Pseudonagoda zhejiangensis, Wu & Yang & Han, 2022
|
publication ID |
https://doi.org/10.11646/zootaxa.5099.2.9 |
|
publication LSID |
lsid:zoobank.org:pub:446A8FC4-FB91-4ADD-8146-DAA6B936137C |
|
DOI |
https://doi.org/10.5281/zenodo.6309259 |
|
persistent identifier |
https://treatment.plazi.org/id/50228794-FFE5-E613-F8CF-FA25FE31271D |
|
treatment provided by |
Plazi |
|
scientific name |
Pseudonagoda zhejiangensis |
| status |
sp. nov. |
Pseudonagoda zhejiangensis View in CoL sp. nov.
Figures 1, 2, 4, 5, 7, 8, 10, 12–27
Type-material. Holotype: ♂, China, Zhejiang Province, Hangzhou City, Hangzhou Botanical Garden , 9 m a.s.l., 30°15'14.48" N, 120°07'24.67" E, 11.VII.2020, reared from Castanopsis tibetana Hance , pupated 14.VII.2020, emgd. 6.VIII.2020, leg. X.F. Yang, genit. prep. WuJ-615-1, in NEFU. GoogleMaps
Diagnosis. Externally, the new species (Figs 1, 2) is similar to P. siniaevi (Fig. 3), but it can be separated from the latter by the following morphological characters (character states for P. siniaevi are parenthesized): P. zhejiangensis sp. nov. is smaller than P. siniaevi , with the length of forewing is 8 mm and a wingspan of only 19 mm in the holotype ( P. siniaevi is larger than P. zhejiangensis sp. nov., forewing length is 12 mm and the wingspan approximately 27 mm in the holotype); the ground colour of the whole body is dark ochreous to fuscous (in P. siniaevi is black).
The male genitalia are clearly diagnostic: i) in P. zhejiangensis sp. nov., each apical lobe of the gnathos (Figs 5, 8) is crescent-shaped (in P. siniaevi each apical lobe of the gnathos is rectangle-shaped; Figs 6, 9); ii) the shape of valva is broad triangular with straight costa and narrower cucullus (in P. siniaevi the shape of valva is triangular with arc-curved costa and rounded cucullus); iii) the horn-shaped processes of the juxta are large and robust (in P. siniaevi the same processes are smaller); iv) the finger-shaped process of the aedeagus (Fig. 10) is more slender (in P. siniaevi the same process is slightly thicker; Fig. 11).
Description. Adult (Figs 1, 2, 7). Wingspan 19 mm, forewing length 8 mm in male.
Head dark ochreous. Labial palpus up-curved, pale yellow to dark ochreous; proboscis absent; antenna strongly bipectinate in basal third then filiform, fuscous (Fig. 7).
Thorax ground colour dark ochreous mixed with fuscous scales dorsally, golden-brown ventrally; tegula fuscous. Forewing elongated, extensively hyaline covered with rare dark tiny scales. Costa and inner margin areas, as well as venations of forewing and hindwing densely covered with dark ochreous scales; fringe short, dark ochreous. Medial stem of forewing well developed, not branched distally; vein R 5 branched form R 3 +R 4. Scales on legs ochreous to pale yellow.
Abdomen black, with a greyish yellow longitudinal median band ventrally.
Male genitalia (Figs 5, 8, 10). Uncus broad, blunt apically. Gnathos highly modified, bilobed apically, each lobe crescent-shaped with a small triangular process on medial part of lower margin (Fig. 8). Tegumen broad. Valva broad triangular in shape; costa pretty straight; cucullus blunt and rounded; sacculus swollen with rounded saccular process, and a sclerotized, sawblade-shaped internal crest extended from apical of saccular process to medial part of valva base. Juxta broadly V-shaped, bearing a pair of strongly sclerotized, horn-shaped, slightly spiralled processes. Saccus not obvious. Aedeagus tube-shaped, with several small spines and a weakly spiralled, finger-shaped process apically (Fig. 10). Sternite VIII with a sclerotized triangular process (Fig. 4, arrowed).
Female genitalia. Unknown.
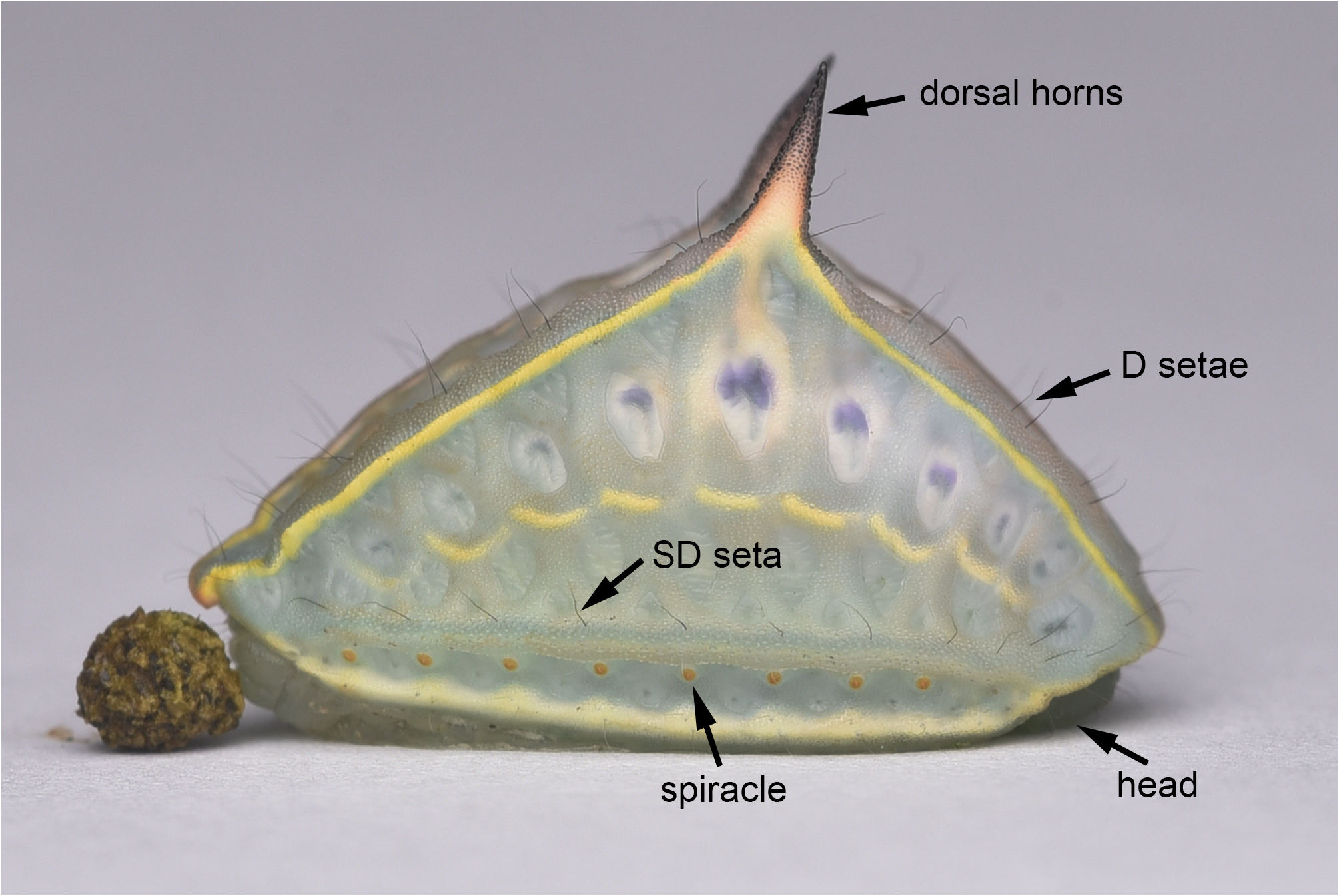
Immature stages ( Figs 12 View FIGURE 12 –27). Final instar larva ( Figs 12 View FIGURE 12 –15) 10 mm in length. Larva is of a non-stinging form, with two pairs of black D (= dorsal) setae and one black SD (= subdorsal) seta per segment on each side of the larva. Head dark brown, not visible from above. Ground colour creamy white dyed with light blue.
From lateral view, the body is triangular in shape, bears a pair of large dorsal horns that are pink to black, robust, pointed apically, and conical-shaped. Lateral side of body with an arc-curved, broken, pale yellow medial line, and eight orange spiracles arranged in a line located above lower margin; upper margin of lateral body is bright yellow whereas lower margin bright white dyed with pale yellow. Dorsal side rhombohedral, with an interrupted white medial line throughout the whole body; two deep blue dots spread out on both sides of white medial line at each segment.
Cocoon hard and oval-shaped, greyish white with diffused ochreous, sparsely distributed with several ochreous patches (Fig. 23).
Biology. Two larvae were collected on Castanopsis tibetana Hance (Fagaceae) on 11.VII.2020, and on Quercus sp. (Fagaceae) on 12.IX.2021, then reared with the samples of its host plants in the laboratory. The larva from Castanopsis tibetana Hance began cocooning on 14.VII.2020, emerged on 6.VIII.2020, as a male adult that later served as the holotype of this species; the cocoon period lasts approximately 23 days. The second larva reared on Quercus sp. was collected at the same locality as the holotype. We presume that this is a 4 th instar larva (Fig. 16) and possibly the second generation of the year. But unfortunately, the pupation was unsuccessful.
The larvae feed on the underside of the leaf of the family Fagaceae , starting from one side of the apex of the leaf and feeding on the leaf in a line perpendicular to the midrib, turning when they encounter an older midrib (Figs 16, 17). The soft underbelly is completely covered by the hard dorsal surface when larvae sense attack on a flat surface. When resting on the curved leaves or branches, the body is more tightly wrapped by the hard dorsum on either side and making the underbelly unexposed.
When disturbed (e.g. touching its body with a finger or brush), the larvae defend themselves from potential predators by rapidly swinging the body from side to side and scraping the leaves vigorously with the mandibles to make a fricative sound. It will continue for several minutes (the related videos are available online in the Suppl. Videos S1, S2). If the stimulation does not continue, the amplitude and frequency of the swing will gradually diminish until it stops. We hypothesize that feeding on the underside of the leaf not only is effective in avoiding predators, but perhaps the louder scraping sound can be made than the sound on the smooth upper leaf surface. For example, when the larvae are on a glass petri dish they still swing back and forth but they do not make any sounds.
Before the larva builds a cocoon, the dorsal horn will bend inwards, and the dorsal cuticle will soften; the parts of the front and terminus of cuticle will strongly up-curve that will make the larva looking more oval. The larva makes a silk mat in the corner on which they will then make a cocoon around their entire body; the body is extremely tight to the inside of the cocoon, with almost no gaps; the constructing of cocoon is estimated to be completed within 3–4 hours (Figs 18–23). The cocoon is similar to descriptions of most limacodid species ( Holloway 1986; Epstein 1996), it is hard, oval, with a circular lid at one end after the adult crawls out (Figs 24, 25). The adult is very active after eclosion and flies wildly within the rearing box, resulting in rapid breakage of its forewings (Figs 26, 27).
Host plants. Fagaceae : Castanopsis tibetana Hance , Quercus sp.
Distribution. China: Zhejiang Province: Hangzhou.
Etymology. The specific name is named after the type locality in Zhejiang Province, China.
Molecular data. The holotype (GenBank accession number OL807620 View Materials ) and a larva (GenBank accession number OL807621 View Materials ) of the new species, were barcoded ( Hebert et al. 2003). The molecular marker of two sequences COI- 5P. Both sequences are of 662 base pairs corrected and aligned (see below) :
Pseudonagoda zhejiangensis sp. nov. (Adult, holotype, GenBank accession number OL807620 View Materials )
ATCTGAGCTGGAATAGTAGGAACATCTCTAAGATTAATAATTCGAGCTGAATTAGGAAATCCAGGTTCTTTAATTGGAGATGATCAAATTTATAATACTATTGTAACTGCTCATGCTTTTATTATAATTTTTTT - TATAGTTATACCTATTATAATTGGAGGATTTGGTAATTGATTAGTACCTTTAATATTAGGAGCTCC TGATATAGCTTTCCCACGAATAAATAATATAAGATTTTGATTATTACCCCCTTCTCTTATACTTTTAATTTCAAGAAGTATTGTAGAAAATGGGGCAGGAACAGGTTGAACAGTCTATCCTCCTTTATCTTCCAATATTGCACATAGTGGTAGATCAGTAGATTTAGCAATTTTTTCCCTTCATCTAGCGGGAATTTCTTCTATTTTAGGGGCTGTAAATTTCATTACCACTATTATTAATATACGACCTAATGGAATATCATTTGACCAAATACCTTTATTTGTTTGAGCAGTAGGAATTACTGCATTATTATTACTCCTTTCTTTACCAGTATTAGCCGGAGCTATTACTATATTATTAACTGATCGAAATTTAAATACTTCATTCTTTGATCCAGCTGGAGGAGGAGATCCTATTTTATATCAACATTTATTTTGATTTTTTGGTCACCCTGAAGTTTA
Pseudonagoda zhejiangensis sp. nov. (Larva, GenBank accession number OL807621 View Materials )
ATCTGAGCTGGAATAGTAGGAACATCTCTAAGATTAATAATTCGAGCTGAATTAGGAAATCCAGGTTCTTTAATTGGAGATGATCAAATTTATAATACTATTGTAACTGCTCATGCTTTTATTATAATTTTTTT - TATAGTTATACCTATTATAATTGGAGGATTTGGTAATTGATTAGTACCTTTAATATTAGGAGCTCC TGATATAGCTTTCCCACGAATAAATAATATAAGATTTTGATTATTACCCCCTTCTCTTATACTTTTAATTTCAAGAAGTATTGTAGAAAATGGGGCAGGAACAGGTTGAACAGTCTATCCTCCTTTATCTTCCAATATTGCACATAGTGGTAGATCAGTAGATTTAGCAATTTTTTCCCTTCATCTAGCGGGAATTTCTTCTATTTTAGGGGCTGTAAATTTCATTACCACTATTATTAATATACGACCTAATGGAATATCATTTGACCAAATACCTTTATTTGTTTGAGCAGTAGGAATTACTGCATTATTATTACTCCTTTCTTTACCAGTATTAGCCGGAGCTATTACTATATTATTAACTGATCGAAATTTAAATACTTCATTCTTTGATCCAGCTGGAGGAGGAGATCCTATTTTATATCAACATTTATTTTGATTTTTTGGTCACCCTGAAGTTTA
Remarks. The sequence with GenBank accession number OL807621 View Materials came from the larva (Fig. 15) that died during the rearing process. After death, the larva was stored in 100% alcohol and subsequently used for DNA extraction and amplification. To date, all three known species of this genus are represented by a single male holotype and, with the exception of this new species, molecular data and immature stages are lacking. In this article, the life history of P. zhejiangensis sp. nov. was recorded, as well as the DNA was extracted and sequenced. The barcode sequences were deposited in GenBank.
Mr.John Horstman once collected and reared a larva that successfully eclosed as a male adult in Pu'er City of Yunnan Province, China, and posted the photos online. Although we cannot be certain which species it is, we were able to identify it as belonging to the genus Pseudonagoda (https://twitter.com/sinobug/status/1090597104706248705). In addition, Ms. Marina Roth has also shared some photographs of the larvae of this genus from Shangrao City of Jiangxi Province on iNaturalist platform. The collection site is not far from the type locality of the new species P. zhejiangensis sp. nov., and we speculate that these samples could be probably conspecific (https://www.inaturalist. org/observations/104722689). Additional adult specimens from both localities are still needed to confirm the presence of P. zhejiangensis sp. nov. in Pu'er City of Yunnan Province and Shangrao City of Jiangxi Province, China. What is certain, is that the genus Pseudonagoda occurs in at least two other provinces in China besides Zhejiang.
Females of this genus remain unknown. Among the cup moths known as having transparent patchy wings females are recorded for the following genera Nagodopsis Matsumura, 1931 , Nagoda Moore, 1887 , and Cheromettia Moore, 1883 . All these three genera are sexually dimorphic. It seems that in Limacodidae the clearwing or with clear patches on the wing species tend to be sexually dimorphic (Dr. Marc E. Epstein, pers comm. 18 January 2022). Therefore, we presume that it is highly possible that species in the genus Pseudonagoda are also sexually dimorphic. Female characters can be better studied and recorded by collecting and rearing more larvae or pupae, so female adult specimens are obtained.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |