Aceria rhodiolae ( Canestrini, 1892 )
|
publication ID |
https://doi.org/ 10.1080/00222933.2015.1103910 |
|
DOI |
https://doi.org/10.5281/zenodo.4323734 |
|
persistent identifier |
https://treatment.plazi.org/id/4E6287AC-7718-160C-BCD6-FA9626BDFBE7 |
|
treatment provided by |
Carolina |
|
scientific name |
Aceria rhodiolae ( Canestrini, 1892 ) |
| status |
|
Aceria rhodiolae ( Canestrini, 1892)
Phytoptus rhodiolae Canestrini, 1892 : Canestrini 1892: 722; Nalepa 1893: 294; Canestrini 1894: 803.
Phytoptus sp.: Löw 1881: 5; Löw 1885: 454; Szépligeti 1890: 20. Löw (1881, 1885) mentioned ‘Phytoptus’ mites collected from ‘ mite galls ’ (‘ Phytoptocecidien ’) on R. rosea View in CoL . Whereas Löw ’ s illustrations of galls are only vaguely reminiscent of those induced by A. rhodiolae , his text seems more concordant ( Löw 1881), suggesting conspecificity of the mite with A. rhodiolae .
Eriophyes rhodiolae ( Canestrini, 1892) : Nalepa 1898: 23; Nalepa 1911: 232; Nalepa 1923: 43; Ross and Hedicke 1927: 268; Nalepa 1929: 112; Kari 1932: 212; Kari 1936: 14; Julin 1936: 547; Baudyš 1938: 34; Moesz 1938: 151; Liro 1941: 4; Wahlgren 1948: 179; Liro and Roivainen 1951: 47, 125.
Aceria rhodiolae ( Canestrini, 1892) : Roivainen 1950: 7; Leatherdale 1959: 31; Boczek 1961: 14; Buhr 1965: 1144; Farkas 1965: 23; Davis et al. 1982: 96; Amrine and Stasny 1994: 80; Bernini et al. 1995: 62; Skoracka et al. 2005: 60.
[The most of these publications merely list the species, with its host, sometimes with the
type of gall it produces; a few include morphological descriptions.]
Diagnosis and similar species
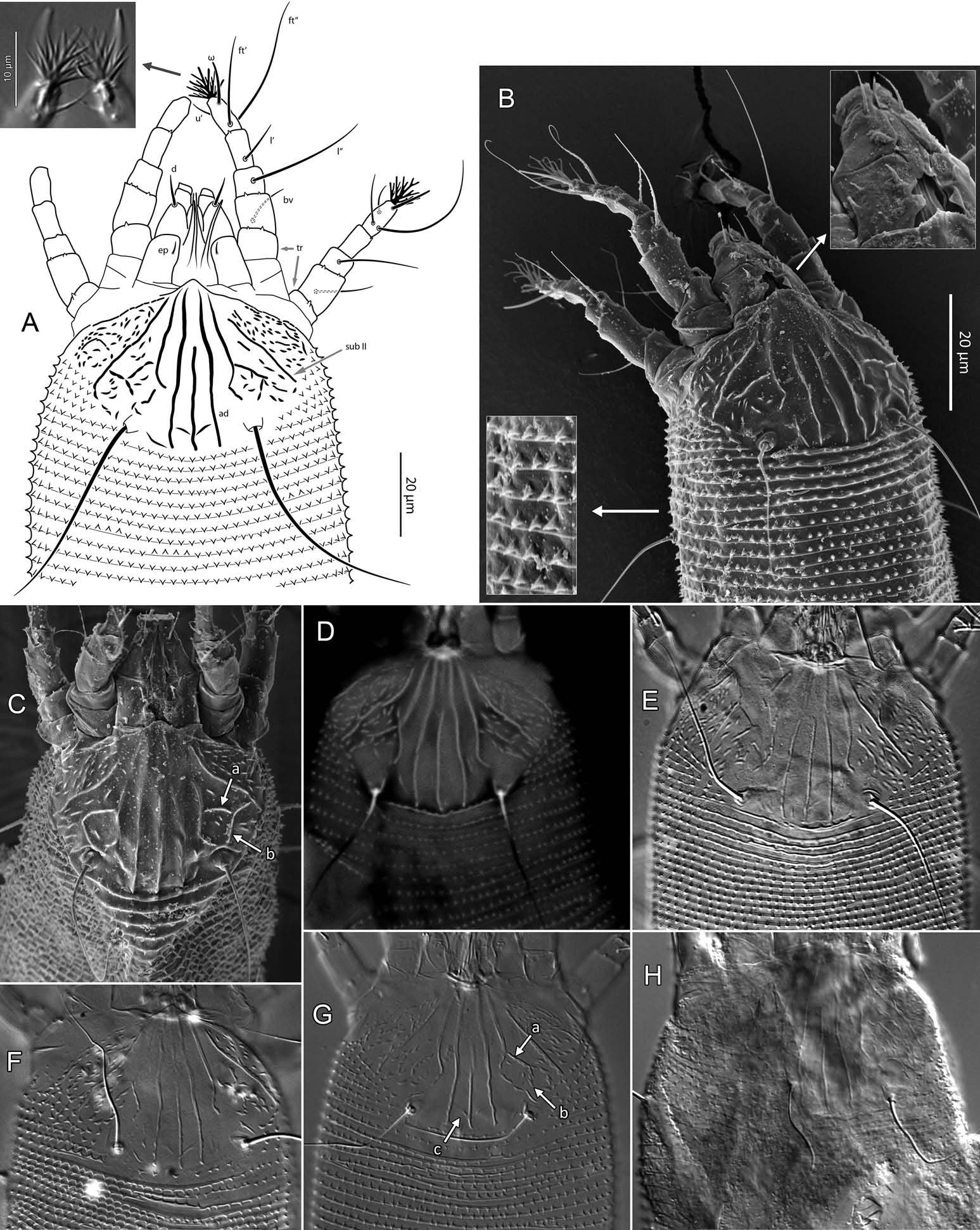
Adults can be distinguished from other Aceria species by the following combination of characters. Only one female form is known. Prodorsal shield 37 ‒ 44 long, including apically a small frontal lobe, pointed or narrowly rounded; Setae sc 27 ‒ 35 apart; first pair of submedian lines reaching posterior third of shield, where they curve inwards and then outwards (curved portion sometimes interrupted into short lines); submedian I usually branching posteriorly before its curving, roughly forming a broad, reversed ‘ Y ’ facing sc tubercles; many short ridges or nodules scattered posterolaterad submedian I, and more densely scattered laterad submedian II. Empodium with four pairs of rays. Coxal plates with conspicuous rounded ridge(s) surrounding medially setae 1a tubercles, and diagonal ridges laterad tubercles of setae 1b. Prosternal apodeme strong, slightly broadened posteriorly. Epigynial coverflap 12 – 15 long × 23 – 27 wide with 8 ‒ 12 longitudinal ridges; a pair of small, rounded lateral flaps flanking the coverflap. Opisthosoma covered by pointed microtubercles throughout, slightly larger dorsally than ventrally. Setae c2 36 – 51, d 44 – 68, e 15 – 21, f 26 – 40.
Aceria destructor may be a close relative, in part based on similarity in prodorsal shield pattern, particularly submedian line I and the ridge laterally branching from it ( Figure 3C View Figure 3 , ‘ a ’) ( Nalepa 1891; however, illustration in Farkas (1965) differs). Aceria rhodiolae differs from A. destructor by ( Table 2): generally shorter sc setae; fewer opisthosomal annuli, and accordingly, by setae d‒f being inserted on different annuli number; a transversally narrower genital coverflap that bears fewer longitudinal ridges, with a few that are usually broken or abbreviated; posteromedian region of prodorsal shield, between sc tubercles, smooth or with few rather indistinct lineae ( Figure 3 View Figure 3 ) (several nodules or very short ridges present in this region in A. destructor ; Figure 7 View Figure 7 ; Nalepa 1891). In addition to the small V-shaped ridge crossing the median line near the posterior shield margin, often visible in A. rhodiolae ( Figure 3G View Figure 3 , ‘ c ’) and A. destructor ( Figure 7 View Figure 7 , ‘ a ’), another V-shaped ridge is generally present in A. destructor , at about 15 μm from the posterior shield margin [partly discernible on Figure 7 View Figure 7 , ‘ b ’; clear on Figure 4 View Figure 4 in Nalepa (1891); not in Farkas (1965)].
Aceria stinsonis ( Keifer 1939) is moderately similar to A. rhodiolae . Based on comparison with description in the literature ( Keifer 1939), A. rhodiolae may be primarily differentiated from A. stinsonis by: its longer sc; prodorsal shield with submedian I branched posteriorly; and coxisternal region ornamented with several nodules, and ridge(s) mesad seta 1a and laterad 1b (only a few scattered nodules for A. stinsonis ; see also Table 2).
Based on the literature ( Nalepa 1895; Liro and Roivainen 1951), Aculus kochi (Nalepa and Thomas) appears similar to A. rhodiolae , particularly in the ornamentation of the dorsal shield. The main difference may be the well-developed frontal lobe of the prodorsal shield, which probably was the reason why it was assigned to Aculus by Amrine and Stasny (1994).
Description ( Figures 2‒6 View Figure 2 View Figure 3 View Figure 4 View Figure 5 View Figure 6 , Table 1)
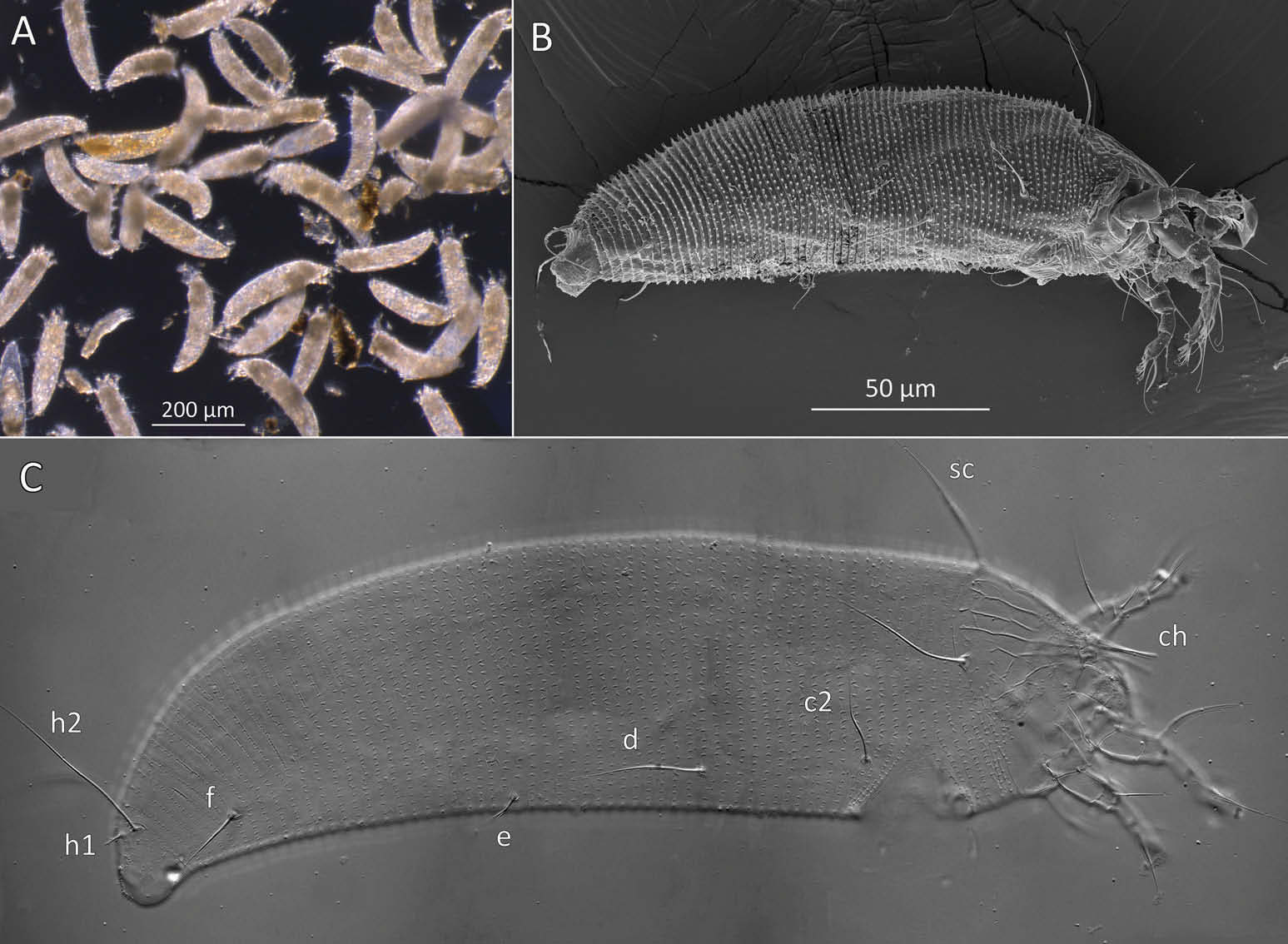
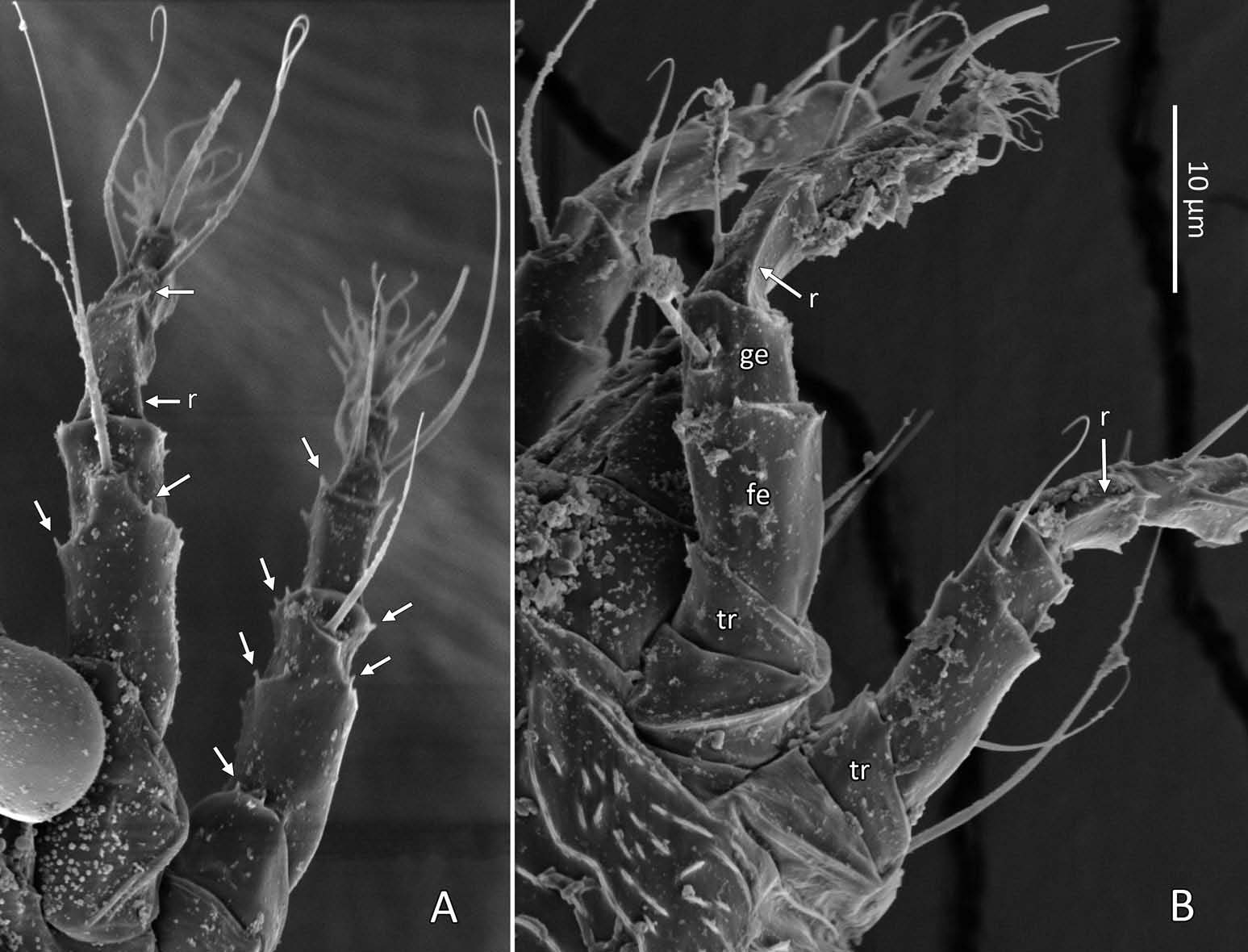
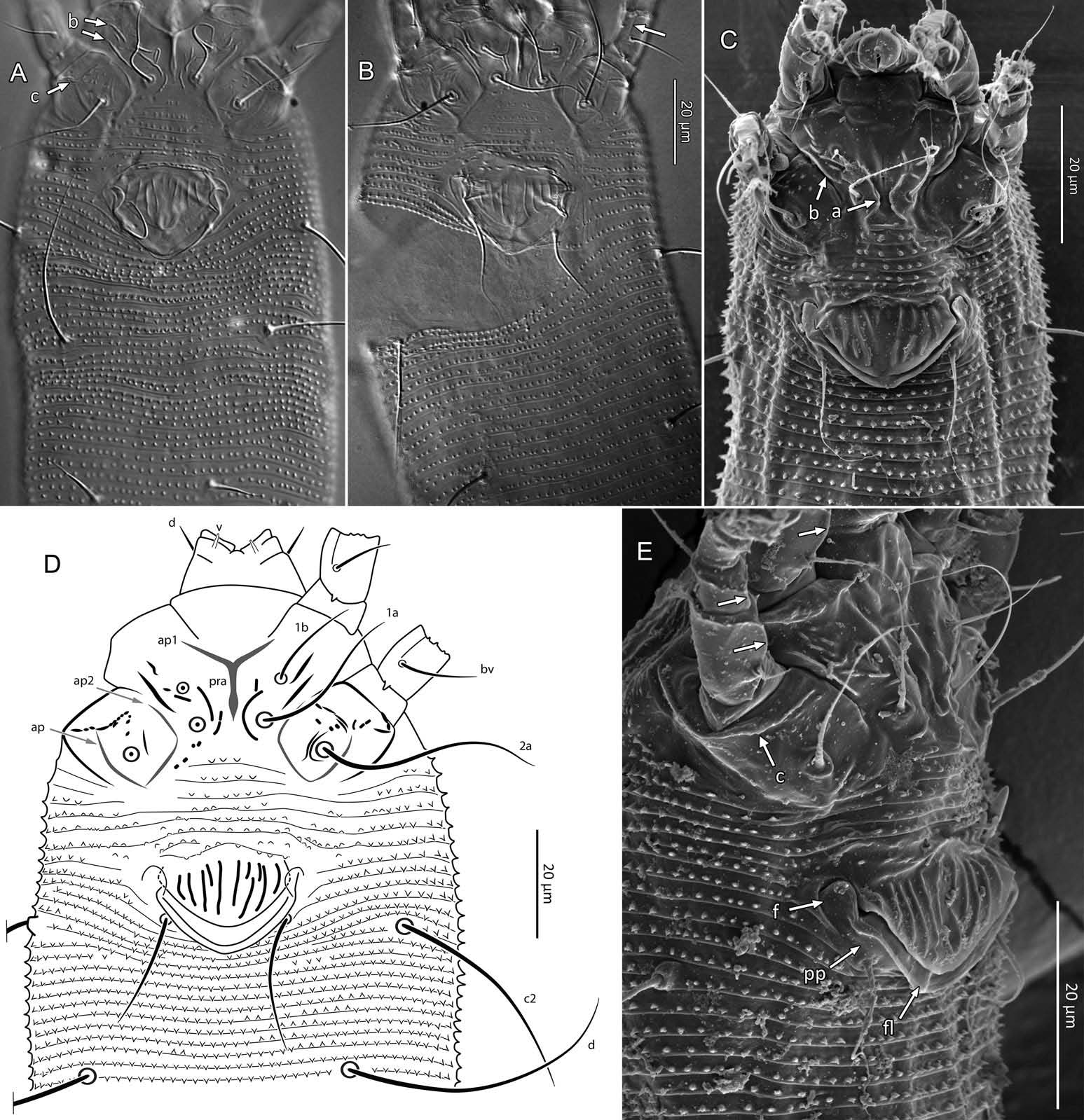
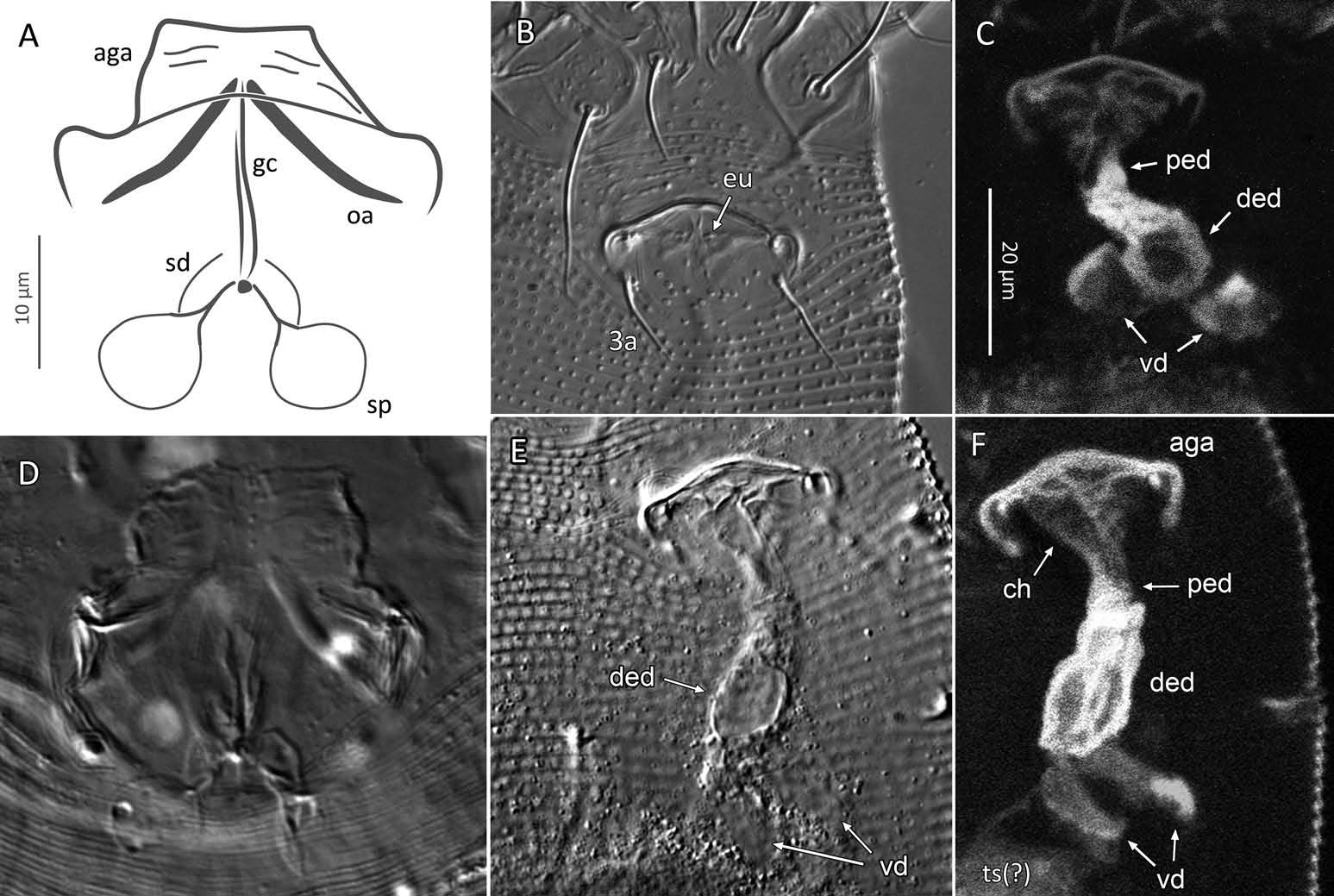
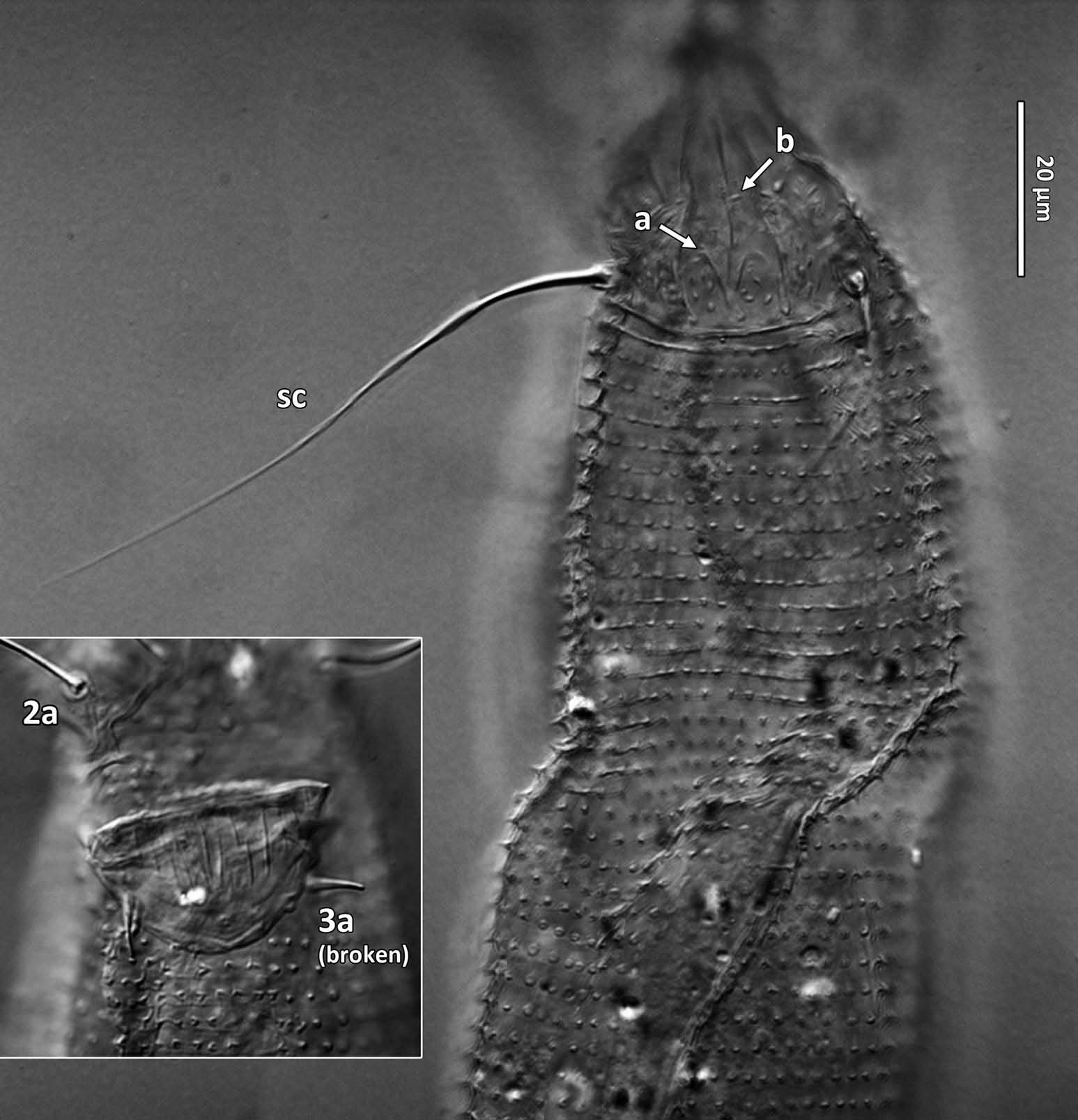
Female (n = 22, some measurements based on fewer specimens). Beige to pale orange (in alcohol; Figure 2A View Figure 2 ). Idiosoma somewhat vermiform 177 ‒ 327 long (depending on level of body contraction), 71 ‒ 88 wide at level of setae c2‒d. Gnathosoma ( Figures 3A ‒ C View Figure 3 , 5C View Figure 5 ) curved downward, 23 ‒ 30; palpcoxal seta ep 2.4 ‒ 3.8, palpgenual seta d 5.4 ‒ 8.3, palptarsal seta-like process v 1.7 – 2.8; cheliceral stylets 16 ‒ 21. Cheliceral retainer a broad flap bent above cheliceral stylets; these stylets largely exposed for about the proximal half of the length of palpcoxae, and covered anteriorly by the stylet sheath ( Figure 3B View Figure 3 ). Prodorsal shield ( Figure 3 View Figure 3 ) somewhat subpentagonal (or subtriangular, if not considering the region laterad submedian lines II); 37 ‒ 44 long, 47 ‒ 59 wide, with a small frontal lobe, narrowly rounded ( Figure 3A,C,E View Figure 3 ) or pointed apically ( Figure 3D,F View Figure 3 ), 2.5 – 4.8 long × 6.4 – 10 wide (may appear broadly rounded in some SEM photographs, e.g. Figure 3B View Figure 3 , possibly due to different angle of view); lobe continuous and not clearly delineated from the rest of the shield anterior margin. Setae sc typically about 50 (43 ‒ 61, exceptionally 35 on a single specimen), inserted at posterior margin of shield, projecting upwards and posterad in natural position ( Figure 3B,C View Figure 3 ) and typically posterolaterad in slide-mounted specimens; sc tubercles with their basal axes transversal (hence, setae sometimes directed anteriorly on slide), setae 27 ‒ 35 apart. Shield ornamentation: median line (ridge) conspicuous for about the posterior two-thirds of shield length, and faint or absent in anterior third; a pair of short lines (ridges) often present posteriorly on each side of median line, directed anterolaterad, forming a broad ‘ V ’ ( Figure 3A,E,F,G View Figure 3 ); admedian lines stretching from posterior margin of shield to about the base of frontal lobe, gradually converging anteriorly; submedian lines I subparallel to admedians, reaching posterior third of shield, where it curves mediad (inwards) and then laterad (outwards), approaching sc tubercles; curved portion of submedian lines sometimes interrupted into short ridges ( Figure 3F,G View Figure 3 ). Submedian lines II running from posterolateral margins of shield to near anteromedian shield margin, at about a 90° angle from each other, and slightly curving outwards in their anterior quarter. A short ridge (‘ a ’, Figure 3C,G View Figure 3 ) (sometimes represented by a series of about four or five shorter ridges) running posterolaterad originates from submedian line I, posteriorly forming a reversed Y-shaped bifurcation, facing sc tubercles (Y-shape may not be discernible, depending on other ridges present); occasionally another ridge (or series of short ridges; ‘ b ’) running perpendicular to the previously described ridge (‘ a ’), and sometimes reaching laterally submedian line II, creating the impression of an eye-like structure ( Figure 3C,G View Figure 3 ). Region between submedian lines I and II scattered with about 10 ‒ 16 short ridges or nodules; region laterad submedian line II densely scattered with 30 ‒ 38 ridges or nodules. Legs ( Figures 3A,B View Figure 3 , 4 View Figure 4 , 5 View Figure 5 ) with setation normal as described for Eriophyidae ( Lindquist and Amrine 1996) . Leg I 38 – 45; femur 10.3 – 13.0, bv 9.6 – 12.4, position of bv 3.8 ‒ 5.0; genu 5.4 – 7.7, l” 27 – 32, position of l” 2.7 ‒ 3.6; tibia 9.0 – 11.0, l’ 9 – 13, position of l’ 1.4 ‒ 2.6; tarsus 9.5 – 11.3, ft’ 20 – 22, ft” 22 – 36, u’ 5.6 – 7.5; ω 10.0 – 11.3 without knob; empodium 7.9 – 9.0, with four pairs of rays; the two most basal pairs of rays with two (visible) secondary branches on each side, the third pair with one secondary branch, and terminal pair simple. Leg II 36 – 40; femur 10.3 – 13.4, bv 12.4 – 15.0, position of bv 3.4 ‒ 4.3; genu 4.5 – 6.2, l” 16 – 21, position of l” 2.0 ‒ 3.4; tibia 7.2 – 8.8; tarsus 9.0 – 11.1, ft’ 11 – 13, ft” 29 – 34, u’ 5.3 – 7.7; ω 9.8 – 11.0 without knob; empodium 7.0 – 10.5, with four pairs of rays, with branching as that of leg I. Apical margins of trochanter, femur, genu and tibia with spinules (more easily discerned under SEM): two dorsally on trochanters I ‒ II; two dorsally on femora I ‒ II; a few spinules laterally and ventrally on femora and genua I ‒ II; ventral spinules more tightly packed and aligned along a transversal ridge that curves medioventrally into a longitudinal ridge, which crosses genu I ‒ II entirely, and posteriorly reaches bv tubercles on femora I ‒ II; tibiae I ‒ II each with two dorsolateral ridges and a medioventral longitudinal ridge that each end apically in a spine ( Figure 4 View Figure 4 ). Based on SEM, femora I ‒ II apparently superficially fused mediodorsally with genu (between the two dorsal spinules), with apicolateral margins of femora sometimes extending dorsally on each side into a ridge that meets with the tubercle of genual setae l”; such ridges and fusion not apparent under light microscopy (although femoro-genual boundary may appear weak mediodorsally). Distal extension of coxal regions I and II (before connection with trochanters) with strong dorsal ridges running diagonally. Coxal region ornamented with a few conspicuous ridges, in coxal field I: one or two, moderately long, curving ridges surrounding medially tubercles of setae 1a (‘ a ’, Figure 5C View Figure 5 ); a moderately long ridge running close and parallel to coxal apodeme II, accompanied anteriorly by one or more shorter ridges laterad setae 1b tubercles (‘ b ’, Figure 5A,C View Figure 5 ); on coxal field II: a ridge running close and parallel to trochanter II margin (‘ c ’, Figure 5A,E View Figure 5 ); all these ridges are entire or interrupted into a series of shorter ridges or nodules. A few additional short ridges or nodules, particularly anterad 2a tubercles. Prosternal apodeme (or sternal line) strong (hardly discernible under SEM, because it is mostly internal), bifurcating anteriorly into coxal apodemes I (sensu Lindquist 1996), and posteriorly broadened and tapering to end at level of setae 1a; coxal field II delimited antero- and postero-medially by a linea (may represent apodeme 2, sensu Lindquist 1996; Figure 5D View Figure 5 , ‘ ap2 ʹ); another linea (or apodeme, ‘ ap ’) flanking posterolaterally tubercles of 2a; the junction of these lineae posteriorly leading (more internally) to a poorly defined, inversely Y-shaped apodeme (vaguely discernible, Figure 5B View Figure 5 ). Subcapitular plate 10 – 14 long × 15 – 21 wide. Setae 1b 11 ‒ 23 long, 15 – 18 apart; 1a 23 – 37 long, 8.8 – 12.2 apart; 2a 30 – 57 long, 27 – 36 apart. About six or seven microtuberculate coxigenital annuli, of which the two to four anteriormost annuli are incomplete (i.e. segregated from lateral annuli); microtubercles on coxigenital annuli smaller, and rounder or more weakly pointed than other ventral microtubercles posterior to epigynium. External genitalia ( Figure 5 View Figure 5 ) with genital coverflap 12 – 15 long × 22 – 27 wide, more or less semicircular, anterolateral margins angled (just before meeting with anterior hinge), ornamented with 8 ‒ 12 longitudinal ridges extending for most of coverflap length, except for a few abbreviated ridges (mostly medially); genital ‘ flange ’ (on which sits the coverflap at rest; see Chetverikov et al. 2013) often visible for 2 ‒ 6 behind coverflap (even when coverflap ‘ closed ’); a pair of small, rounded lateral flaps flanking coverflap (sometimes not visible on slides); setae 3a 15 – 37 long, 18 – 23 apart. Opisthosoma ( Figure 2 View Figure 2 ) more or less parallel-sided for its anterior half (from setae c2 to e); evenly rounded dorsally and ventrally, bearing 66 – 75 dorsal and 61 – 68 ventral annuli, microtuberculate throughout. First dorsal annulus behind prodorsal shield often interrupted (i.e. without microtubercles) at level of sc tubercles; that first dorsal annulus is three to five annuli posterad the first complete annulus behind coxal plate II. Dorsal microtubercles ( Figure 3B View Figure 3 , inset) subconical (or subtriangular if bent or flattened on slides), sharply pointed, with slightly concave sides, straight or slightly curving posteriorly in natural position (SEM); similar throughout, 1.0 ‒ 1.4 long × 0.9 ‒ 1.3 wide basally, except smaller on the first three to five dorsal annuli, and narrower and progressively shorter on posterior third of opisthosoma, 0.5 ‒ 1.2 long × 0.5 ‒ 0.8 wide (microtubercles may appear rounded if oriented upwards, especially in laterally mounted or highly contracted specimens). Laterally, microtubercles similar to dorsal ones, becoming smaller, more weakly pointed ventrally (0.7 ‒ 1.2 long × 0.8 ‒ 1.2 wide; Figure 5 View Figure 5 ); microtubercles narrow, ridge-like on the last five ventral annuli. Setal lengths: c2 36 – 51, d 44 – 68, e 12 – 21, f 26 – 40, h1 5.4 – 7.0; h2 54 – 97. Distances between pairs of setae: c2–c2 56 – 69, d–d 40 – 48, e–e 21 – 26, f–f 28 – 34, h1–h1 6.5 – 9.0, h2–h2 12.5 – 14.5, h1– h2 2.3 – 3.4. Position of setal tubercles (the number of annuli counted from coxal plate II): c2 9 – 12, d 20 – 24, e 34 – 39, f 56 – 62; there are five or six annuli past seta f. Internal genitalia ( Figure 6A,D View Figure 6 ). Anterior genital apodeme more or less flat anteriorly for 12 ‒ 17 µm, with anterolateral walls bent posteriorly or posterolaterally (i.e. at a 45° ‒ 90° angle), and ending in two short curves or folds. A pair of oblique apodemes (‘ oa ’; sensu Chetverikov et al. 2015), thick, 13 ‒ 16 long, each slightly curved, sometimes bent midway, oriented more or less perpendicular with each other. Longitudinal bridge 13 ‒ 17 long, with walls (of the genital channel; see Chetverikov 2014) sometimes discerned apart (‘ gc ’, Figure 6A View Figure 6 ). Spermathecae spherical, usually about 6 (4.5 ‒ 7.3) in diameter; spermathecal duct 3.4 ‒ 4.2 long; duct connected near or at the posterior apex of longitudinal bridge, at an angle of 38 ‒ 62° from bridge axis.
Male (n = 4; Figure 6B,C,E,F View Figure 6 ). Fundamentally the same as female, except for genitalia and minor quantitative differences, including shorter idiosoma, shorter leg segments, and slightly fewer dorsal and ventral annuli ( Table 1). Ornamentation of prodorsal shield and coxigenital region as that of female. Epiandrium 22 ‒ 24 wide, 14.5 ‒ 15.3 long, including the region of irregularly scattered, rounded microtubercles between setae 3a ( Figure 6B View Figure 6 ); eugenital setae c. 1 μm long, bases 3.0 ‒ 3.5 apart. Internal structures identified include anterior genital apodeme (‘ aga ’), genital chamber (‘ ch ’), proximal and distal ejaculatory ducts (the latter acting as a spermatophore pump), both highly sclerotized (based on their high autofluorescence under CLSM), connected posteriorly to a pair of vasa deferentia (‘ vd ’), leading to a putative testis (Chetverikov 2015).
Taxonomic remarks
The specimens that we borrowed from the Canestrini collection had been preserved in vials (containing an alcohol-based fluid) labelled as from Verona, Italy, the presumed type locality for A. rhodiolae ( Canestrini 1892: ‘ place of origin, Veronese ’; note, however, that Amrine and Stasny (1994) mentioned ‘ woods near Trentino, Italy ’ as the type locality). Unfortunately, the specimens that Canestrini used to describe ‘Phytoptus’ rhodiolae (syntypes) are probably lost, because no slides labelled as P. rhodiolae were retrieved from his collection (now hosted by the MZUP; Paola Nicolosi pers. comm.).
Canestrini ’ s description (1892) of A. rhodiolae is the only one that includes morphological illustrations. His illustrations of the ventral habitus and prodorsal shield partly agree with our observations; they show 58 ventral opisthosomal annuli (61 ‒ 68 for our specimens), 12 longitudinal ridges on the coverflap (8 ‒ 12 on our specimens), and sc setae about two-thirds the length of the dorsal shield (his text says sc at least as long as the shield, which is more concordant with our observations). The text mentions ‘ approximately 60 annuli ’, which is, again, near the lower end of the range of our count of dorsal annuli (66 ‒ 75). More importantly, his illustration of the prodorsal shield shows differences (no nodules or ridges other than the main median, admedian and submedian lines, and the two pairs of submedian lines show different paths) from the specimens we examined, and only three pairs of rays can be seen on all the four empodia illustrated (the text also mentions three pairs of rays only). This may in part be due to the suboptimal microscope qualities and standards of taxonomic descriptions of that period. Roivainen (1950) mentioned that A. rhodiolae from Sweden had more opisthosomal annuli than specimens studied by Canestrini (1892), with 70 ‒ 75 opisthosomal annuli, which is more consistent with our results. The few other descriptions of A. rhodiolae ( Nalepa 1898, 1911; Liro and Roivainen 1951; Farkas 1965) appear as a subset of, or equivalent to Canestrini ’ s description (e.g. they all mention ‘ 60 annuli ’, and three-rayed featherclaws), with no additional information. We consider that three-rayed empodia is probably a mistake made by Canestrini, and which was duplicated by other authors.
Certain characters shared by A. rhodiolae and relatives have unclear phylogenetic significance. For instance, the ridges and spinules present on the leg segments of A. rhodiolae are also present in other genera of eriophyoids ( Baker et al. 1996; Chetverikov et al. 2014). Second, the median, basal V-shaped ridge on the prodorsal shield of A. rhodiolae and A. destructor occurs in other Aceria species, such as Aceria anthonoma (Nalepa) , Aceria chrysopsis (Keifer) , Aceria lappae (Liro) , Aceria saxifragae (Rostrup) , as well as in species in other eriophyid genera, such as ‘Aculus’ kochi , Aculus ligustri (Keifer) , Aculops maculatus (Hodgkiss) (sensu Keifer) , and Paraphytoptus mcgregori Keifer , (see Liro and Roivainen 1951; Farkas 1965; Baker et al. 1996).
Galls
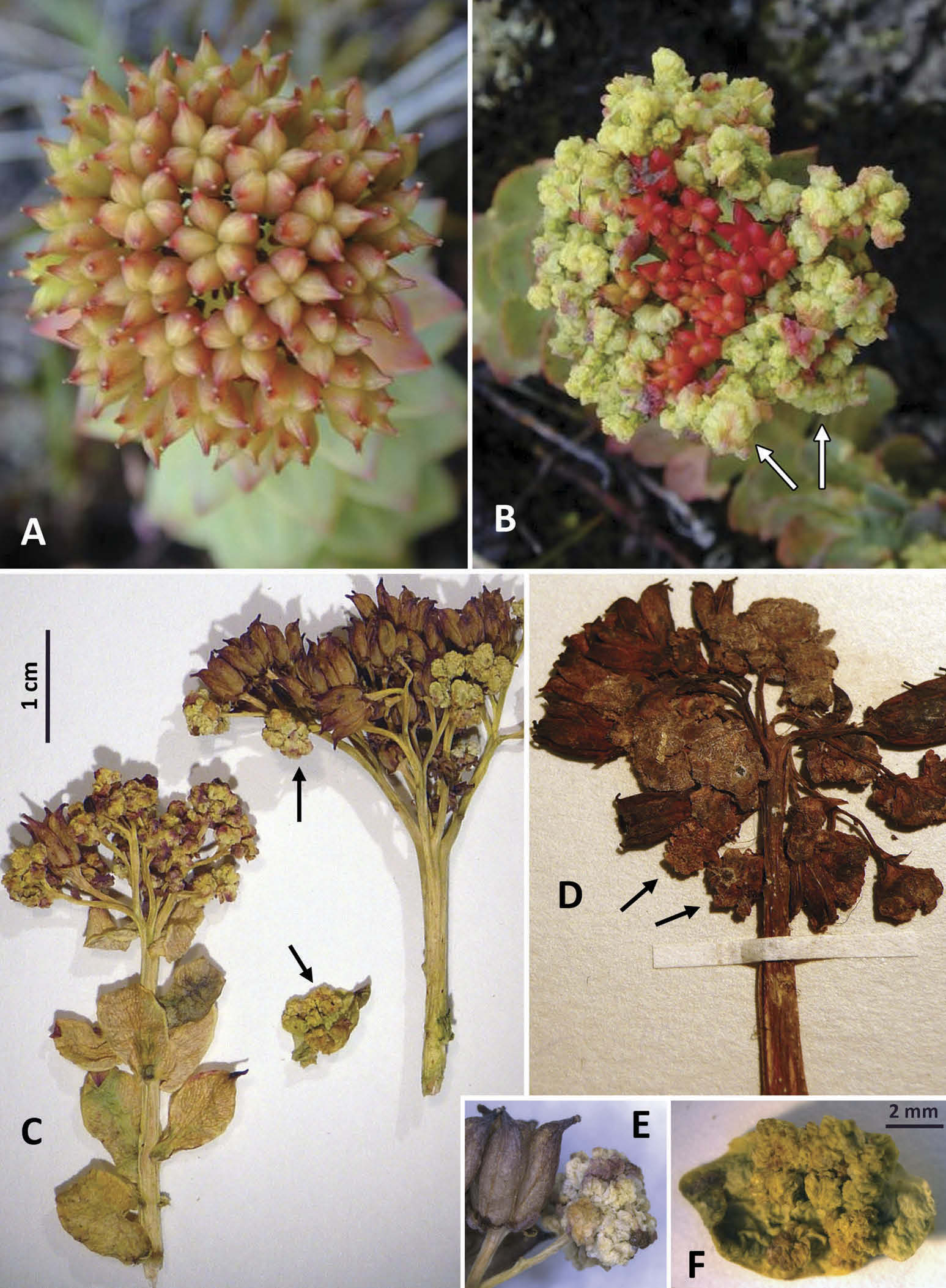
Galls were observed primarily on the female fruiting inflorescences of R. rosea (= Sedum rosea (L.) Scop., = Sedum rhodiola DC.) and occasionally on the upper leaves surrounding the infructescence ( Figure 8 View Figure 8 ). Galled tissues turned fleshy, wrinkled, and whitish or yellowish green ( Figure 8B,C View Figure 8 ). The galled flowers were patchily distributed within inflorescences ( Figure 8C,D View Figure 8 ), and sometimes comprised most or all of the inflorescence, giving a cauliflower-like appearance ( Figure 8B View Figure 8 ).
Local distribution
Our survey has found galled R . rosea at many sites scattered along the shore of Ungava Bay GoogleMaps , from the extreme northwest point (near Quaqtaq: 61.047°N, 69.634°W) to near the northeast extreme of the surveyed area (south of Killiniq: 60.359°N, 64.850°W) and as far south as Kuujjuaq (58.148°N, 68.336°W) ( Figure 1B View Figure 1 ). From 92 sites studied in Nunavik, 29 (31.5%) had at least a few (often numerous) galled individuals of R . rosea, and the remaining 63 had apparently no infested individuals. In addition, we have observed galled R . rosea in Labrador, at multiple sites on Base island GoogleMaps (56.633°N, 61.586°W) and in the vicinity of Saglek Fjord (58.51°N, 63.25°W), Nain (56.54°N, 61.70°W) and Rigolet (54.18°N, 58.44°W).
Impact on phytochemistry
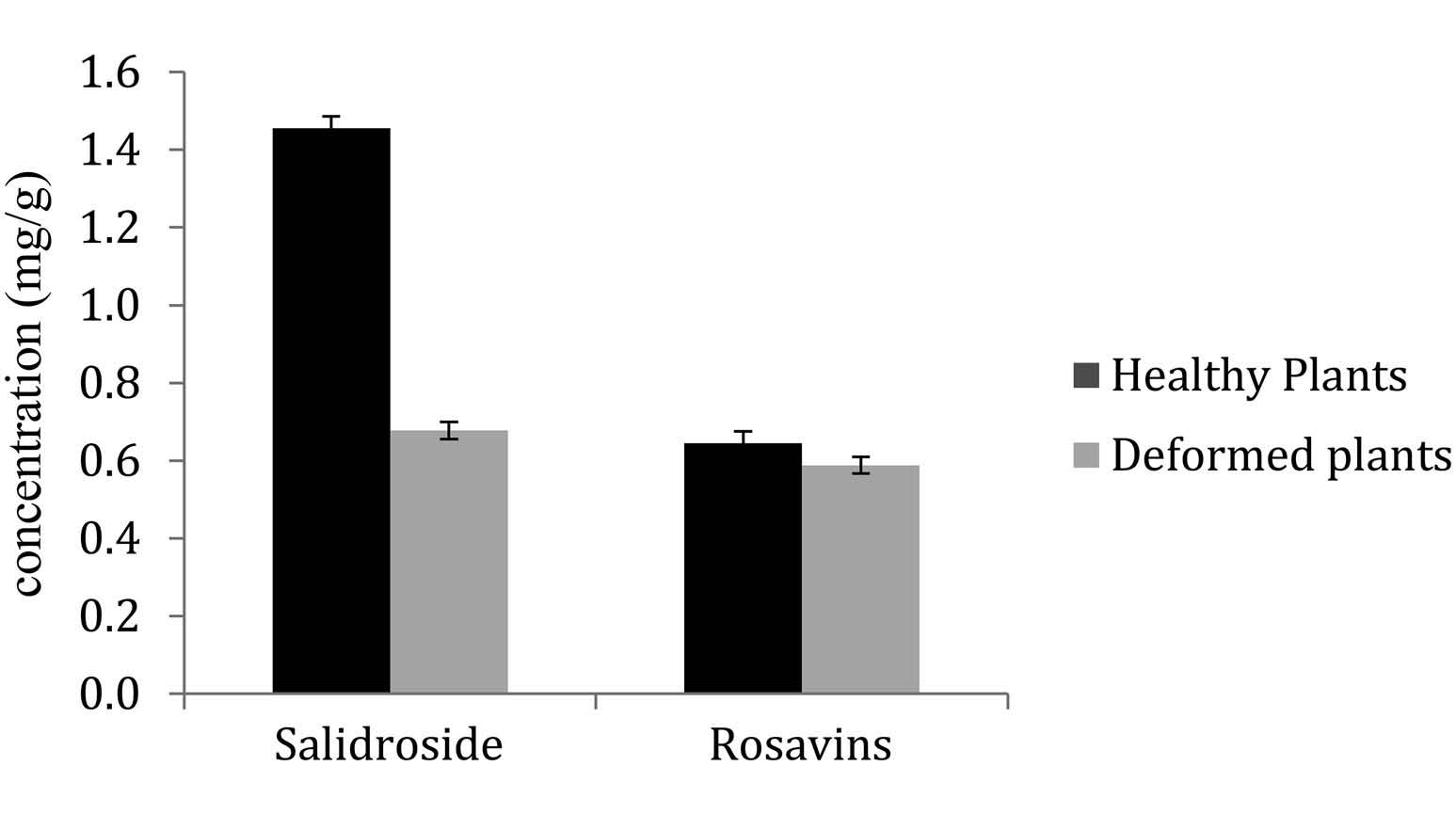
Concentrations in rosavins did not differ between healthy (0.63 ± 0.05 mg /g) and infested (0.61 ± 0.05) plants. In contrast, salidroside concentration in galled plants (0.65 ± 0.05 mg /g) was less than half the concentration in healthy plants (1.42 ± 0.05) and this was significant (p = 0.01) ( Figure 9 View Figure 9 ).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Aceria rhodiolae ( Canestrini, 1892 )
| Beaulieu, F., Cuerrier, A., Filion, V. J., Saleem, A. & Arnason, J. T. 2015 |
Aceria rhodiolae ( Canestrini, 1892 )
| Skoracka A & Lewandowski M & Boczek J 2005: 60 |
| Bernini F & Castagnoli M & Nannelli R 1995: 62 |
| Amrine Jr JW & Stasny TA 1994: 80 |
| Davis R & Flechtmann CHW & Boczek JH & Barke HE 1982: 96 |
| Buhr H 1965: 1144 |
| Farkas H 1965: 23 |
| Boczek J 1961: 14 |
| Leatherdale D 1959: 31 |
| Roivainen H 1950: 7 |
Eriophyes rhodiolae ( Canestrini, 1892 )
| Liro JI & Roivainen H 1951: 47 |
| Wahlgren E 1948: 179 |
| Liro JI 1941: 4 |
| Baudys E 1938: 34 |
| Moesz G 1938: 151 |
| Kari LE 1936: 14 |
| Julin E 1936: 547 |
| Kari LE 1932: 212 |
| Nalepa A 1929: 112 |
| Ross H & Hedicke H 1927: 268 |
| Nalepa A 1923: 43 |
| Nalepa A 1911: 232 |
| Nalepa A 1898: 23 |
Phytoptus rhodiolae
| Canestrini G 1894: 803 |
| Nalepa A 1893: 294 |
| Canestrini G 1892: 722 |
Phytoptus
| Szepligeti G 1890: 20 |
| Low F 1885: 454 |
| Low F 1881: 5 |