Elaphoidella jaesornensis, Watiroyram, Santi, Brancelj, Anton & Sanoamuang, La-Orsri, 2015
|
publication ID |
https://doi.org/ 10.11646/zootaxa.3919.1.4 |
|
publication LSID |
lsid:zoobank.org:pub:81B81187-EC8F-42B6-BF74-2988A289B4C0 |
|
DOI |
https://doi.org/10.5281/zenodo.6115438 |
|
persistent identifier |
https://treatment.plazi.org/id/5A3587EF-E035-FFA7-7EEA-FD2B10343A4F |
|
treatment provided by |
Plazi |
|
scientific name |
Elaphoidella jaesornensis |
| status |
sp. nov. |
Elaphoidella jaesornensis sp. nov.
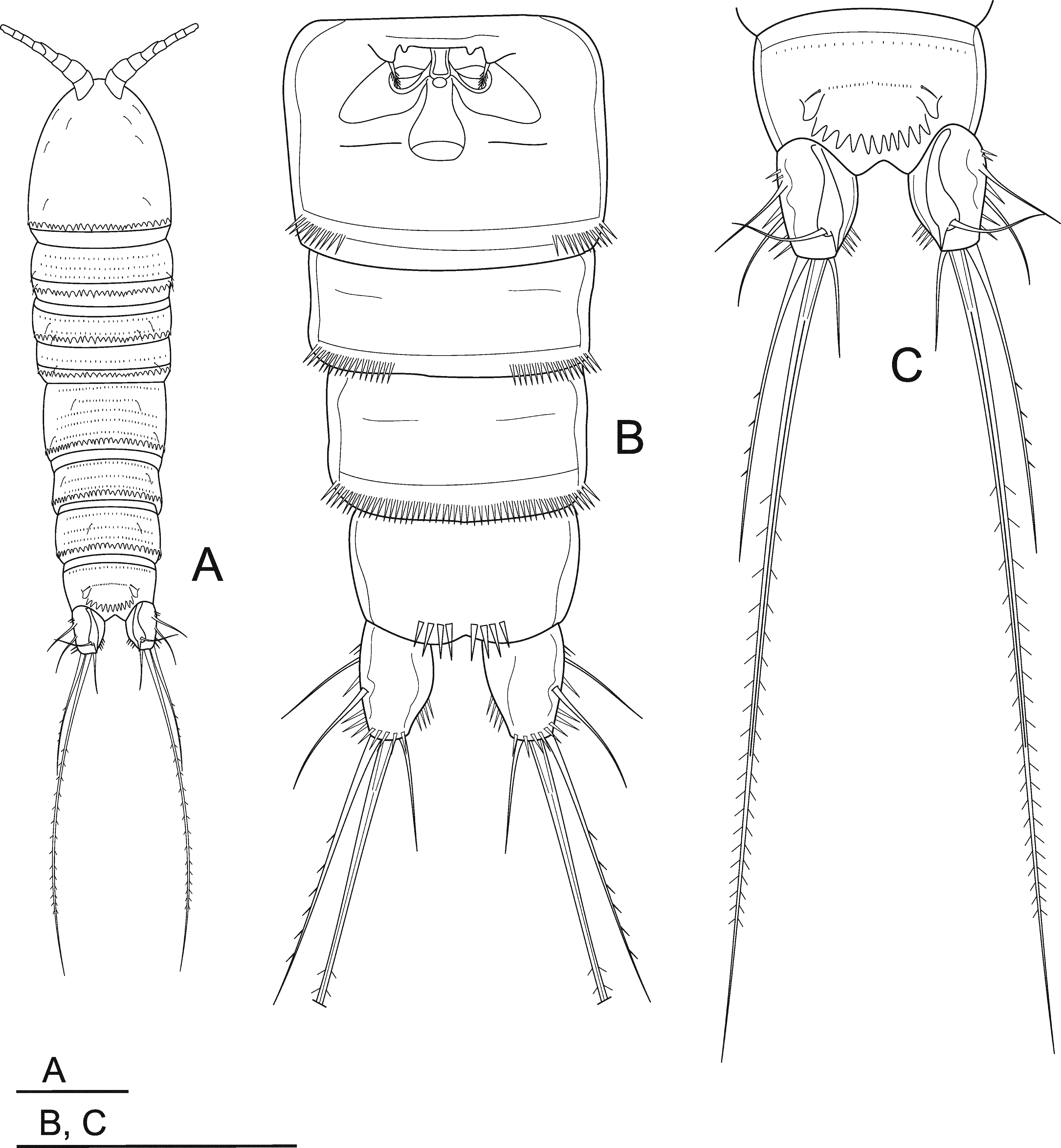
( Figs. 6 View FIGURE 6 –7)
Etymology. The new species is named after Jaesorn National Park, the place where it was found for the first time. The name is a noun composed from the name Jaesorn, and the Latin suffix –ensis, denoting a place (meaning: “from Jaesorn”).
Type locality. Tham Phar Ngam cave is located in Jaesorn National Park (Lampang Province, Northern Thailand). The coordinates of the entrance are: 19° 06΄ 39.6˝ N, 99° 34΄ 42.4˝ E, 305 m above sea level. The cave is about 1 km long, represented by a horizontal gallery. For the fauna survey, several small pools filled with dripping water from the ceiling were sampled at about 100 m from the entrance. Some pools were on solid rock and others were on a muddy substrate. Their volume varied between less than 0.5 to 2 L. Specimens of the new species were found only in the drips forming the small pools on the muddy floor. On 5 October 2009, water temperature at the nearest pool to the entrance where specimens were selected as type specimens, was 22.7 °C, pH was 8.0, and conductivity was 255 µ S cm −1.
Material examined. Holotype: adult female, completely dissected and mounted on a slide: NHMUK 2012. 301. Paratypes: three females without egg sac (stored in 70% alcohol): NHMUK 2012. 302–304; two females without egg sac (stored in 70% alcohol): KKU-COP-2011-005. Additional material, nine females stored in 70% alcohol: KKU-COP-2011-006. All material collected by S. Watiroyram on 5th October 2009.
Description. Female. Body length, measured from anterior margin of rostrum to posterior end of caudal rami, 520–640 µm (mean = 550 µm, n = 5); elongated; cephalothorax wider than rest of body, colourless, naupliar eye absent. Rostrum small. Cephalothorax with three pairs of sensilla and with well-developed dorsal integumental window ( Fig. 6 View FIGURE 6 A). Posterior margins of thoracic and abdominal somites smooth dorsally. Prosomites with one pair of sensilla each. Genital double-somite wider than long ( Fig. 6 View FIGURE 6 B–C), without trace of former division between genital and third urosomites; division between genital and third urosomite evidenced only by row of spinules. All urosomites, including genital double-somite, with pair of sensilla and medially interrupted row of rather strong spinules dorsally, ventrally with row of tiny spinules. Genital complex ( Fig. 6 View FIGURE 6 D) with a single large, bell-shaped copulatory pore; paired seminal receptacles well developed. Additional row of tiny spinules on ventro-distal side of 4th and 5th urosomite. Anal somite with paired dorsal sensilla at base of operculum ( Fig. 6 View FIGURE 6 B, 6E), interconnected with transversal row of small spinules. Two-four ventral strong and robust spinules decreasing in length from innermost to outermost, inserted at base of each caudal ramus ( Fig. 6 View FIGURE 6 C). Anal somite with rounded operculum flanked by one pair of dorsal sensilla ( Fig. 6 View FIGURE 6 B), and ornamented with transverse row of small spinules. Anal operculum ( Fig. 6 View FIGURE 6 B) large, rounded, with 20–25 spinules along free margin; not reaching beyond distal margin of anal somite.
Caudal rami ( Fig. 6 View FIGURE 6 A–C, E) approximately conical, parallel, short, as long as wide, with dorsal keel along 2/3 of ramus length. Inner margin smooth. Each ramus with 7 setae; seta I very small. Setae II, III, VI and VII bare; anterolateral seta (II) inserted at about proximal third of caudal ramus; posterolateral seta (III) slightly longer than anterolateral (II), with few spinules along outer margin; outer terminal seta (IV) about two times as long as caudal ramus, without breaking plane; inner terminal seta (V) long, distal two-thirds bipinnate, fracture plane not visible; inner terminal seta (VI) thin, slightly shorter than caudal ramus; dorsal seta (VII) thin and short, inserted at distal end of keel on middle point of each ramus.
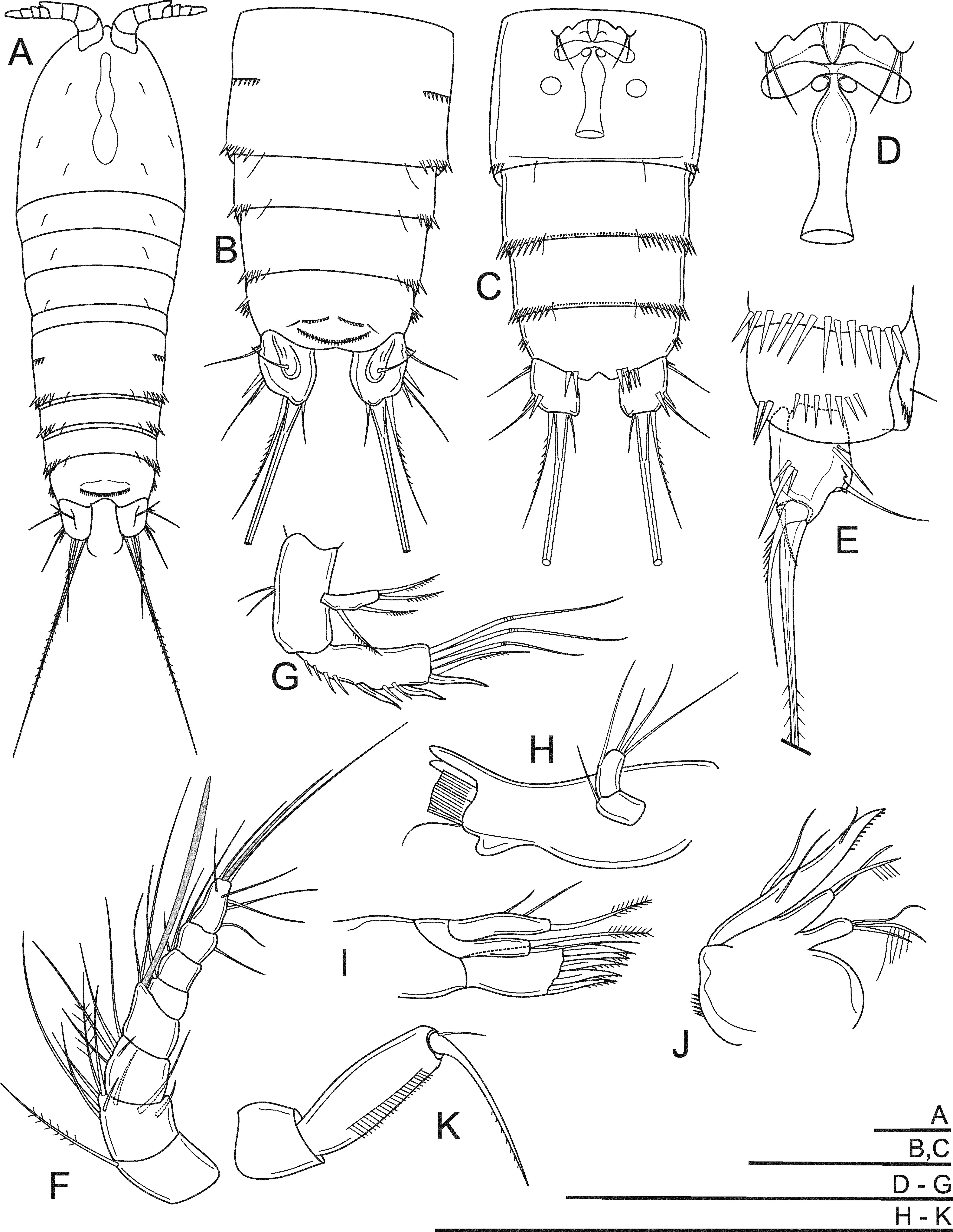
Antennule ( Fig. 6 View FIGURE 6 F) relatively short and stout, eight-segmented, not reaching posterior margin of cephalothorax ( Fig. 6 View FIGURE 6 A). Aesthetasc on fourth segment cylindrical. Aesthetasc on terminal segment long and slim, overreaching tip of the first aesthetasc. Setal formula as follows: 1, 9, 5, 3+A, 1, 2, 2, 7+A. All setae thin and bare except for one bipinnate seta on first and second segment. No additional ornamentation.
Antenna ( Fig. 6 View FIGURE 6 G) robust, with allobasis; one-segmented Exp and Endp. Allobasis with two thin setae midway on outer margin. Exp with two apical and two lateral setae, subequal in length; all setae unipinnate. Endp with two strong outer spines accompanied by several strong, short spinules; distally with three geniculate setae, one normal seta and one strong spine.
Mandible ( Fig. 6 View FIGURE 6 H) short and robust, with two strongly chitinized teeth and one row of smaller teeth on gnathobase; with short seta at dorsal corner and knob. Mandibular palp two-segmented; proximal segment with one seta, distal segment with one subapical and three apical setae. All setae thin and bare.
Maxillule ( Fig. 6 View FIGURE 6 I) consists of robust praecoxa, coxa, and basis. Praecoxal arthrite with five strong apical spines. Coxa with cylindrical endite bearing one pinnate and one smooth seta. Basal endite with one smooth and one pinnate seta. Exp and Endp represented by one and three thin, smooth setae, respectively.
Maxilla ( Fig. 6 View FIGURE 6 J) two-segmented, composed of syncoxa and allobasis. Syncoxa with two endites; proximal endite with three setae, distal endite with two setae. Allobasis drawn out into strong serrate claw with one accompanying seta. Endp fused to basis, represented by two thin, smooth setae.
Maxilliped ( Fig. 6 View FIGURE 6 K) three-segmented, composed of syncoxa, basis, and one-segmented Endp. Syncoxa small, without ornamentation. Basis 3.2 times as long as wide, with about 20 outer spinules. Endp represented by strong, acutely curved, unipinnate claw; accessory armature represented by short seta at base of Endp.
P1 (Fig. 7A) with three-segmented Exp and Endp. Basis with thin inner pinnate seta and robust outer spine. Endp longer than Exp. Endp-1 longer than Endp-2 and Endp-3, reaching middle of second exopodal segment, with robust and pinnate inner seta. Endp-2 as long as Endp-3, with long thin inner seta. Endp-3 with three terminal setae; innermost thin, as long as outer one, middle one geniculate and longest, outermost spiniform, about 0.5 times as long as middle one. Exp-2 with thin inner seta. Exp-3 with strong outer spine, and one spine and two long geniculate setae distally.
P2 (Fig. 7B) with three-segmented Exp and two-segmented Endp. Basis with robust outer spine. Endp-1 shorter than wide, without armature. Endp-2 about 2.5 times as long as wide, with two inner sub-equal setae (distalmost unilaterally feather-like, proximal one unipinnate); with two setae and one spine apically; setae long and pinnate, innermost shorter. Exp-1 as long as Exp-2. Exp-2 with one long seta with unilaterally feather-like tip. Exp-3 3 times as long as wide, with outer spine, one spine and two setae apically; one long unilaterally feather-like seta inserted at about midway of inner margin.
P3 (Fig. 7C) with robust outer basal spine. Endp 2-segmented, Endp-1 shorter than wide, without armature. Endp-2 about 2.5 times as long as wide, with two long, apical setae (innermost slightly shorter) and one outer distal spine; with three inner setae slightly decreasing in length from distalmost seta (apicalmost seta unipinnate, other two bare). Exp similar to that of P2 except for additional unilaterally feather-like seta on inner distal corner of Exp 3.
P4 (Fig. 7D) with long and thin outer basal seta. Two-segmented Endp, Endp-1 small, without inner seta. Endp-2 with inner unipinnate seta at midlength of segment, one inner pinnate seta and one outer pinnate spine apically; inner pinnate seta about 3.0 times as long as segment, outer spine as long as segment. Three-segmented Exp, Exp-1 and Exp-2 similar to those of P3. Exp-3 with strong outer lateral spine, two long, unilaterally featherlike setae on inner margin, and two terminal setae and spine.
Armature formula of P1– P4 as follows (Arabic numerals = setae; Roman numerals = spines):
Exopod Endopod
1 2 3 1 2 3 P1 0-I 1-I 0-2,I-I 1-0 1-0 1-2-0 P2 0- I 1-I 1-2,I-I 0-0 2-2,I-0 - P3 0- I 1-I 2-2,I-I 0-0 3-2,I-0 - P4 0- I 1-I 2-2,I-I 0-0 1-1,I-0 - FIGURE 7. Elaphoidella jaesornensis sp. nov., female (holotype): A, P1; B, P2; C, P3; D, P4; E, Enp-2 P4 (variability); F, P5. Scale bar = 100 µm.
Additional ornamentation of P1–P4 as in Fig. 7A–D.
P5 (Fig. 7F) with separate Exp and baseoendopod, without surface ornamentation. Baseoendopod welldeveloped, about 2.0 times as long as wide, with four strong spiniform setae of unequal length; the second innermost element longest, followed by the second outermost, and innermost seta; outermost seta shortest. Outer basal seta long and bare. Exp small, rounded; with five setae of unequal length, all setae spiniform except for outermost element. Second innermost longest, about 7.0 times as long as segment; middle seta about 0.5 times as long as second innermost element; other setae short, sub-equal in length.
P6 ( Fig. 6 View FIGURE 6 D) fused, small, forming single plate; with two thin, smooth setae (innermost longest).
Egg sac: with 8– 9 eggs.
Male unknown.
Variability. In two specimens the anal somite exhibits two rows of spinules ventrally at base of caudal rami, 2 upper spinules, and additional 3 lower spinules on ramus ( Fig. 6 View FIGURE 6 C). In one specimen, the number of inner setae on Endp-2 P4, differed between the two legs, i.e., carrying one or two setae (Fig. 7E).
Differential diagnosis. The new species described herein was allocated into the genus Elaphoidella Chappuis, 1929 , given the presences of a three-segmented Endp-P1, a two-segmented Endp of P2–P4, and four and five setae on the baseoendopod and exopod of P5, respectively (Fig. 7F). The armature formula of P5 of E. jaesornensis sp. nov. suggests it belongs to IV group sensu Lang (1948) (i.e. elaphoides). Other Elaphoidella species belonging to the same group collected in Thailand are E. intermedia Chappuis, 1931 and E. namnaoensis Brancelj, Watiroyram & Sanoamuang, 2010 .
Elaphoidella jaesornensis is clearly distinguished from E. intermedia Chappuis, 1931 by several characters. Regarding the original description of E. intermedia given by Chappuis (1931), E. jaesornensis differs in the armature of P2–P4 Endp-1 and 2; in the new species this is 0, 0, 0 and 5, 6, 3 respectively, but 1, 1, 1 and 5, 6, 4 respectively in E. intermedia . The P5 of E. jaesornensis has a well-developed baseoendopod, which is about 2.0 times as long as wide, but less than 1.5 as long as wide in E. intermedia . The P5 Exp is oval, with short lateral spinules in E. intermedia , but it is round, small and without surface ornamentation in E. jaesornensis . The posterior margin of the prosomites and urosomites is smooth in E. jaesornensis , but serrate in E. intermedia .
The new species is similar to E. namnaoensis described from a cave in North-western Thailand ( Brancelj et al. 2010). However, a number of morphological differences between those species were observed. The anal operculum of E. namnaoensis is ornamented with well-developed spinules which reach beyond the distal margin of the anal somite, but these spinules are rather short in E. jaesornensis and not reach the distal end of the anal somite. P2–P3 Endp-1 lack inner seta in E. jaesornensis , but an inner seta is present in P2–P3 Endp1 of E. namnaoensis . Elaphoidella jaesornensis possesses 4, 6, and 3 setae/spines on the P2–P4 Endp-2, but there are 5, 6, 4 setae/spines in E. namnaoensis . There are also differences in the armature of P5. Elaphoidella jaesornensis possesses 5 setae on the P5 Exp, but there are 3 long setae, 1 spiniform element, and 2 outer spines in E. namnaoensis (for more details see Brancelj et al. 2010; Figs. 1 View FIGURE 1 B–D, 3B–E). There are also obvious differences in the shape of the P5 Exp of these two species. The P5 Exp of E. namnaoensis is about twice as long as wide, reaching beyond the baseoendopod, but it is rounded and does not reach beyond the distal margin of the baseoendopod in E. jaesornensis . All segments of Exp and Endp P1–P 4 in E. namnaoensis are more elongated, compared to those in E. jaesornensis . However, some other features, especially the unique feather-like transformation of the setae on the inner margin of Exp and Endp P1–P4 as well as the similar shape of integumental window indicates close relationship between both taxa as a result of allopatric speciation. Relatively close localities of both taxa indicate that allopatric speciation is the most likely option especially as epikarst aquifers are highly fragmented even on short distance and prevailing water flow there is in vertical direction and thus lateral connections are weak or non-existing ( Brancelj & Culver 2006).
The morphological comparisons among known species of the genus Elaphoidella from Thailand are presented in Table 1.
Character (female only) E. thailandensis E. jaesornensis E. margaritae Inner spine on Endp-1 P4 - Absent Absent Inner marginal seta on Endp-2 P4 - Longer than Endp-2 P4 Ve r y s ho r t Spinules at the base of caudal ramus 4–6 2 5 Shape of caudal ramus Sub-conical Asymmetrically conical Sub-conical Length of Exp P5 vs. tip of baseoendopod Exceeding the tip Shorter than the tip Exceeding the tip lobe
Geographical distribution of Harpacticoida from Thai caves. One hundred and fifty four copepod samples were collected from 17 caves in Northern Thailand (Fig. 8) during years 2007–2011. In total, 905 specimens of 11 harpacticoid species, belonging to two families, were collected ( Table 2). The family Laophontidae Scott, 1905 was represented by a single species: Onychocamptus mohammed (Blanchard & Richard, 1891) . The Canthocamptidae Sars, 1903 were represented by five species of Elaphoidella Chappuis, 1929 , two species of Attheyella Brady, 1880 and one species of the genera Bryocamptus Chappuis, 1928 , Epactophanes Mrázek, 1893 and Moraria T. & A. Scott, 1893. Two of those taxa, Attheyella sp. and Moraria sp., are still undescribed and are considered as stygophilic when considered on their habitats. However, they were found in low numbers, thus their ecological affinities (i.e. either stygobionts or stygophiles) are not yet clear. Epactophanes richardi Mrázek, 1893 and Onychocamptus mohammed (Blanchard & Richard, 1891) are cosmopolitan. Some evidence indicates that further detailed analyses of microcharacters could eventually reveal them to be cryptic species (Schizas & Shirley 1994; Bruno & Cottarelli 1999). The ecology of the two species in Thai caves differs. Epactophanes richardi was collected from the pools in Tham Chiang Dao cave (Chiang Mai Province) and Tham Phar Ngam cave (Lampang Province) filled exclusively by dripping water and was mainly colonized by epikarstic species as a result of limitation of water bodies connection. On the other hand, O. mohammed was collected in a spring with many water bodies connection as well as species interactions, from Tham Yai Nam Nao cave, Phetchabun Province (see Fig. 8).
We collected Bryocamptus cf. echinatus (Mrázek, 1893) in several sites either in pools filled partly by swallow streams after heavy rain and partly by dripping water or in a spring. The species has been recorded in caves from two provinces: Phetchabun Province (Tham Yai Nam Nao cave (c. 500 m from the entrance) and Tham Bar Dahn cave (10 m from the entrance) of Nam Nao National Park) and Lampang Province (Tham Nam cave of Jaesorn National Park). The species was always collected in low numbers. Copulas were not observed but copepodites were present in some localities, suggesting that caves are also an alternative habitat for this taxon. Sometimes it cooccurs with E. namnaoensis and E. intermedia . Bryocamptus echinatus (Mrázek, 1893) , which was initially described from Europe, has been so far reported from different habitats, mainly from the Palaearctic zooregion, including porous aquifers (=alluvium), caves, springs and lakes ( Stoch et al. 2011).
Females Males juveniles Unsaturated Saturated zone zone
Family Canthocamtidae Sars, 1906
Attheyella vietnamica * 3 - - stygobite + - Attheyella sp. 2 1 - stygophile - + Bryocamptus cf. echinatus * 26 - 16 stygophile - + Elaphoidella bromeliaecola * 223 60 25 stygophile + + Elaphoidella intermedia * 136 - 11 stygophile + + Elaphoidella namnaoensis 248 - 66 stygobite + + Elaphoidella thailandensis 24 9 - stygobite + - Elaphoidella jaesornensis 30 - 3 stygobite + - Epactophanes richardi * 19 - - stygophile + - Moraria sp.** 2 - - stygobite(?) + - Family Laophontidae Scott, 1905
Onychocamptus mohammed 1 - - stygoxene - +
* = new record for Thailand; ** = species presumed to be new to science.
FIGURE 8. The geographical distribution of harpacticoids in caves from Northern Thailand: dots = sampling sites (sites K–O are represented by one common dot); stars = towns; numbers = species numbers as in Table 2; Sampled caves: A, Tham Lod cave; B, Tham Chiang Dao cave; C, Tham Phar Ngam cave; D, Tham Nam cave; E, Tham Keaw Komon cave; F, Tham Nam Phar Pha Ngam cave; G, Tham Mae Usa cave; H, Tham Khun cave; I, Tham Bot Wangna cave; J, Tham Duean Tham Dao cave; K, Tham Yai Nam Nao cave; L, Unnamed cave; M, Tham Song Hong cave; N, Tham Phar Pha Rai cave; O, Tham Payanaak cave; P, Tham Bar Dahn cave; Q, Tham Phar Hong cave.
Attheyella vietnamica Borutzky, 1967 , which was originally described from a cave water reservoir in Vietnam, was also found in Thailand. They were collected only in pools filled by percolating water in Tham Bot Wangna cave (Phitsanulok Province).
Representatives of the genus Elaphoidella are, beyond any doubt, the most common inhabitants of karstic environments in Thailand. So far, representatives of six Elaphoidella species (including the two new species described here) have been recorded from groundwater habitats in Thailand. Five of them ( E. bromeliaecola ( Chappuis, 1928) , E. intermedia Chappuis, 1931 , E. namnaoensis Brancelj, Watiroyram & Sanoamuang, 2010 , E. thailandensis sp. nov. and E. jaesornensis sp. nov.) inhabit the unsaturated zone in karstic caves. From porous aquifers in Thailand only E. margaritae Pesce & Apotolov, 1985 is known so far, which was found in a dug well in Phuket Province (southern Thailand). All these species still seem to be restricted to Southeast Asia.
Among the six Elaphoidella species recorded from Thailand, E. thailandensis and E. jaesornensis are rare. The former species has been so far found only in two caves located 1 km apart, Tham Bot Wangna cave and the type locality (Phitsanulok Province). The latter species seems to be restricted to a single cave in Lampang Province, Tham Phar Ngam cave. Three other species, E. intermedia , E. namnaoensis and E. bromeliaecola are more frequent and are discussed in detail below.
Ecology of Harpacticoida from Thai caves. Some preliminary conclusions on the ecology of cave-dwelling harpacticoids could be drawn only for the most common species, i.e., those which were collected on several occasions and in different locations.
Elaphoidella intermedia was the most frequently encountered species and widely distributed throughout the study areas, except in Chiang Mai Province. This is probably the result of the low number of samples taken from the latter locality (see Fig. 8). Also, it was found in a wide range of habitats: pools on muddy floor fed by percolating water or/and stream water, pools on solid rock and on the bottom of streams. Egg-carrying females (9– 15 eggs per clutch) were abundant in dripping pools (on 8 January 2011) compared to those in samples from amphibious and saturated zones; actually no males were found in habitats connected with subterranean streams. Elaphoidella intermedia co-occurred with E. namnaoensis , E. bromeliaecola and B. cf. echinatus .
Elaphoidella bromeliaecola was found in both unsaturated and saturated zones in three caves of Nam Nao National Park. More than 200 individuals were collected in one single sample taken from a Buddha’s pot filled exclusively with dripping water (on 27 January 2008). It was much less common in pools in a gallery filled with percolated water and/or stream water. In the case of the pot sample, the sex ratio was 2: 1 (female: male). Among all Harpacticoida , it was the only species with specimens found also in copula (found in pot only): 20 pairs in copula (with 8 females already with egg sacs (ranging from 9– 24 eggs per clutch) and 12 females with spermatophores). In addition, there were 40 females with egg-sacs and 18 females with spermatophores. Presence of copulas, egg-sacs and spermatophores indicates that E. bromeliaecola can probably breed in small cracks in the ceiling (i.e. subterranean habitat) which are inhabited by true stygobionts. As mentioned in the introduction, E. bromeliaecola was originally described from phytothelmata (i.e. epigean habitat) which could somehow characterised it as a “stygophilic species”. In fact, connection between phytothelmata and dripping water is not unlikely in karstic areas, as animals from phytothelmata can be easily washed into the epikarstic zone during heavy rain. A high population in a single pot suggests that the cave habitat is suitable for this species, but additional studies on its local distribution in epigean habitats (phytothelmata) are needed.
The two new species have been found only in pools filled with dripping water in small caves, with no connection to surface waters (for details on water characteristics see Table 3). This indicates that the original habitat of the species is the unsaturated karstic zone, inhabited by true stygobionts ( Table 2). Additional support for their stygobiotic nature comes from the fact that specimens of both new species are eyeless, a common characteristic of stygobiotic species worldwide.
A comparison of the distribution of three stygobiotic species ( E. namnaoensis , E. thailandensis and E. jaesornensis ) revealed that E. namnaoensis is rather common, both in unsaturated as well as in saturated zones throughout the study areas (present in 75 % of samples), whereas E. thailandensis and E. jaesornensis are rare, each of them present in about 7 % of the samples. The two new species appear to be well specialized to epikarst and thus cannot survive for a longer time in the pools, filled with percolating water, whilst E. namnaoensis which is less specialized species is different. We suppose that due to different physical and chemical properties of water in the pools as competitors/predators were rare or absent.
Species Altitude Water temperature (°C) pH Conductivity (m a.s.l.) (µS cm -1)
Attheyella vietnamica 202 25.8 8.0 320
Attheyella sp. 165 22.2 8.1 390
Bryocamptus cf. echinatus 167–740 20.0–22.2 7.2–8.1 410–558
Elaphoidella bromeliaecola 684 16.2–21.6 7.9–8.4 593–752
Elaphoidella intermedia 298–878 17.6–24.0 7.3–8.5 380–834
Elaphoidella namnaoensis 457–878 16.7–23.8 7.2–8.7 360–745
Elaphoidella thailandensis 159–202 25.2–26.3 8.0–8.2 320–445
Elaphoidella jaesornensis 167 22.0–22.8 8.0–8.1 210–348
Epactophanes richardi 457 23.1 7.9 531
Moraria sp. 684 not measured not measured not measured
Onychocamptus mohammed 650 18.4 7.8 698 In E. thailandensis , the inner seta on P1 Endp2 are reduced and very short, while P1 Endp1 and P2–P4 Exp2 and 3 are transformed into spine-like setae (see fig. 3A–D). Morphological adaptations, the feather-like transformed seate on P2–P4 Exp of E. jaesornensis resemble more those of E. namnaoensis , with which it may be closely related, especially if the hypothesis about allopatric speciation is correct. Elaphoidella thailandensis also shares some more pronounced morphological adaptations with stygobiotic species living in the epikarst in the temperate zone (i.e. E. franci Petkovski, 1983 , E. tarmani Brancelj, 2009 or E. millennii Brancelj, 2009 ), than the other two Elaphoidella species, including the reduction in number of segments of swimming legs (Endp P4 completely absent in E. thailandensis ), short antennules, an elongated body and reduction of the setae, which are frequently spine-like ( Brancelj 2009). These morphological similarities are present also in species of Morariopsis and Paramorariopsis ( Brancelj 2006, 2009; Brancelj et al. 2010).
Differences in species composition and abundance were observed between unsaturated and saturated zones. Elaphoidella thailandensis , E. jaesornensis , and E. bromeliaecola were relatively common in the unsaturated zone. This is especially evident for E. bromeliaecola , which was represented by numerous females with eggs (as mentioned before). Elaphoidella namnaoensis and E. intermedia were common to both zones, while B. cf. echinatus appeared to be restricted to the saturated zone. In samples originating directly from the epikarst, usually only one species was found, but it was represented by numerous specimens. This reflects the environment, where possibility for horizontal dispersion of aquatic fauna is rather limited, thus inhabitants (i.e. copepods) can have proportionally less predators or competitors than in larger water bodies ( Brancelj 2006). The hypothesis, in which number of specimens and species depend on size, inter-connections, and predation within the drainage basin of each dripping point is supported by Brancelj (2002). In contrast, the species inhabiting amphibious and/or saturated zones were less abundant, but up to 4 harpacticoid species were found together ( E. intermedia , E. bromeliaecola , B. cf. echinatus , and Attheyella sp.) along with cyclopoid copepods ( Eucyclops serrulatus (Fischer, 1851) and Paracyclops fimbriatus (Fischer, 1853)) . In saturated systems, there is a greater dispersion for animals in a horizontal distribution as a result of good interconnection between water bodies. Therefore, different species could be found together easier than those in epikarst, but their abundance is controlled by the co-occurring species.
| NHMUK |
Natural History Museum, London |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Elaphoidella jaesornensis
| Watiroyram, Santi, Brancelj, Anton & Sanoamuang, La-Orsri 2015 |
E. namnaoensis
| Brancelj, Watiroyram & Sanoamuang 2010 |
E. tarmani
| Brancelj 2009 |
E. millennii
| Brancelj 2009 |
E. margaritae
| Pesce & Apotolov 1985 |
E. franci
| Petkovski 1983 |
Attheyella vietnamica
| Borutzky 1967 |
E. intermedia
| Chappuis 1931 |
E. intermedia
| Chappuis 1931 |
Elaphoidella
| Chappuis 1929 |
Bryocamptus
| Chappuis 1928 |
E. bromeliaecola (
| Chappuis 1928 |
Laophontidae
| Scott 1905 |
Laophontidae
| Scott 1905 |
Canthocamptidae
| Sars 1903 |
Epactophanes Mrázek, 1893
| Mrazek 1893 |
Epactophanes richardi Mrázek, 1893
| Mrazek 1893 |
Bryocamptus cf. echinatus (Mrázek, 1893)
| Mrazek 1893 |
Bryocamptus echinatus (Mrázek, 1893)
| Mrazek 1893 |
Onychocamptus mohammed
| Blanchard & Richard 1891 |
Onychocamptus mohammed
| Blanchard & Richard 1891 |
Attheyella
| Brady 1880 |
Paracyclops fimbriatus
| Fischer 1853 |
Eucyclops serrulatus
| Fischer 1851 |