Coella gloriae, Kajihara, 2020
|
publication ID |
https://doi.org/ 10.12782/specdiv.25.251 |
|
publication LSID |
lsid:zoobank.org:pub:718AB490-40FA-4C06-B862-FA64D4804FDE |
|
persistent identifier |
https://treatment.plazi.org/id/577E87FA-633E-FFF2-FEC8-58FDFDB6FDD6 |
|
treatment provided by |
Felipe |
|
scientific name |
Coella gloriae |
| status |
sp. nov. |
Suborder REPTANTIA View in CoL Infraorder EUREPTANTIA Family Coellidae Stiasny-Wijnhoff, 1936 Genus Coella Stiasny-Wijnhoff, 1936 Coella gloriae sp. nov. ( Figs 11–15 View Fig View Fig View Fig View Fig View Fig ; Table 4)
Reptantia View in CoL sp.: Kajihara et al. 2007: 127.
Polystilifera View in CoL sp.: Sundberg et al. 2016: Nembar0831.
Etymology. The specific name is dedicated to Dr Gloria Gomez-Delan (Cebu Technological University), a Filipino fisheries scientist, who helped my sampling trip in Cebu.
COI barcode. KU 840158 (513 bp; Sundberg et al. 2016) from the holotype .
Material examined. Holotype, PNM 4648, male, 6 series of serial transverse sections (99 slides in total): anteri- or end of body (13 slides), foregut region (19 slides), anterior intestinal region (22 slides), posterior intestinal region (13 slides), posterior end of body (7 slides), proboscis (25 slides). The specimen everted its proboscis during anaesthetization and was not completely relaxed while fixation, causing contraction to a certain degree.
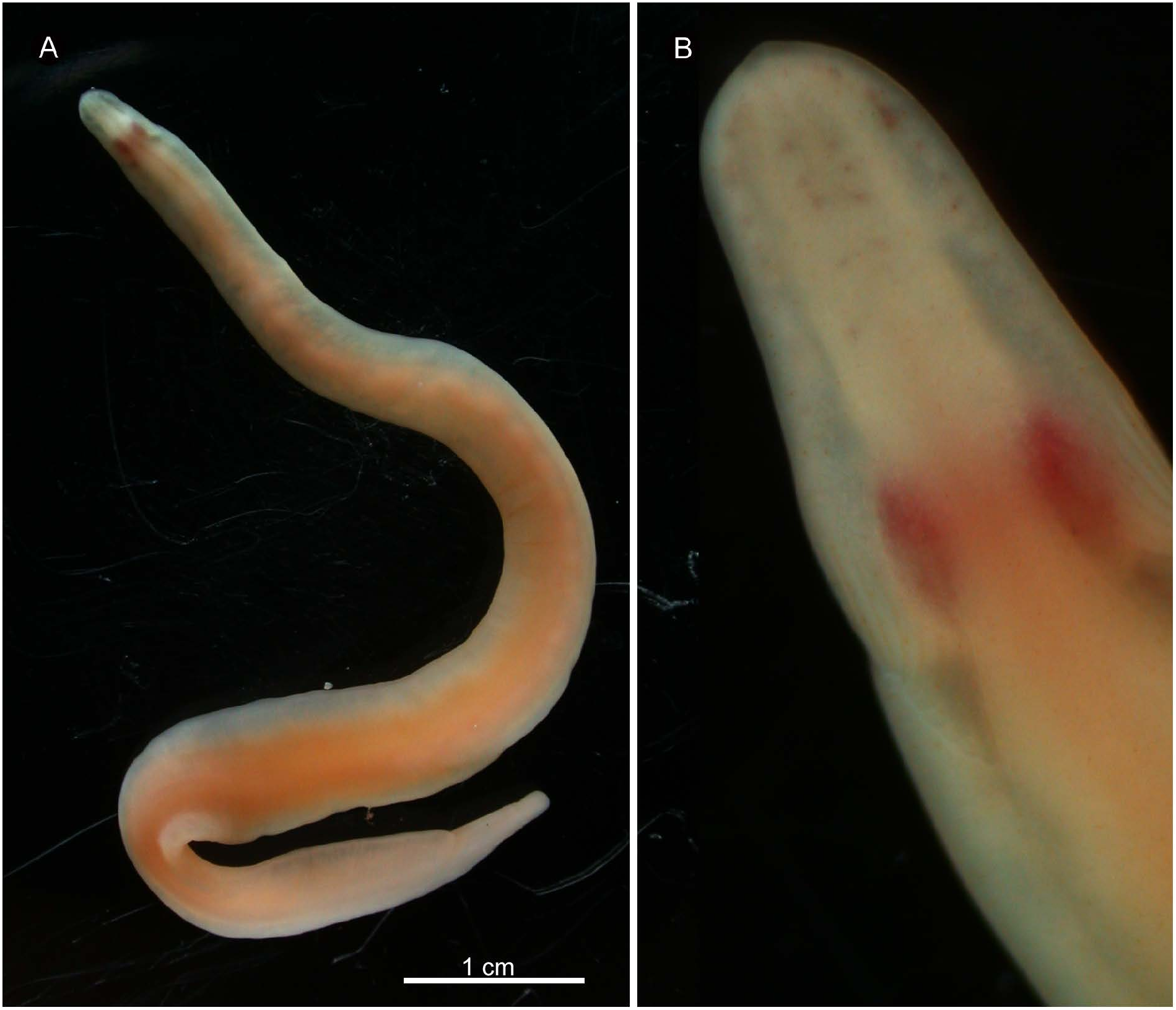
External features. Body about 5 cm long, 3.5 mm wide, with thin lateral margins, posteriorly rounded; pale orange in colour except cephalic region and body margins being more or less transparent ( Fig. 11A View Fig ). Head not wider than succeeding region of body, possessing mid-dorsal ridge; edges of ridge anteriorly connected at near tip of head, posteriorly curving latero-ventrally along body surface before turning forward on latero-ventral surface ( Fig. 11B View Fig ). Brain seen as pair of red antero-posteriorly elongated ellipses, situated in front of region where edges of mid-dorsal ridge starting to curve. Eyes arranged precerebrally in two rows on each side, median rows situated in mid-dorsal cephalic ridge; peripheral rows outside ridge. Cephalic furrows comprising primary and secondary furrows; primary, transverse furrow beginning from lateral portion at level near cerebral commissures running obliquely postero-mediad to middorsal ridge; numerous (~ 10 in number), secondary cephalic furrows running anteriorly or dorsally from primary furrows, not exceeding frontal end of brain. Cerebral sensory organs visible as dark hue immediately posterior to brain.
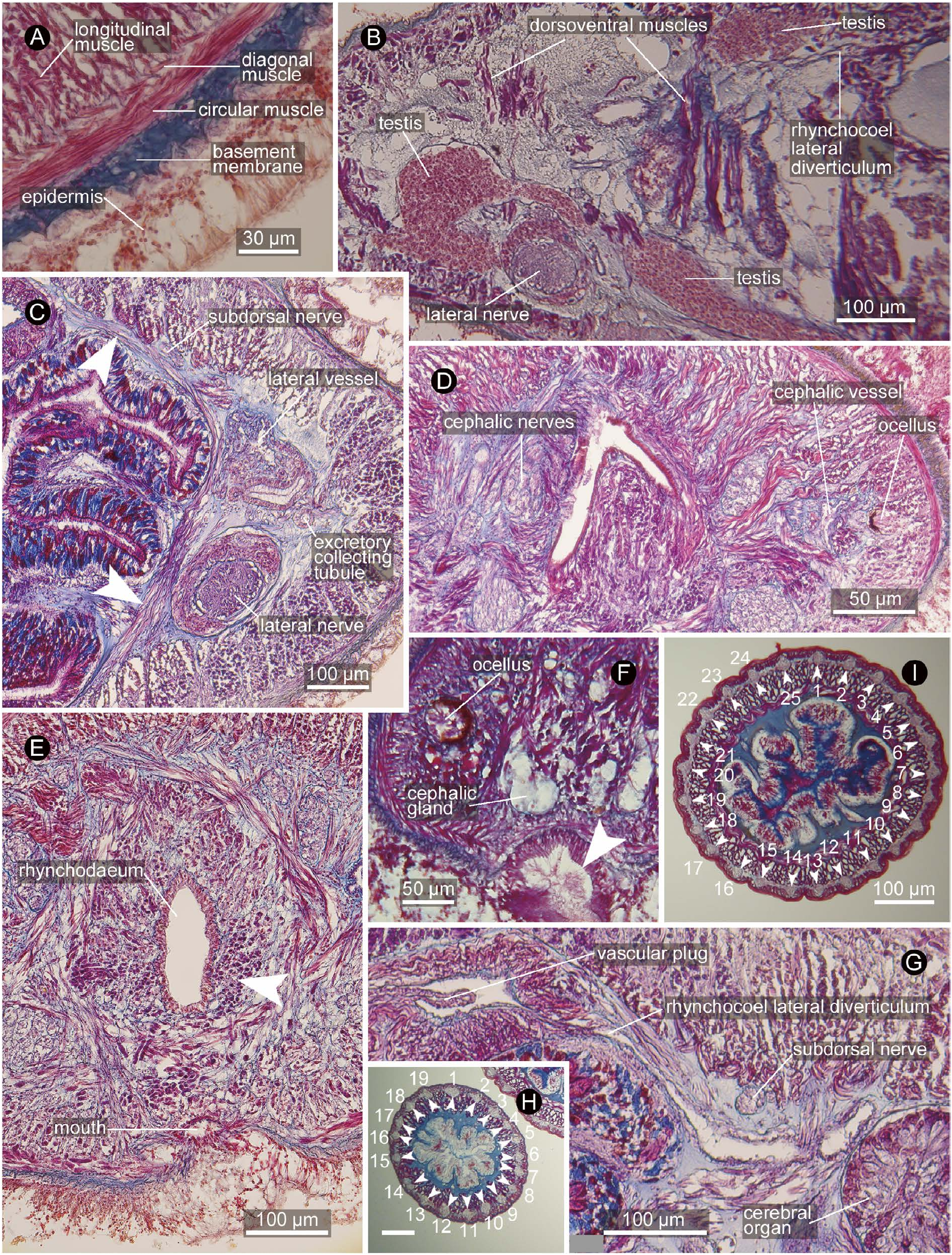
Body wall and musculature. In foregut region, ciliated epidermis 40–60 µm thick, connective tissue basement membrane 10–20 µm thick, body-wall outer circular muscle layer 4–6 fibres thick, diagonal muscle layer, and body-wall longitudinal muscle layer ( Fig. 12A View Fig ). Dorsoventral muscles present, running both medial and lateral sides of lateral nerve cord ( Fig. 12B View Fig ); in foregut region, medial ones appearing as if comprising incomplete inner circular muscle ‘layer’ ( Fig. 12C View Fig ).
Just in front of brain, fibres from body-wall longitudinal muscle layer partially turning inward to form proboscis insertion ( Fig. 12D View Fig ); rest of fibres running further anteriorly around rhynchodaeum as peri-rhynchodaeal longitudinal muscle layer ( Fig. 12E View Fig ), eventually diffusing near extreme tip of head. Anterior to proboscis insertion, precerebral radial muscles present, comprised of numerous bundles of fibres from body-wall longitudinal muscle layer crosssectionally giving out from one side of body to another in various angles, medially abutting peri-rhynchodaeal longitudinal muscle layer ( Fig. 12E View Fig ).
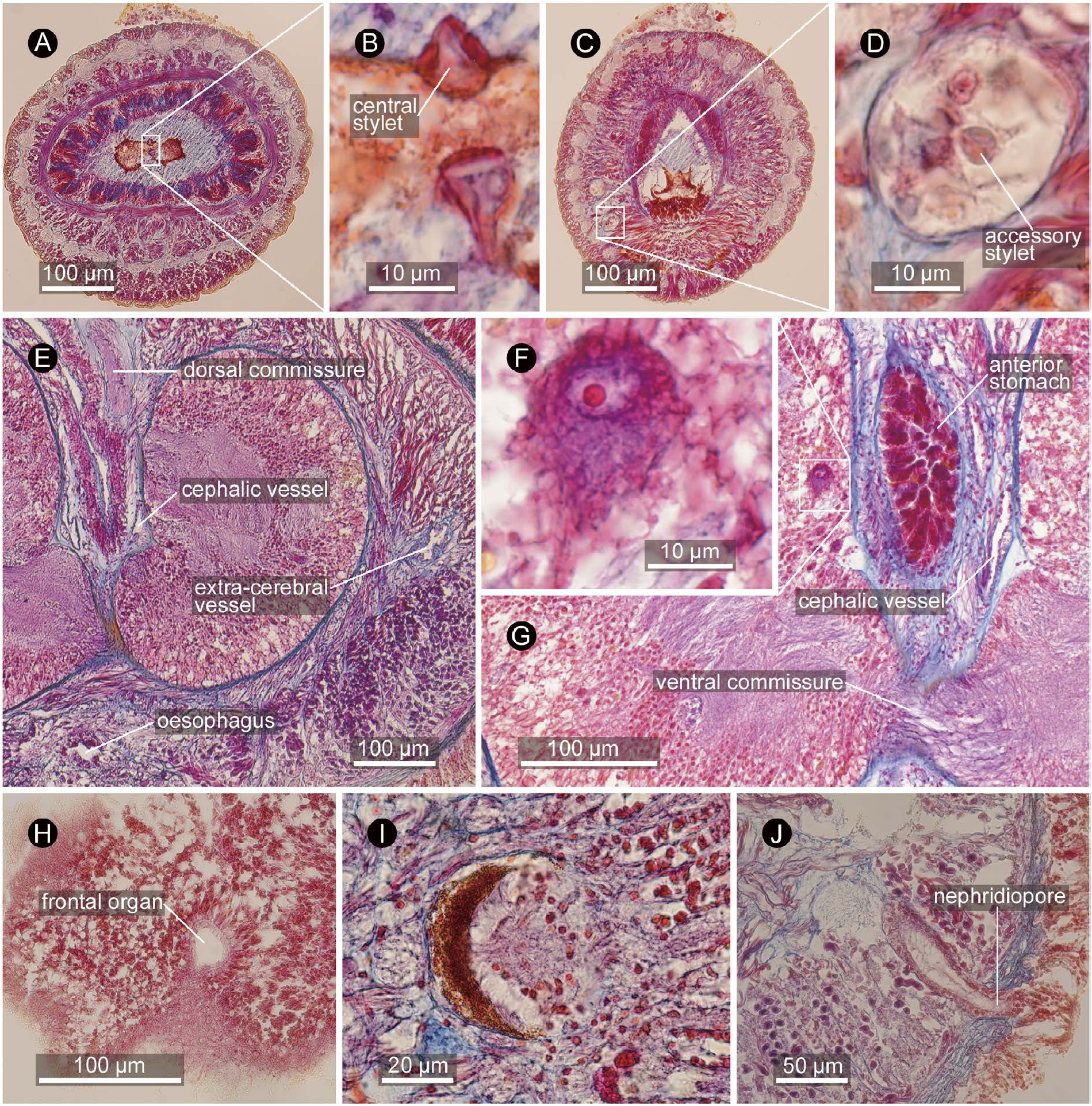
Proboscis apparatus. Proboscis detached from body during fixation. Proboscis pore situated subterminally, independent from mouth opening ( Fig. 12F View Fig ). Rhynchodaeal wall unciliated except its extreme anterior end, composed of cells with acidophilic cytoplasm. Proboscis insertion just in front of brain ( Fig. 12D View Fig ). Rhynchocoel composed of interwoven longitudinal and circular muscle fibres, extending to posterior end of body, with thin-walled lateral diverticula ( Fig. 12B, G View Fig ) arranged pseudometamerically; anterior-most diverticula found just posterior to brain near cerebral organ ( Fig. 12G View Fig ). Proboscis anterior chamber composed of inner glandular epithelium, inner circular, middle longitudinal, and outer circular muscle layers; proboscis nerves anteriorly 19, posteriorly 25 (mostly 24) in number, embedded in longitudinal muscle layer ( Fig. 12H, I View Fig ). Middle chamber with sickle-shaped basis spanning for 43 serial sections (=344 µm) in length, about 100 µm in maximum width ( Fig. 13A View Fig ); central stylets up to 15 µm long ( Fig. 13B View Fig ); at least 10 central stylets confirmed from serial sections, arranged along median line of basis; 10 accessory-stylet pouches on each side of middle chamber, each pouch containing about eight accessory stylets ( Fig. 13D View Fig ). Posterior chamber composed of outer longitudinal and inner circular muscle layers, filled with basophilic contents.
Alimentary system. Mouth opening ventrally ( Fig. 12E View Fig ), 400 µm posterior to proboscis pore, about 300 µm ahead of dorsal cerebral commissure, leading to oesophagus, about 100 µm in diameter, passing below ventral cerebral commissure to connect to stomach. Anterior stomach wall acidophilic, posterior basophilic; in holotype, anterior stomach pushed forward into brain ring, possibly as a result of contraction during fixation ( Fig. 13G View Fig ). Pylorus opening to dorsal wall of intestinal caecum. Intestine, as well as intestinal caecum, possessing numerous lateral diverticula.
Blood system. Pair of cephalic vessels mid-ventrally meeting above rhynchodaeum, posteriorly running between precerebral radial muscles. Each cephalic vessel bifurcating into medial and lateral branches at level of proboscis insertion; lateral branch posteriorly continues as extracerebral vessel between brain and body-wall longitudinal muscle layer ( Fig. 13E View Fig ) before eventually ending blindly at level of cerebral-organ-canal opening; medial branch on both sides meeting medially above ventral cerebral commissure below rhynchocoel, from where three post-cerebral vessels (one mid-dorsal and two lateral vessels) leading posteriorly. Soon after its origin, mid-dorsal vessel penetrating rhynchocoel to form vascular plug ( Fig. 12G View Fig ), exposed to rhynchocoel lumen to run backward for 600 µm before descending below rhynchocoel wall above alimentary canal to extend for the rest of body. Lateral vessel tangling with excretory collecting tubules above lateral nerve cord in anterior stomach region. Pseudometameric transverse connectives between lateral and mid-dorsal vessels present in intestinal region. Blood corpuscles not found.
Nervous system. Brain comprised of dorsal and ventral ganglia on each side, covered with outer neurilemma, but no inner neurilemma separating outer neuronal layer and inner fibrous core; dorsal ganglion not forked posteriorly; single neurochord cell present on each side, about 20 µm in diameter, situated medially to posterior end of dorsal cerebral fibrous core ( Fig. 13F View Fig ). Ventral ganglion bending laterally at right angle, then curving posteriorly at right angle again to lead to lateral nerve ( Fig. 14A, B View Fig ). Lateral nerve with inner and outer neurilemma ( Fig. 12C View Fig ); longitudinal muscle bundles arranged just inside inner neurilemma. Single middorsal nerve originating from dorsal commissure, situated between basement membrane and body-wall outer circular muscle layer. Pair of subdorsal nerves originating from medial sides of dorsal ganglia and moving dorsolaterally along dorsal ganglia, then running posteriorly just beneath bodywall inner longitudinal muscle layer dorsolaterally to foregut ( Fig. 12C, G View Fig ).
Glandular system. Single frontal organ about 30 µm in diameter, 40 µm in length, situated subterminally ( Fig. 13H View Fig ), from which basophilic cephalic glands discharge. Cephalic glands poorly developed ( Fig. 12F View Fig ), situated inside bodywall longitudinal muscle layer anterior to level of mouth opening: basophilic lobules ~30 µm diameter extend posteriorly to level of mouth opening; acidophilic glandular masses situated latero-ventrally near proboscis pore.
Sensory system. Pigment-cup ocelli ( Fig. 13I View Fig ) up to 50 µm in diameter, arranged precerebrally in four rows (15–18 lateral and 5–6 dorsolateral on each side; about 40 in total), all restricted anterior to proboscis insertion, embedded just below body-wall longitudinal muscle layer.
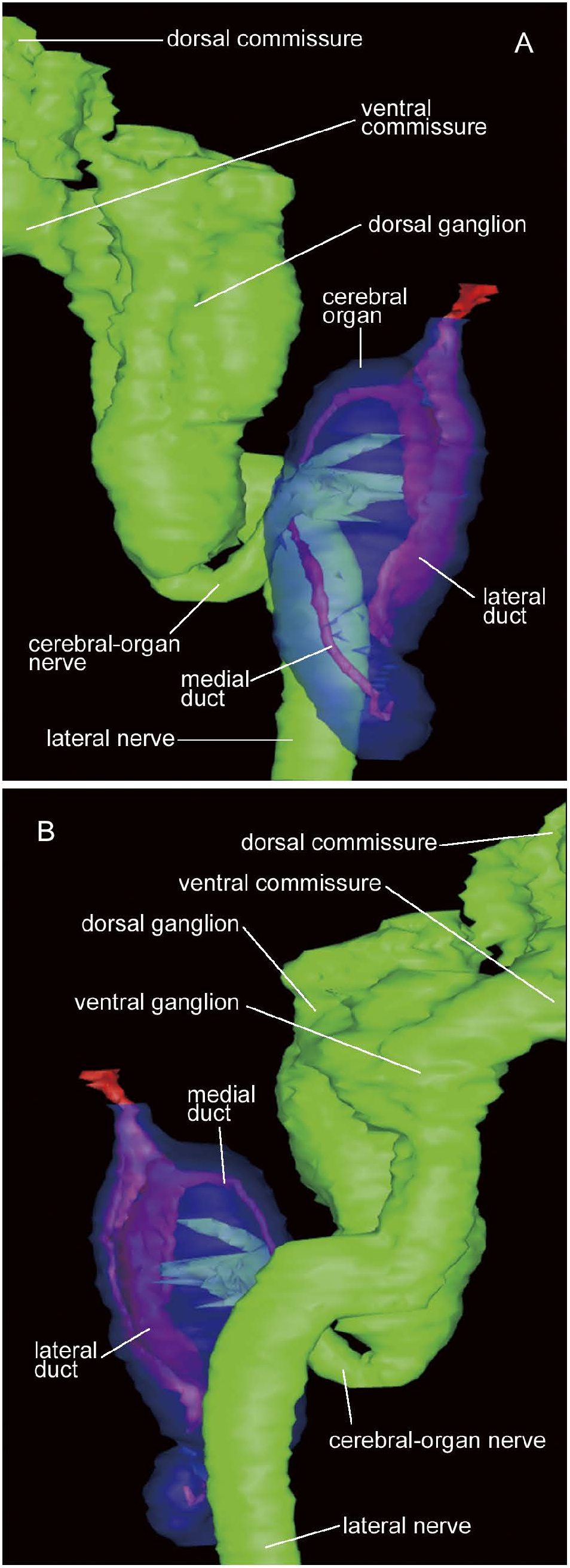
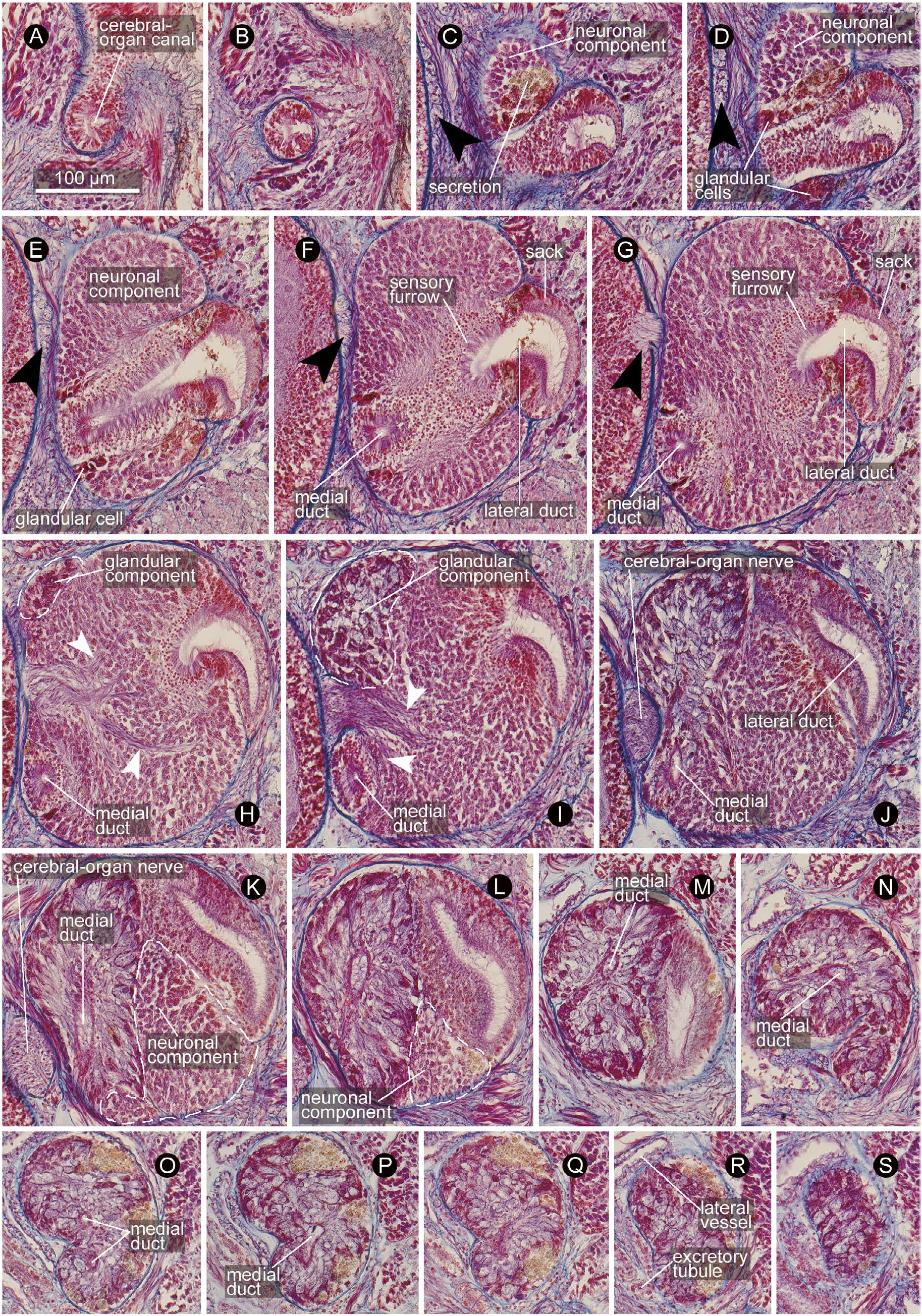
Cerebral organ comprised of i) canal, ii) neuronal component, and iii) glandular component. Canal opening dorso-laterally at primary transverse cephalic furrow (almost equivalent to level of ventral cerebral commissure), leading medio-posteriorly ( Fig. 15A–D View Fig ) to fuse with neuronal component, where it bifurcates into lateral and medial ducts ( Fig. 15E, F View Fig ); lateral duct wider than medial one; initially, lateral duct medially associated with ‘sensory furrow’ sensu Kirsteuer (1973) [thus the organ is of Aequifurcata sensu Stiasny-Wijnhoff (1926, 1936), as opposed to Inaequifurcata, where the lateral duct is not surrounded by sensory cells in any place and directly leads to sack] and laterally comprised of ‘sack’ sensu Kirsteuer (1973) lined with ciliated epithelium ( Fig. 15F–I View Fig ), posteriorly comprised entirely of sack-type epithelium ( Fig. 15J–M View Fig ), ending blindly without bifurcation; medial duct anteriorly lined with sensory cells ( Fig. 15F–I View Fig ), posteriorly bending upward after passing below insertion of cerebral-organ nerve ( Fig. 15J View Fig ) and fused with glandular component ( Fig. 15K View Fig ), slightly twisted ( Fig. 15O, P View Fig ) before terminating blindly. Neuronal and glandular components both antero-posteriorly accounting for about 60% of entire length of organ, overlapping for about 20%, where glandular component situated medio-dorsally, while neuronal component ventrally ( Fig. 15H–L View Fig ). Neuronal component innervated by single bundle (50 µm thick) of nerve fibres sent antero-laterally from posterior end of dorsal cerebral fibre core ( Figs 14A, B View Fig , 15H–K View Fig ). Glandular component posteriorly tapered ( Figs 14A, B View Fig , 15N–S View Fig ), in close contact with blood vessel and excretory collecting tubules ( Fig. 15R View Fig ).
Excretory system. Excretory collecting tubules ramified and convoluted, embedded in extracellular matrix around lateral blood vessels ( Fig. 12C View Fig ), distributed from rear end of cerebral sensory organ to anterior portion of intestinal caecum. Single pair of excretory ducts leading laterally above lateral nerve cord to single nephridiopore, opening at foregut region ( Fig. 13J View Fig ).
Reproductive system. The single specimen was immature male; testes arranged in single lateral row on each side. Each testis comprised of single dorsal and single ventral branches, each above and below intestinal lateral diverticulum, extending laterally to merge above lateral nerve cord, further extending laterally ( Fig. 12B View Fig ); testes thus assuming 90°-rotated Y-shape. Gonopore not found, but probably opening ventrolaterally.
Remarks. The benthic body form ( Fig. 11A, B View Fig ) and the minute central stylets mounted on a sickle-shaped basis ( Fig. 13A, B View Fig ) in the present Cebu specimen undoubtedly affiliate the species in the Reptantia within the Polystilifera . However, its generic placement had to be admittedly arbitrary because no synapomorphy is apparent as to all the currently known ~15 genera in Reptantia ( Härlin and Sundberg 1995; Härlin and Härlin 2001). At the moment, the reptantian systematics is not based on molecular data. Of the reptantian sequences currently available in public databases, those that are tagged to species names pertain to only two species in two genera: Drepanophorus spectabilis (Quatrefages, 1846) and Paradrepanophorus crassus (Quatrefages, 1846) ( Andrade et al. 2012; Beckers et al. unpubl.); others are left unidentified, such as “Reptant nemertean sp.” ( Thollesson and Norenburg 2003), “ Polystilifera sp.” ( Andrade et al. 2012; Sundberg et al. 2016), and “ Reptantia sp.” ( Kvist et al. 2014).
I included the present Cebu species in the genus Coella on the basis of an a priori assumption that vascular-system morphology would have greater phylogenetic significance than some other characters; needless to say, however, this assumption should be tested in future studies. In addition to Coella gloriae , seven other species of Reptantia are reported to possess extracerebral vessels that branch off from the cephalic vessels and run backward outside the cerebral ring before terminating blindly without turning forward or making a loop: these are Coella tiurensis Stiasny-Wijnhoff, 1936 ; Drepanophorina guineensis Stiasny-Wijnhoff, 1936 ; Drepanophorina savuensis Stiasny-Wijnhoff, 1936 ; Drepanophoringia waingapuensis Stiasny-Wijnhoff, 1936 ; Punnettia maldivensis Stiasny-Wijnhoff, 1936 ; Punnettia micrommata Stiasny-Wijnhoff, 1936 ; and Xenonemertes rhamphocephalus Gibson, 1983 ( Stiasny-Wijnhoff 1936: text-fig. 75b, c; Gibson 1983: fig. 12B). Of these seven species, Coella tiurensis , Drepanophorina savuensis , and Drepanophoringia waingapuensis are the type species of the respective genera. Of these three, Coella gloriae shares with Coella tiurensis the greatest number—25 out of 40—of morphological characters that were utilized in a cladistic analysis by Härlin and Härlin (2001); it shares 23 with Drepanophorina savuensis and 18 with Drepanophoringia waingapuensis ( Table 4). Along with Coella tiurensis , Coella gloriae may also be equally similar to Drepanophorina guineensis by sharing the same number of characters ( Table 4). Therefore, Coella gloriae could have been placed in Drepanophorina Gibson, 1995 if the latter was a distinct genus from Coella . Should the two genera turn out to be synonymous, that genus must be referred to as Coella because it has nomenclatural precedence over Drepanophorina . Drepanophorina was established by Stiasny-Wijnhoff (1936: 60) for four nominal species— Drepanophorina argus Stiasny-Wijnhoff, 1936 ; Drepanophorina guineensis ; Drepanophorina savuensis ; and Drepanophorus latus Bürger, 1890 —without type-species fixation. The name Drepanophorina became nomenclaturally available when Gibson (1995: 357) fixed Drepanophorina savuensis as the type species.
Apart from the characters listed in Table 4, Coella gloriae also differs from the seven species by the following characteristics: from Coella tiurensis by the rhynchodaeal sphincter (present in C. gloriae ; absent in C. tiurensis ); from Drepanophorina guineensis by the direction of the innervation into the cerebral organ (laterally in C. gloriae ; from anterior backward in D. guineensis ); from Drepanophorina savuensis by the pigments in the outer portion of the epithelium (absent in C. gloriae ; present in D. savuensis ); from Drepanophoringia waingapuensis by the number of the eyes (~ 40 in C. gloriae ; ~ 100 in D. waingapuensis ) and by the innervation from the lateral nerve to the cerebral organ (absent in C. gloriae ; present in D. waingapuensis ); from Punnettia maldivensis by the number of the proboscis nerves (19–25 in C. gloriae ; 14 in P. maldivensis ); from Punnettia micrommata by the length of the cerebral organ relative to the ventral ganglion (longer in C. gloriae ; shorter in P. micrommata ) ( Stiasny-Wijnhoff 1936); and from Xenonemertes rhamphocephalus by the blood corpuscles (absent in C. gloriae ; present in X. rhamphocephalus ) ( Gibson 1983). Indeed, Coella gloriae is morphologically unique among all the known rep- tantians in that it has i) four rows of eyes, ii) the cephalic furrows, iii) no dorsal marking, iv) separate mouth and proboscis openings, v) blind-ending extracerebral vessels, vi) non-forked fibre core in the dorsal ganglia, vii) the subdorsal nerve, and viii) cerebral organs partly overlapping the brain ( Härlin and Sundberg 1995; Härlin and Härlin 2001).
“Reptant nemertean sp. 500” of Thollesson and Norenburg (2003) from Shirahama, Japan, would be congeneric with Coella gloriae , between which it shows 7.6% uncorrect- ed p -distance in terms of 513-bp COI sequence.
| KU |
Biodiversity Institute, University of Kansas |
| PNM |
Philippine National Museum |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Coella gloriae
| Kajihara, Hiroshi 2020 |
Reptantia
| Kajihara, H. & Olympia, M. & Yap, E. S. & Gomez-Delan, G. & Quilantang, M. B. & Shimomura, M. & Taniyama, S. & Asakawa, M. 2007: 127 |