Phaeolus sharmae Hembrom, A. Parihar, K. Das & A. Ghosh, 2022
|
publication ID |
https://doi.org/10.5252/cryptogamie-mycologie2022v43a2 |
|
DOI |
https://doi.org/10.5281/zenodo.7828933 |
|
persistent identifier |
https://treatment.plazi.org/id/511E879F-FFF7-F041-A51C-FC981A3AFD5F |
|
treatment provided by |
Felipe |
|
scientific name |
Phaeolus sharmae Hembrom, A. Parihar, K. Das & A. Ghosh |
| status |
sp. nov. |
117. Phaeolus sharmae Hembrom, A. Parihar, K. Das & A. Ghosh , sp. nov.
( Figs 13-15 View FIG View FIG View FIG )
DIAGNOSIS. — Differs from other Phaeolus by its habitat as it grows in the upper part of its host tree Abies densa Griff. at high altitude in the Himalayas, also by its basidiomata with pinkish orange tainted hymenophore when young, duplex context, larger basidia (16- 53 × 7-12 µm) and basidiospores (6-11 × 6-7.8 µm).
HOLOTYPE. — India. Sikkim, North district, Yumthang valley Shingba Rhododendron sanctuary, attached to the bark of a living tree trunk of A. densa Griff. , 3470 m, 27°46’53.2”N, 88°42’34.8”E, 19.VII.2019, K. Das & M. E. Hembrom, KMA-19-014 (holo-, CAL [ CAL1843 About CAL ]!). GoogleMaps
MYCOBANK. — MB840191.
GENBANK. — MT762941 View Materials (nrITS, holotype), MT762940 View Materials (nrITS, paratype); MT764209 View Materials (nrLSU, holotype), MT764236 View Materials (nrLSU, paratype).
ETYMOLOGY. — Named in honour of J. R. Sharma for his contribution to Indian macrofungi.
ADDITIONAL MATERIAL STUDIED. — India. Sikkim, North district, Dombang valley, on living tree trunk of A. densa Griff. attached to bark, 3540 m, 27°46’06.2”N, 88°48’21.3”E, 20.VII.2019, K. Das, M. E. Hembrom & A. Parihar, KMA-19-026 (CAL 1844).
DESCRIPTION
Basidiomata
Annual, lignicolous, narrowly and loosely attached to host, single or imbricate, up to 100 mm broad, 150 mm wide and 20-50 mm thick, spongiose watery to leathery and heavy when fresh, rigid to brittle and lightweight when dry.
Pileus
70-190 × 70-320 mm, 8-20 mm thick near base, sessile, spathulate to applanate when young, then gradually becoming semicircular to almost dimidiate; upper surface covered with dense hispid hairs forming a thick tomentum in actively growing regions, glabrous and rough in older parts, concentrically zonate, weakly sulcate, mustard yellow to olive yellow (3B6-C7) when young, turning light brown to brown (7D5- E6) when mature; finally, becoming pale reddish brown to blackish with age.
Margin
Sterile, up to 3 mm wide, acute to obtuse, entire to more or less undulating, sometimes forming narrow lobes, distinctly incurved when dry, lemon yellow or yellowish when actively growing, turning concolorous to pileus surface at maturity.
Hymenophore
Poroid to irpicoid to often daedaleoid near base; pores 1-2 per mm, often widening up to 3-4 mm in mature parts while staying minute towards pileus margin, glancing, pinkish orange to ochraceous orange when young, then gradually changing into almost yellowish brown to sulphur yellow, finally becoming darker coffee brown with age, turning charcoal black when bruised.
Context
5-10 mm wide, divided in a compact lower and loose upper partthat are not separated by a black demarcation line, spongy to cheese-like when fresh, often fibrillose, becoming hard and brittle on drying, light brown to brown (7D5-E6) to dark reddish brown in the lower compact part, upper loose part and tomentum light brown (7D4-6).
Tubes
3-10 mm long, distinct from context, yellowish brown or concolorous with the context, brittle on drying, orange to dark blonde (5C5-D4) when young, then turning brown to dark brown (7E3-F4) when mature; dissepiments thin, entire to lacerate.
Hyphal system of context
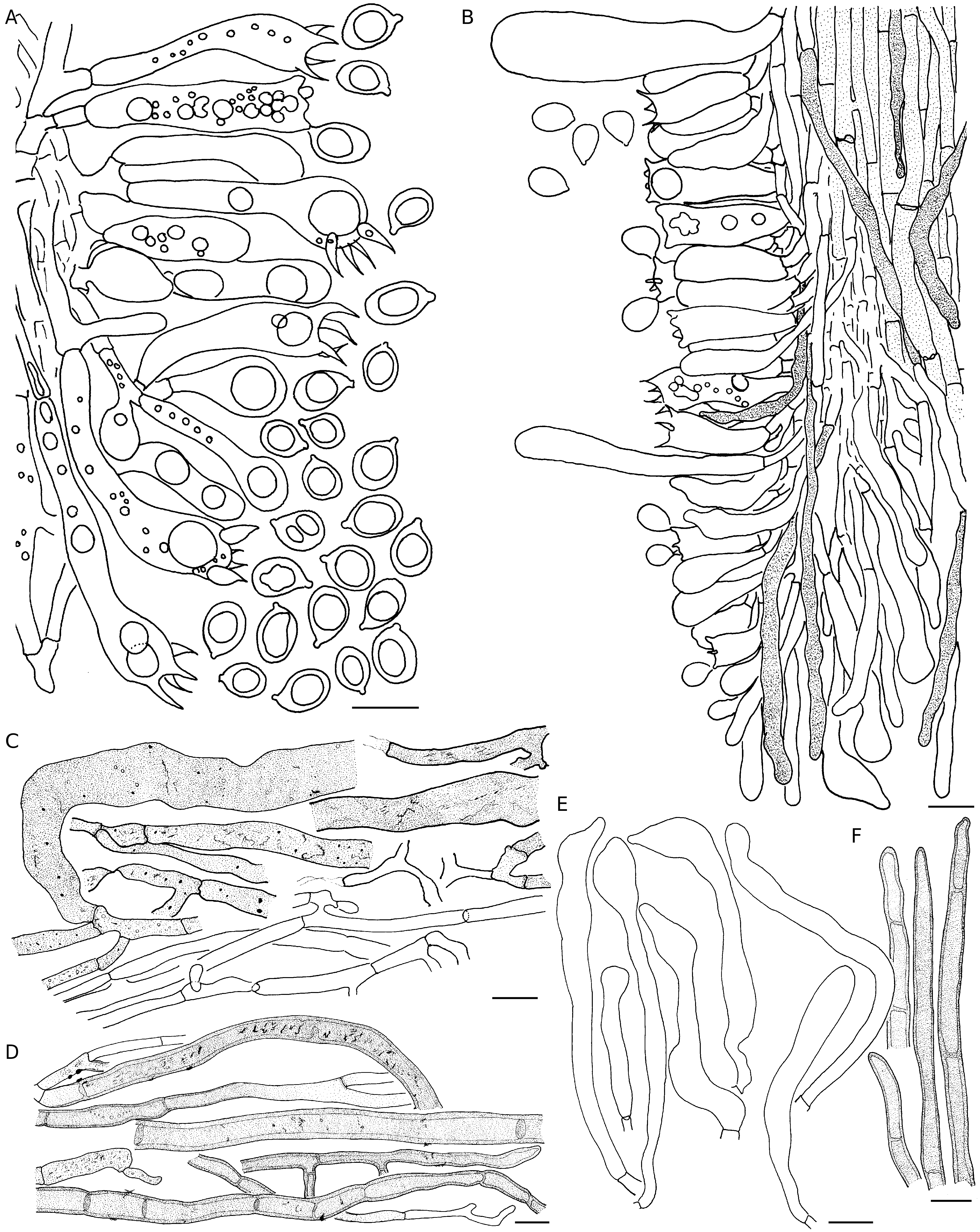
Monomitic, generative hyphae 3-15 µm wide, simple septate, frequently to occasionally branched, thin- to thick-walled (<1.5 µm), hyaline or pale yellowish to dark brownish, becoming collapsed when old; walls smooth or sometimes with crystal deposits.
Hymenophoral trama
Composed of parallel and compactly arranged, thin- (mostly) to moderately thick-walled generative hyphae mixed with submerged gloeocystidial hyphae; generative hyphae 2-6 µm wide; submerged gloeocystidial hyphae 40-105 × 4-10 µm, septate, unbranched (mostly) to rarely branched, thin-walled, smooth, pale coffee brown to dark brown, filled with dense cytoplasmic contents.
Hymenial gloeocystidia
Measuring 10-105 × 4-15 µm, clavate to cylindrical, irregularly capitate, thin- to moderately thick-walled, smooth, projected up to 55 µm beyond hymenial layer, filled with dense pale yellowish contents before becoming empty in older specimens.
Basidia
16-53 × 7-12 µm, clavate to pedicellate-clavate, thin-walled, smooth, 4-spored; sterigmata 6-8 µm long, hyaline.
Basidiospores
6-(8.97)-11 × 6-(6.75)-7.8 µm, Q = 1-(1.32)-1.57, ellipsoid to ovoid, thin-walled, smooth, distinctly apiculate, hyaline, acyanophilic, inamyloid.
NOTES
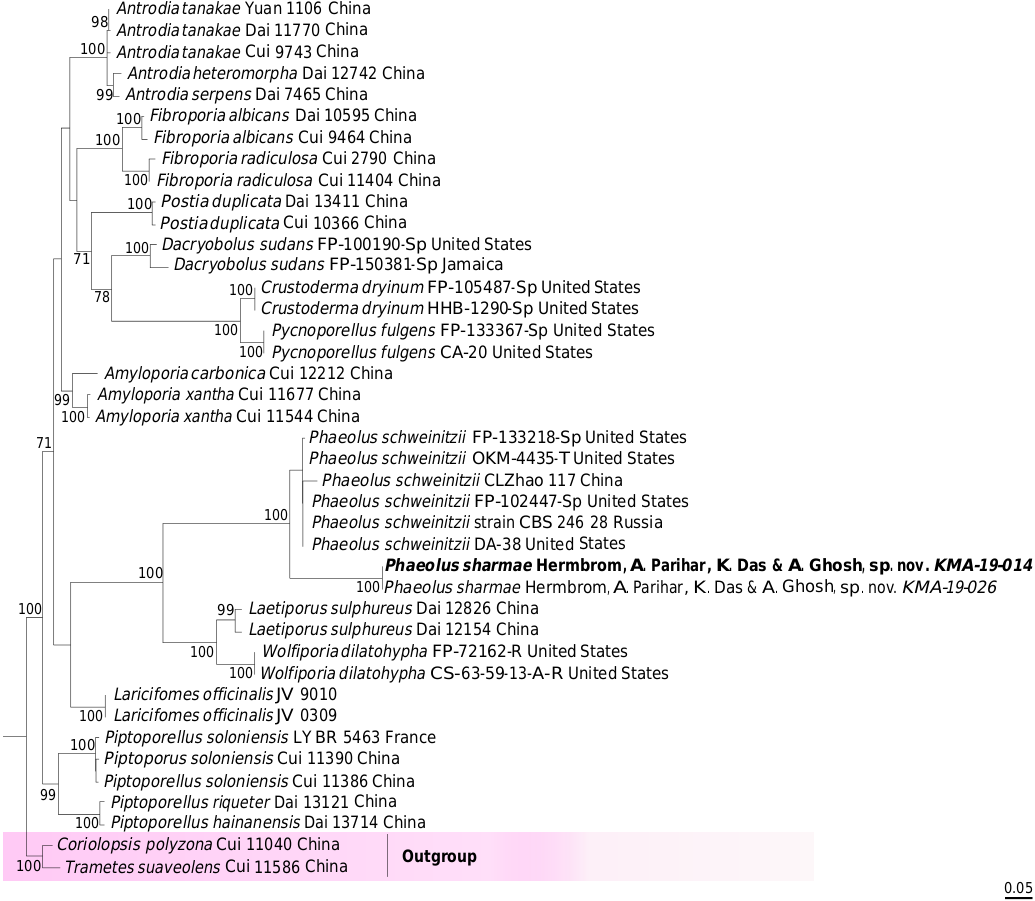
During fungal forays to the North district of Sikkim in 2018 and 2019, three of us (KD, MEH and AP) repeatedly came across populations of an unknown species growing on bark of standing trees of Abies densa . This species is quite distinct based on phylogenetic analyses including obtained ITS & LSU sequences that place it sister to Phaeolus schweinitzii , a species widely distributed in the northern hemisphere ( Gilbertson & Ryvarden 1987; Ryvarden & Gilbertson 1994; Núñez & Ryvarden 2001; Sharma 2012; Prasher 2015).
Within Polyporales , species of Phaeolus (Pat.) Pat. are easily confused with various xanthochoric polypores but the genus is phylogenetically distinct and causes a brown rot. Within family Laetiporaceae , Phaeolus can be separated from Laetiporus Murrill and Wolfiporia Ryvarden & Gilb. because these lack gloeoplerous elements. Also Inonotus hispidus (Bull.) P. Karst. , which lacks hymenial setae and forms lightweight, brittle basidiocarps with a strongly hispid pileus surface and large hymenial pores, may resemble our species in the field. Yet, it equally lacks gloeoplerous elements in context and hymenium.
Phaeolus harbours six species, half of these described by Patouillard, from which P. sharmae sp. nov. can be distinguished by its combination of having broadly attached basidiomata with rough pilear surface forming irregular papillae, a shiny pinkish orange young hymenophore and larger basidia and basidiospores. Berkley’s (1845), Léveillé’s (1844) and Patouillard’s (1900) descriptions for P. tabulaeformis (Berk.) Pat. , P. javanicus (Pat.) Henn. , and the description of P. rigidus (Lév.) Pat. lack microscopic details to compare these with our species. Moreover, P. tabulaeformis has been considered as synonym of P. schweinitzii ( Overholts 1953; Bakshi 1971). The African Phaeolus manihotis R. Heim has stipitate (6-7 × 3-4 mm) basidiomata and minute basidia (11-14 × 6-8 µm) and smaller spores (5.5-7 × 3.2-4.3µm) ( Heim 1931). The medium sized (up to 60 × 50 × 10 mm), laterally stipitate (40 × 20 mm) basidiomata with whitish yellow context and smaller basidiospores (5-6 × 4-4.3 µm) of P. amazonica M. A. De Jesus & Ryvarden ( De Jesus & Ryvarden 2010) separate it from our novel species, while P. subbulbipes (Henn.) O. Fidalgo & M. Fidalgo possesses much smaller spores (3.5-4 µm).
In our combined (nrITS+nrLSU) phylogenetic analysis ( Fig. 1 View FIG ), our species appeared as sister to the American, European and Asian samples of P. schweinitzii (Fr.) Pat. But P. sharmae sp. nov. always occupies upper parts of living tree trunks and branches rather than growing on the ground or on bases of trees as found in P. schweinitzii ( Overholts 1953; Gilbertson & Ryvarden 1987; Zhao & Zhang 1992; Sharma 2012). The distinctly shiny pinkish orange hymenophore that changes on bruising, observed in young specimens of our species, is also worth mentioning, along with its non-decurrent tubes attached to a duplex context, thus clearly distinguishing it from P. schweinitzii ( Overholts 1953; Ryvarden & Gilbertson 1994; Sharma 2012; Ryvarden & Melo 2014) where context is homogeneous and continuous with tube layer. Microscopically, the larger basidiospores (6-11 × 6-7.8 µm) and basidia (16-53 × 7-12 µm) distinguish our species from P.schweinitzii (usually with spores 5.5-9 × 2-5.6 µm and basidia 20-30 × 6-8 µm) known from India and abroad ( Overholts 1953; Bakshi 1971; Ryvarden & Johansen 1980; Gilbertson & Ryvarden 1987; Zhao & Zhang 1992; Ryvarden & Gilbertson 1994; Sharma 2012; Ryvarden & Melo 2014). Another Indian report of P. schweinitzii made by Prasher (2015) from Shimla Himachal Pradesh should be recollected and re-examined under the light of phylogenetic estimations as sizes of basidiospores (6-11.5 × 4-6.8 µm) and clavate basidia (12.4-15.3 × 5-6.8 µm) are deviating from report of similar kind of standard Indian and extralimital materials ( Overholts 1953; Bakshi 1971; Ryvarden & Johansen 1980; Gilbertson & Ryvarden 1987; Zhao & Zhang 1992; Ryvarden & Gilbertson 1994; Sharma 2012; Ryvarden & Melo 2014).
| K |
Royal Botanic Gardens |
| M |
Botanische Staatssammlung München |
| E |
Royal Botanic Garden Edinburgh |
| CAL |
Botanical Survey of India |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |