Centruroides chanae, Goodman & Prendini & Francke & Esposito, 2021
|
publication ID |
https://doi.org/10.1206/0003-0090.452.1.1 |
|
DOI |
https://doi.org/10.5281/zenodo.5745651 |
|
persistent identifier |
https://treatment.plazi.org/id/510E87A5-6D44-FFB0-242D-FBF5FC7FFBA2 |
|
treatment provided by |
Carolina |
|
scientific name |
Centruroides chanae |
| status |
sp. nov. |
Centruroides chanae View in CoL , sp. nov.
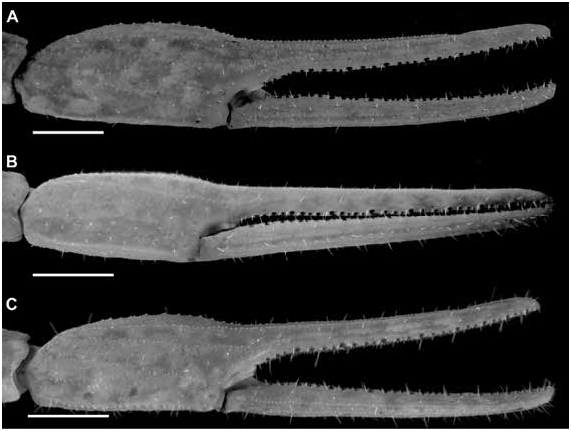
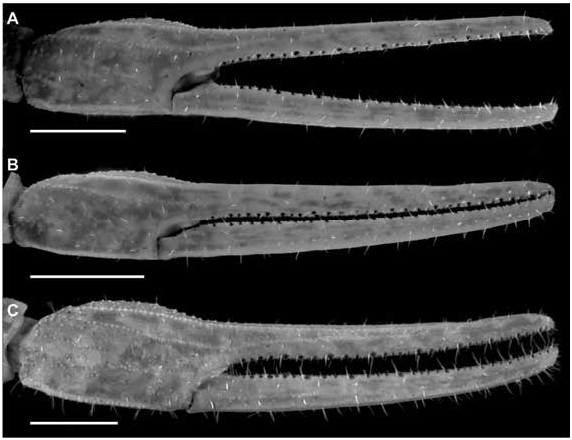
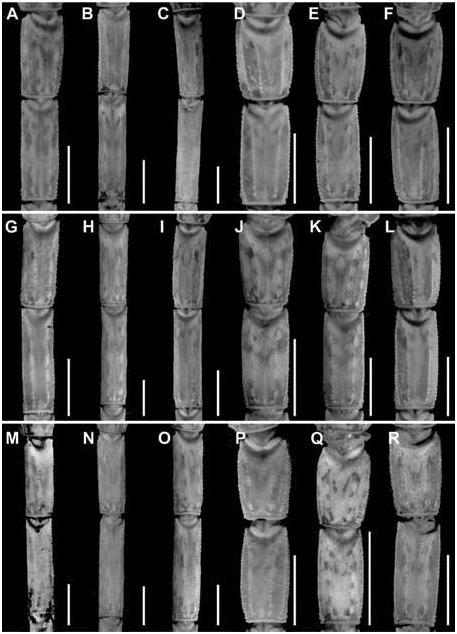
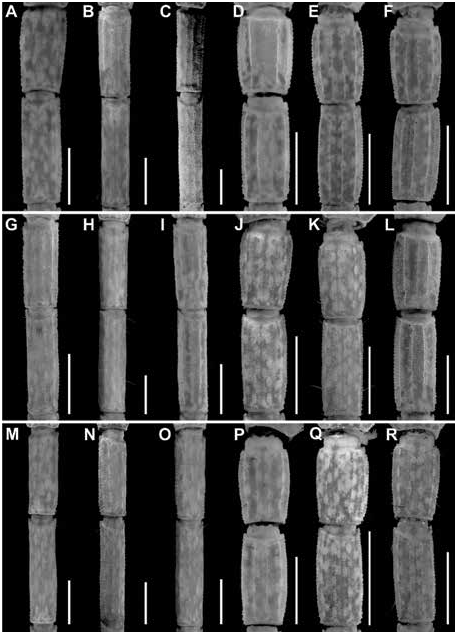
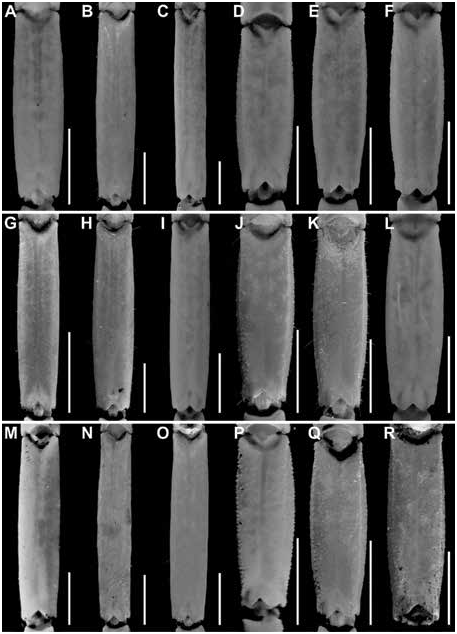
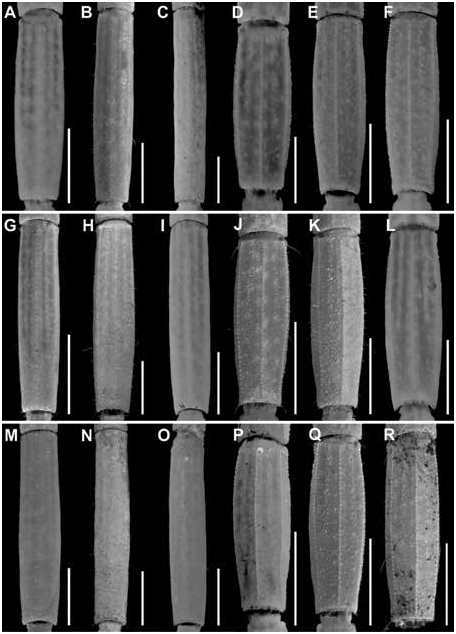
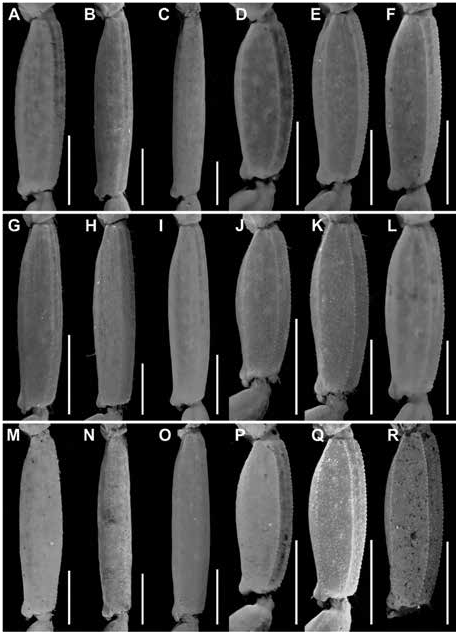
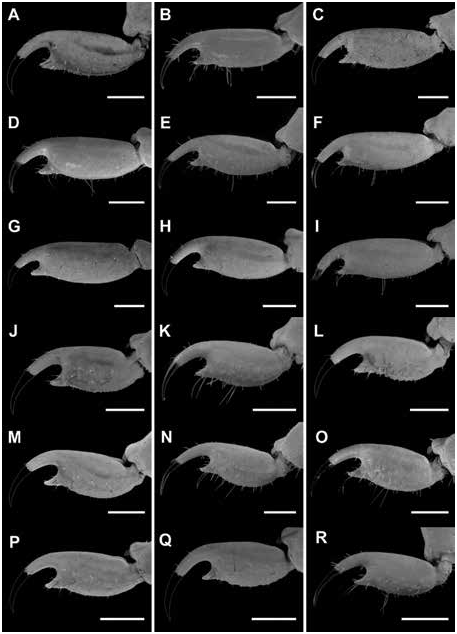
Figures 2 View FIGURE 2 , 3 View FIGURE 3 , 7C, D View FIGURE 7 , 10C, D View FIGURE 10 , 15B View FIGURE 15 , 16B View FIGURE 16 , 17I, L View FIGURE 17 ,
18I, L View FIGURE 18 , 19I, L View FIGURE 19 , 20I, L View FIGURE 20 , 21I, L View FIGURE 21 , 22I, L View FIGURE 22 , 23I, L View FIGURE 23 , 24I, L View FIGURE 24 , 25I, L View FIGURE 25 , 40,41, tables 1 View TABLE 1 , 9 View TABLE 9 , 10 View TABLE 10
TYPE MATERIAL: MEXICO: Guerrero: Município Copala: Holotype ♂ ( CNAN T01407 ), Microondas Fogos , 16°33′59.5″N 98°53′18.1″W, 14 m, 2.xi.2007, O.F. Francke, H. Montaño, and A. Ballesteros; GoogleMaps 2 ♂ paratypes (CNAN T01403, T01404), 2 ♀ paratypes (CNAN T01405, T01406), same data except: 103 m, 22.vi.2007, O.F. Francke, M. Escalante, H. Montaño, and J. Ballesteros.
ETYMOLOGY: The species name honors Kendra Chan, a friend of the first author, who passed away in 2018.
DIAGNOSIS: Centruroides chanae is most closely related to C. hoffmanni , from which it differs as follows. A dark line along the lateral margins of the carapace and mesosomal tergites I–III, and pale stripe medially on the carapace and tergites, present in C. chanae , are absent in C. hoffmanni (fig. 7A, D). The carapace, pedipalps, tergites, and metasoma are less infuscate, creating a less mottled appearance, in C. chanae than C. hoffmanni . More reticulate infuscation is present on the chelicerae of C. chanae than those of C. hoffmanni . The interocular triangle is less darkly infuscate in C. chanae than C. hoffmanni . The marbled infuscation of the mesosomal sternites is faint or absent in C. chanae but pronounced in C. hoffmanni . The carapace is shorter, its length and width similar, in C. chanae , but longer, its length greater than its width, in C. hoffmanni . The carapace surfaces are more finely granular, the carinae less developed and the sulci broader and shallower in C. chanae than C. hoffmanni . The pedipalp chela manus of the male is less incrassate in C. chanae (figs. 15, 16A) than C. hoffmanni (figs. 15, 16B). The ventral surfaces of the telotarsi of leg I are more coarsely and densely setose in C. chanae than C. hoffmanni . The pectinal tooth count of the male is higher in C. chanae , usually 17 (fig. 10A) than C. hoffmanni , usually 15 (fig. 10C, tables 8 View TABLE 8 , 9 View TABLE 9 ). The ventrolateral carinae of mesosomal sternite VII are distinct, granular and the ventrosubmedian carinae weakly developed, granular in C. chanae , whereas the ventrolateral carinae are granular, and the ventrosubmedian carinae weakly granular and restricted to the posterior half of the segment in C. hoffmanni . Although the metasomal segments of the male are shorter and broader in C. chanae than C. hoffmanni , the metasoma is more than 3× (up to 3.3×) the length of the mesosoma in C. chanae , but less than 3× its length in C. hoffmanni ( table 10 View TABLE 10 ). The ventrolateral and ventrosubmedian carinae of metasomal segments I–IV are less pronounced in C. chanae , being finely granular to subserrate on I–III and obsolete, smooth on IV (figs. 18I, L, 19I, L), compared with slightly serrate on I–IV in C. hoffmanni (fig. 18C, F, 19C, F). The ventrosubmedian carinae of metasomal segments I and II are absent or obsolete in C. chanae but very pronounced in C. hoffmanni . The telson of the male is shorter, the vesicle rounded posteriorly, in C. chanae (figs. 23–25I) whereas the telson is elongate and the vesicle bilobed posteriorly in C. hoffmanni (figs. 23–25C).
DESCRIPTION: The following description is based on the holotype male, with differences among other material noted in the section on variation.
Coloration: Base color light yellow, with extensive infuscation, creating mottled or marbled pattern. Carapace with uniformly infuscate marbling, more densely infuscate medially. Pedipalp chela fingers and manus, dorsal and retrolateral intercarinal surfaces with moderately infuscate marbling; prolateral and ventral intercarinal surfaces mostly immaculate. Legs retrolateral surfaces with infuscate marbling; prolateral surfaces pale, immaculate. Tergites with unformly infuscate mottling, pale stripe medially, blackish spots submedially, and faint, narrow bands laterally. Sternites pale, mostly immaculate. Metasomal segments uniformly marbled; segment V and telson markedly infuscate, noticeably darker than preceding segments.
Carapace: Shape trapezoidal; anterior width four-fifths of posterior width ( table 9 View TABLE 9 ); anteromedian sulcus moderately deep, oval; posteromedian sulcus shallow anteriorly, deep posteriorly; median ocular tubercle weakly granular; carinae moderately developed, comprising small to medium-sized granules; lateral ocular and posterosubmedian carinae distinct; intercarinal surfaces finely and evenly granular (fig. 7D).
Pedipalps: Orthobothriotaxic, Type A; femur dorsal trichobothria with α configuration; pedipalp chela fixed finger, trichobothrium db situated slightly distal to et. Femoral carinae strongly developed, serrate; retromedian carinae comprising spiniform granules; prolateral intercarinal surface with series of large spiniform granules. Patella carinae strongly developed, granular; prolateral intercarinal surface with five or six large, subspiniform granules. Chela manus dorsomedian and retrodorsal carinae complete, granular; prodorsal carina absent. Fixed finger, median denticle row comprising eight oblique subrows, each flanked by pro- and retrolateral supernumerary denticles. Movable finger, median denticle row with short terminal row comprising four denticles preceded by eight oblique subrows, each flanked by pro- and retrolateral supernumerary denticles.
Legs: Leg I length 1.79× greater than carapace length ( table 10 View TABLE 10 ). Telotarsi ventral surfaces densely covered with short setae; ungues markedly curved.
Pectines: Pectinal plate 1.65× wider than long; posterior margin distinctly rounded; pectinal tooth count 17/17 ( ♂) (fig. 10C, table 9 View TABLE 9 ).
Mesosoma: Tergites width similar to carapace posterior width; I and II slightly narrower ( table 9 View TABLE 9 ). Pretergites surfaces smooth to finely granular. Posttergites surfaces weakly granular; I–VI with dorsomedian carinae finely granular on I–III, absent on IV–VI; VII surface weakly granular, dorsomedian carina absent, dorsosubmedian and dorsolateral carinae smooth. Sternites III– VI, surfaces smooth; VII surface, ventrolateral and ventrosubmedian carinae smooth.
Metasoma: Metasoma length 3.1× mesosoma length ( table 9 View TABLE 9 ). Segments longer than wide; increasing in length posteriorly, segment V 2× length of I; carinae finely granular on segments I–III, smooth on IV, absent on V; intercarinal surfaces sparsely granular (figs. 17–22I).
Telson: Vesicle elongate, ovoid; ventral surface shallowly convex; ventromedian carina granular, terminating at subaculear tubercle; subaculear tubercle narrow and angular in lateral aspect, directed toward midpoint of aculeus. Aculeus angled ventrally at slightly less than 90° (fig. 25I).
Variation: Base coloration varies from light yellow to orange with considerable variation in infuscation of the carapace and mesosoma (figs. 40, 41A, B). Adult males and females differ as follows. The prodorsal carina of the pedipalp chela manus is absent, the pectinal tooth count higher (16 or 17), the mesosoma proportionally longer and slenderer, the metasoma up to 3× longer, with segment V markedly longer, and the telson more elongate, with the vesicle more rounded and smoother, in males (figs. 23I, L, 24I, L, 25I, L, table 9 View TABLE 9 ). The prodorsal carina is granular and restricted to the distal half of the chela manus, the tegument more densely infuscate, the pectinal plate produced into a rounded lobe posteriorly, which is punctate and slightly infuscate, the pectinal tooth count lower (13 or 14), and the telson shorter and narrower, with the vesicle surfaces weakly granular, in females (figs. 10C, D, 12B, C, 15–16B, 20I, L, 22I, L, 23I, L, 24I, L, 25I, L, table 9 View TABLE 9 ).
DISTRIBUTION: Centruroides chanae is endemic to the states of Guerrero and Michoacán, in southwestern Mexico. The known records extend from eastern Michoacán, near the border of Colima, to western Guerrero, south of the Sierra Madre del Sur and east of the Sierra Madre Occidental (fig. 3).
ECOLOGY: The localities at which C. chanae has been recorded range in altitude from 8 to 221 m. The habitat at these localities varies from low to medium-height deciduous tropical forest and savanna to mangroves and oaks near the coastline. Specimens from Microondas Fogos were collected on fence poles in rangeland at night. The habitat and habitus are consistent with the arboreal, corticolous ecomorphotype ( Prendini, 2001a).
MATERIAL EXAMINED: MEXICO: Guerrero: Município Copala: Microondas Fogos, 16°33′59.5″N 98°53′18.1″W, 103 m, 22.vi.2007, O.F. Francke, M. Escalante, H. Montaño, and A. Ballesteros, 1 ♂ (AMNH [LP 7032]), GoogleMaps 1 juv. ♂ (CNAN SC3983), 14 m, 2.xi.2007, O.F. Francke, H. Montaño, and A. Ballesteros, 3 ♂ (CNAN SC3978), 1 ♀ (AMNH [LP 8582]). Michoacán: Município Aquila: Faro de Bucerias, 18°21′08.3″N 103°30′20.9″W, 13 m, 10.iii.2002, J. Ponce, low deciduous forest, 1 juv. ♀ (CNAN SC3982), GoogleMaps 13.iv.2002, J. Ponce, low deciduous forest, 2 ♂ (CNAN SC3979, SC3980), 3 ♂ (CNAN SC4005), 18°35′50.5″N 103°30′04.3″W, 221 m, 14.iv.2002, J. Ponce and E. González, low deciduous forest, 1 ♂ (AMNH [LP 2009]). GoogleMaps Município Aquila: La Llorona, el Faro, 18°20′17.2″N 103°29′49.2″W, 8 m, 6.v.2000, E. Miranda, beach gap, 1 ♂ (CNAN SC3981). GoogleMaps
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
SubFamily |
Centruroidinae |
|
Genus |