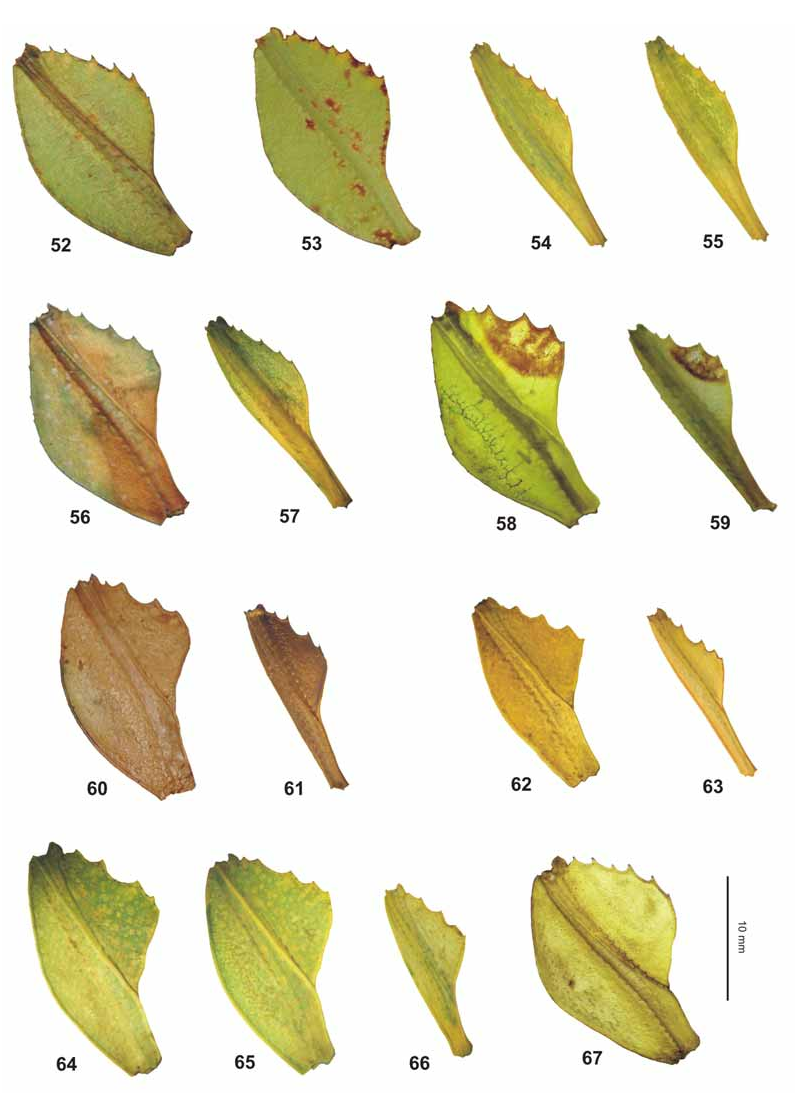
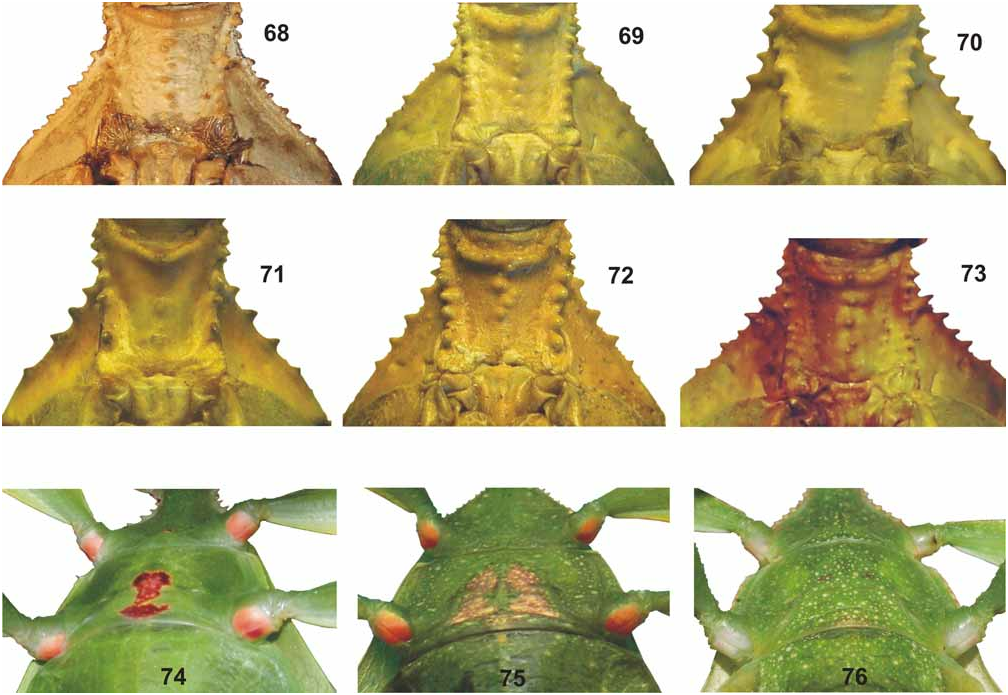
Differentiation: Similar to the New Guinean Ph. zomproi Grösser, 2001 , Philippine Ph. mindorense n. sp. and Ph. hausleithneri Brock, 1999 , the latter representing the Malayan specimens formerly referred to as “ siccifolium ”. The shape and armature of the profemora strongly resemble Ph. zomproi and Ph. mindorense n. sp. ( Fig. 67 View FIGURES 52–67 ). ♀ however differ from all these species by the conspicuous black interior marking on the metacoxae (and mesocoxae in material from Ambon and Banggai – see below) and very indistinct median granules of the anterior portion of the meso-praescutum ( Fig. 70 View FIGURES 68–76 ). Another distinguishing feature is represented by the very distinctive eggs and the distribution in the Moluccas furthermore separate Ph. siccifolium from all three species.
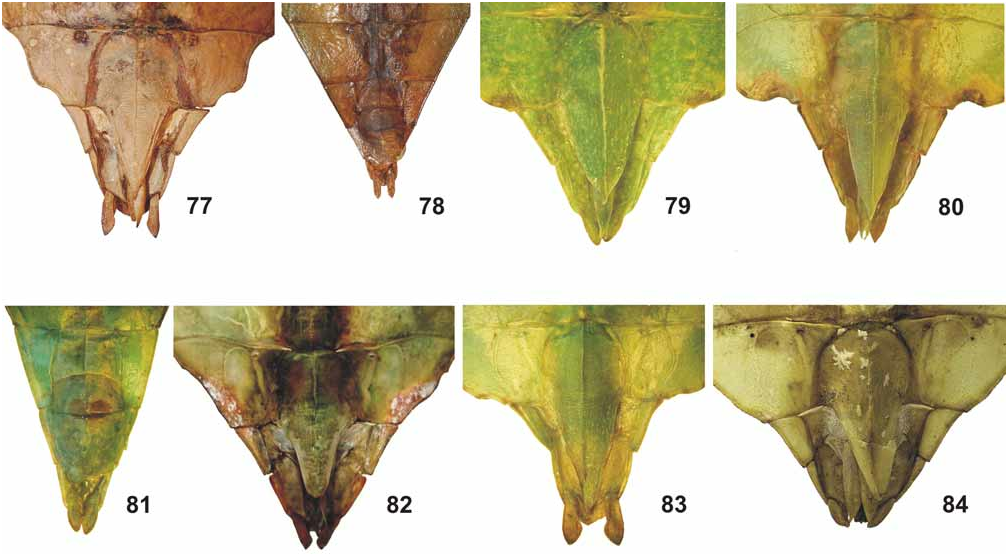
From Ph. zomproi ♀ differ by: the larger size; much less distinct and less numerous granules on the head; having only 30–32 teeth on the pars stridens of antennomere III (48–50 in zomproi ); more distinctly trapezoidal pronotum; smaller and less pointed anterior spine of the meso-praescutum ( Fig. 70 View FIGURES 68–76 ); more prominently granulose mesosternum (almost smooth in zomproi ); ovate abdominal segments V–VII (gradually narrowing in zomproi ) and longer subgenital plate ( Fig. 84 View FIGURES 77–84 ). ♂♂ at once differ by the ovate abdomen (slender and angulate with segments V–VII tapering in zomproi ).
From Ph. mindorense n. sp. ♀ are well distinguished by: the more ovate head (widened towards the posterior in mindorense ); less granulose vertex; having ten instead of nine antennomeres ( Fig. 94 View FIGURES 85–94 ); less distinctly trapezoidal pronotum; less prominent armature of the meso-praescutum ( Fig. 70 View FIGURES 68–76 ); not rounded and convex outer margins of abdominal segments VII and VIII and shorter, posteriorly rounded anal segment (longer than wide and triangular in mindorense ). ♂♂ differ from those of Ph. mindorense n. sp. by the larger size, relatively shorter and much broader mesothorax; wider exterior and interior lobes of the profemora; broader and more distinctly rounded interior lobe of the protibiae and broader, more ovate outline of the abdomen.
Although frequently confused with Ph. hausleithneri there are several striking differences ♀ clearly differing from non-lobed ♀ of this Malayan species by: the lack of a tuberculate longitudinal keel on the meso-praescutum and more prominent spines of the mesopleurae ( Fig. 70 View FIGURES 68–76 ); broader profemora; different dentations of the interior lobe of the profemora ( Fig. 67 View FIGURES 52–67 ); shorter and posteriorly rounded anal segment (longer than wide and triangular in hausleithneri ) and more blunt apex of the subgenital plate ( Fig. 84 View FIGURES 77–84 ). ♂♂ are at once distinguished by: the longer profemora which are>2x longer than the head and have the interior and exterior lobes broader and more decidedly rounded; more prominent armature of the mesothorax; longer antennae which reach as far back as to the middle of abdominal segment IV (posterior margin of II in hausleithneri ) and much broader, oval to elliptical abdomen (rather spear-shaped in hausleithneri ).
Diagnosis: ♀ ( Fig. 50 View FIGURES 50–51 ). With exception of the colouration and measurements given the following description is based solely on the ♀ HT in UUZM in order to present a detailed description of this particular specimen .
Medium-sized for the subgenus (body length 83.0–92.0 mm) with a moderately broad and ovate abdomen (maximum width 33.2–38.0 mm), which has segments VII and VIII very gently rounded. Due to a prior preservation in ethanol the HT is discoloured to pale yellow with the head, prothorax and mesothorax pale to mid brown. The meso- and metacoxae exhibit a distinct and bold black spot on their interior surface. The three ♀ from Halmahera are typically green insects, but have some portions of the body and legs discoloured to yellow, most certainly caused by an injection of ethanol or other chemicals. The interior surfaces of the meso- and metafemora are pale orange and these specimens lack a black interior marking on the mesocoxae (see comments on variation below). Head widest at the posterior with the cheeks very gently rounded; vertex with a small tubercle posteromedially and otherwise set with a few low granules that are roughly arranged in five longitudinal rows. On frons just before centre of dorsal surface with two very small, rounded impressions; two further more distinct and angulate impressions between the bases of the antennae. Antennae slightly longer than cheeks (5.2 mm) and moderately slender, apical antennomere (X) conical and just a little longer than IX, VIII transverse and rather indistinctly separated from IX. Pars stridens on antennomere III with 30–32 teeth ( Fig. 94 View FIGURES 85–94 ). Pronotum angulate and distinctly trapezoidal, gradually narrowed towards posterior with the lateral margins gently convex; anterior margin almost 2x broader than posterior margin. Meso-praescutum slightly narrowed towards the posterior and about 1.3x longer than wide. Lateral margins armed with 4–5 distinct but blunt spines and one or two much smaller intercalated tubercles; disc with 5 small tubercles medially ( Fig. 70 View FIGURES 68–76 ). Anterior margin with a curved, rather strongly raised and medially angulate transverse ridge; in centre protruded into a blunt conical spine. Mesopleurae distinctly and gradually diverging; their lateral margins armed with five prominent but blunt spines and a few small intercalated tubercles. Mesosternum with a rather distinct pair of granules near anterior margin and otherwise irregularly granulose, in particular throughout the anterior portion. Metasternum minutely granulose in anterior half. Tegmina ovate and slightly projecting over posterior margin of abdominal segment VII. Alae lacking. Abdominal segments II–IV gradually widening; IV widest segment and roundly angulate just before posterior margin. V–VIII elongate oval in outline, VIII gently rounded with posterior margin conspicuously narrower than anterior margin ( Fig. 51 View FIGURES 50–51 ). IX and X convergent caudad and together forming a triangle. IX distinctly transverse and about 3x wider than long. Sternum IX with a large, slightly angular impression on both sides of subgenital plate. Anal segment (X) wider than long with posterior margin almost semi-circular. Subgenital plate narrow triangular and with a rather blunt apex; reaching about half way along tergite X. Profemora with a moderately large, widely rounded exterior lobe; outer margin smooth or with a few very minute teeth. Interior lobe slightly broader than exterior lobe, the outer margin in anterior portion armed with six rather slender triangular teeth, I and III considerably smaller than the remaining ( Fig. 67 View FIGURES 52–67 ). Interior lobe of protibiae of moderate width and roundly triangular. Exterior lobe of meso- and metafemora each with eight rather acute teeth in apical portion, the interior lobe with only one or two minute teeth. Posteroventral carina each with six moderately sized teeth and with 3–4 intercalated much smaller teeth. Protarsi roughly ¾ the length of corresponding tibia. Probasitarsus 5.4x longer than wide and as long as combined length of remaining tarsomeres except claw. Meso- and metabasitarsus a little longer than following three tarsomeres combined.
♁♁ ( Fig. 51 View FIGURES 50–51 ). Large for the subgenus (body length 67.0–73.0 mm) with a roundly ovate to elliptical abdomen (maximum width 19.0–20.0 mm). General colour green, the mesothorax pale brown. Femora occasionally irregularly brown in apical portion (♂ in BMNH). Antennae pale to mid brown, darker apically. Eyes dark reddish brown. Vertex very sparsely and minutely granulose. Antennae consisting of 23 segments and reaching about half way along abdominal segment IV. Pronotum and mesothorax structured generally as in ♀, but pronotum proportionally longer and less distinctly trapezoidal. Meso-praescutum about 1.3x longer than wide distinctly trapezoidal and narrowing towards the posterior; structured generally as in ♀ but transverse anterior ridge less distinct. Mesopleurae and mesosternum as in ♀. Tegmina ovate and slightly constricted apically, ± reaching to posterior margin of abdominal segment III. Alae at least reaching half way along segment VIII. Abdominal segment II roughly parallel-sided, III strongly diverging and roundly angulate just before posterior margin. IV–VIII oval to elliptical in outline; V widest segment and with two small brown eye-like spots. Anal segment (X) roundly triangular with the apex rather narrow; base a little wider than length of segment. Vomer broadly triangular with a single, straight terminal hook. Poculum moderately convex with a fine longitudinal median carina and a broad, widely rounded posterior margin; slightly projecting over posterior margin of tergite IX. Exterior lobe of profemora moderately narrow with the broadest point almost 2x wider than the shaft of the femur itself; outer margin gently rounded. Interior lobe almost 2x wider than exterior expansion and strongly rounded. Armature variable (see Fig. 51 View FIGURES 50–51 ); in apical half with 4–5 moderately sized but acute teeth and a slightly bigger gap between the 3 rd and 4 th tooth; the most basal tooth smaller than the remaining and occasionally one or two smaller intercalated tooth present. Protibiae with a distinct, roundly triangular interior lobe. Protarsus moderately elongate and about ¾ the length of corresponding tibia, probasitarsus about 6x longer than wide.
Eggs: The eggs were illustrated and very briefly characterized by Grösser (2007: 17, fig. 3) based on an egg extracted from the abdomen of the HT of Ph. tobeloense Grösser, 2007 and stated to be fully developed. The very distinctive shape and fact that it was removed from the abdomen however suggest it to be not fully developed why care should be taken. Based on the illustration provided by Grösser (2007: 16, fig. 3) the capsule appears to be rather ovoid and all over covered with hairy or feather-like appendages. Grösser (2007: 17) provided the following measurements [mm]: length 4.0, width 2.1, height 2.6.
Variation: ♀ from Ambon are rather constant in most aspect and variation appears to be less developed than in some closely related species. Variation is only seen in the size, length of the tegmina, width of the exterior lobe of the profemora, number and shape of the teeth of the interior lobe of the profemora, armature of the mesothorax and width of abdominal segments V–VIII. Segments V and VI range from almost parallelsided to gradually converging and accordingly VII and VIII are either strongly or just slightly rounded. The three ♀ from Halmahera at hand differ from the HT and material from Ambon by having a black interior marking only on the metacoxae (also present on the mesocoxae in the HT), only 2–4 small medial granules on the mesonotum, less pointed teeth of the interior lobe of the profemora as well as the slightly narrower exterior lobe of the profemora and interior lobe of the mesofemora. All three have the coxae pale orange interiorly, a fact not mentioned in the original description of Ph. tobeloense by Grösser (2007). The only conspicuous variation seen in these specimens concerns to the length of the tegmina which either project over the anterior margin of abdominal segment VIII or merely reach about half way along VII, number, size and shape of the teeth of the interior lobe of the profemora, armature of the mesothorax and colouration. One of the ♀ (No. FH 0657-1) has two large but indistinctly defined black lateral markings on the metasternum.
Comments: The identity and type-locality of this, one of the first three species of Phasmatodea described, has since its description in 1758 been a mystery and obviously no subsequent author working with Phylliidae had ever examined the type-specimen of Linné in UUZM. The type-locality “Indes” has caused much confusion concerning to the geographic distribution of Ph. siccifolium which was estimated differently by previous authors (e.g. Redtenbacher, 1906; Rehn & Rehn, 1933; Klante, 1976; Grösser, 2008). Since neither author has examined the HT in fact all have misinterpreted Linné’s species, with most records based on misidentified material. The Malayan material referred to as Ph. siccifolium is here shown to be Ph. hausleithneri Brock, 1999 and the Philippine material recorded by Grösser (2008: 134) has proven to be an as yet undescribed species, here named Ph. philippinicum n. sp.. Other records listed by Redtenbacher (1906: 176) including the Seychelles, India and New Britain are doubtful and deserve further evaluation by careful examination of the recorded specimens. Examination of the concerned material in NHMW has proven Redtenbacher’s records from “ Siam ” [= Thailand] to relate to Ph. (Ph.) westwoodii Wood-Mason, 1875 and the records from Java to represent Ph. (Ph.) jacobsoni Rehn & Rehn, 1933 (see chapters on these two species above).
The HT in UUZM was examined from a series of very detailed photographs kindly taken by Hans Meijlon (UUZM) and is here illustrated and described in detail for the first time. Comparison with closely related species has shown Ph. tobeloense Grösser, 2007 described from the island of Halmahera ( Moluccas) to be conspecific and hence to represent a junior synonym of Ph. siccifolium (n. syn.). Unfortunately, there is no chance of extracting the internal sclerite of the HT of Ph. siccifolium for further confirmation of the synonymy. The few very slight differences between the HT of Ph. siccifolium and specimens from Halmahera are summarized above and here interpreted merely as individual traits or intraspecific variation.
The ♂ of Ph. siccifolium was illustrated by Stoll (1813, pl. 7: 24–26) and recognized by e.g. Redtenbacher (1906: 176) or Klante (1976: 75) but no detailed description is available so far and measurements were often mixed up with other misidentified material. Two confirmed ♂♂ of Ph. siccifolium with definite data, including the presumed type-locality, are represented in the collections of BMNH and NHMW and have served for the first description presented above.
As for many of the organisms that were described by Linné the exact type-locality of Ph. siccifolium is a mystery. Linné (1758: 425) stated “Habitat in Indiis” for the HT of Mantis siccifolius , which however is a very inexact record. Research on this common locality stated for many animals and plants described by Linné, has revealed very contrary opinions on what particular region it refers to. Subsequent nominotypical records of species stated to be from “Indiis” or “Indes” by Linné have revealed distributions in the West Indies, Central or northern South America (Amphibians in particular) but also throughout SE-Asia. In fact, Linné’s “Indiis” or “Indes” quite certainly means tropical regions more generally, or at least what is nowadays referred to as the Oriental and Neotropical regions.
Like the type-specimens of Phasma gigas (Linné, 1758) , also in the UUZM collection and labelled “Amboina”, the HT of Ph. siccifolium was received amongst a donation by Gustav IV Adolph in 1745 (formerly Crown-Prince Adolf Frederik). Hence it is rather likely that the HT of Ph. siccifolium was collected alongside with the types of Phasma gigas on Ambon or is from a nearby island of the Moluccas, why the typelocality “Indiis” in the case of siccifolium certainly refers to the Moluccas. Ambon is very likely the typelocality since quite numerous conspecific specimens from this island have been recorded subsequently and are represented in several museum collections (see list of material above).
Distribution ( Fig. 123 View FIGURES 119–123 ): Moluccas: Halmahera (Tobelo [DEIC; coll. FH; coll. OC]); Ambon [NHMW; RMNH; MNHU; BMNH]; Ceram [BMNH]; Sula Islands (Taliaboe [ Klante, 1976: 65, RMNH]); Banggai Island [MNHU] and Buru [NHMW].
* according to Grösser (2007: 17)
** according to Klante (1976: 67) for a ♂ from Ambon in RMNH
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Phyllium (Phyllium) siccifolium (Linné, 1758)
| Hennemann, Frank H., Conle, Oskar V., Gottardo, Marco & Bresseel, Joachim 2009 |
Phyllium tobeloense Grösser, 2007: 15
| Grosser, D. 2007: 15 |
Mantis siccifolia
| Wallin, L. 1997: 28 |
Phyllium (Phyllium) siccifolium, Klante, 1976: 65
| Klante, H. 1976: 65 |
Phyllium siccifolium, Redtenbacher, 1906: 176
| Redtenbacher, J. 1906: 176 |
Phasma chlorophylla
| Redtenbacher, J. 1906: 176 |
Phyllium donovani
| Redtenbacher, J. 1906: 176 |
Mantis foliatus
| Westwood, J. O. 1859: 172 |
Phyllium gorgon
| Westwood, J. O. 1859: 172 |
Phyllium brevicorne
| Latreille, P. A. 1806: 272 |
Phasma citrifolium
| Lichtenstein, A. A. H. 1796: 78 |