Chaoborus flavicans (Meigen, 1830)
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4927.2.1 |
|
publication LSID |
lsid:zoobank.org:pub:942E128B-0A2C-4799-9A8A-B87A1A4FF627 |
|
DOI |
https://doi.org/10.5281/zenodo.4564943 |
|
persistent identifier |
https://treatment.plazi.org/id/5B678789-FFC7-144E-A09D-08FEFA52F834 |
|
treatment provided by |
Plazi |
|
scientific name |
Chaoborus flavicans |
| status |
|
Chaoborus flavicans View in CoL species complex
We found evidence for at least four species in the Chaoborus flavicans complex: Chaoborus flavicans , C. albipes , C. posio sp. n. and a lineage from Japan. The monophyly for each of these species is strongly supported by bootstrap values ( Fig. 15 View FIGURE 15 ) and is not attributable to random branching under a coalescent null model (Rosenberg’s P(AB) <0.05 for all species in Table 1). The presence of the subordinate tooth between the second and fourth tooth in the larval mandible is a likely synapomorphy of the C. flavicans complex. Chaoborus crystallinus has been proposed as a sister species of C. flavicans ( Saether 1970, Borkent 1981; note that the close relationship between C. crystallinus and C. flavicans suggested by Berendonk et al. 2003 appears to be a mistake due to C. flavicans being confused for C. crystallinus ). However, we refrained from assessing the proposed sister species of C. flavicans complex in this study. Phylogenetic relationships beyond the species complex are not analysed in detail here as COI tends to lack resolution across species groups for Chaoborus ( Dupuis et al. 2008) . We also found a lack of resolution at the deeper nodes for Chaoborus in the present phylogeny ( Fig. 15 View FIGURE 15 ). Characters that support a close relationship between C. flavicans and C. albipes are a slender male gonocoxite and gonostylus, a relatively short apical claw of paramere and a subapically constricted pupal respiratory organ. In contrast, C. posio sp. n. has several autapomorphic character states, such as a wide male gonocoxite and gonostylus, long apical claw of the male paramere and a club-shaped pupal respiratory organ. A high number of larval mandibular fan bristles and conspicuous lateral teeth of the mandibles are shared characters for C. albipes and C. posio sp. n. This sister group relationship also had strong support in our COI phylogeny. A distinct lineage from Japan appears to be more related to C. posio + C. albipes than to C. flavicans sensu stricto ( Fig. 15 View FIGURE 15 ).
It is not known where and when the ancestor of the C. flavicans species complex evolved. Divergent selection for habitat seems to be important as species occupy different niches (ponds vs. lakes); Holarctic sister species that occupy the same niche are mostly allopatric (e.g., C. americanus and C. obscuripes, Borkent 1981 ). If the pattern of three phylogenetically independent shifts from pond to lake habitats within Holarctic Chaoborus ( Berendonk et al. 2003) is accepted, the ancestor of the C. flavicans complex was a pond-dwelling species and C. flavicans became a lake-dwelling taxon able to withstand high pressure in deep waters and fish predation. Note that the substantial genetic divergence between C. flavicans and other members of the complex (> 30%) indicates that divergence of the complex likely occurred well before the Pleistocene. Moreover, widespread species such as C. albipes and C. flavicans with two or more closely related geographic clades may also have been present before the Pleistocene.
Chaoborus tertiarius (von Heyden) , Upper Oligocene fossil species, is composed of two complete pupae, disarticulated pupal parts, larval mandibles, and anal fans ( Borkent 1978). This taxon may be ancestral to C. ( Chaoborus s.str.) and the subgenus C. ( Schadonophasma Dyar & Shannon 1924 ), or it may be ancestral, or a sister, to species of C. flavicans species complex ( Borkent 1978). Among the fossil material are two types of larval mandibles: the subordinate tooth is either present between teeth two and four or it branches from the second tooth ( Borkent 1978, figs. 3 A-H). However, Borkent admits that the subordinate tooth of the flavicans - type “was very difficult to see”, and hence it is uncertain if C. tertiarius is closely related to the C. flavicans species complex.
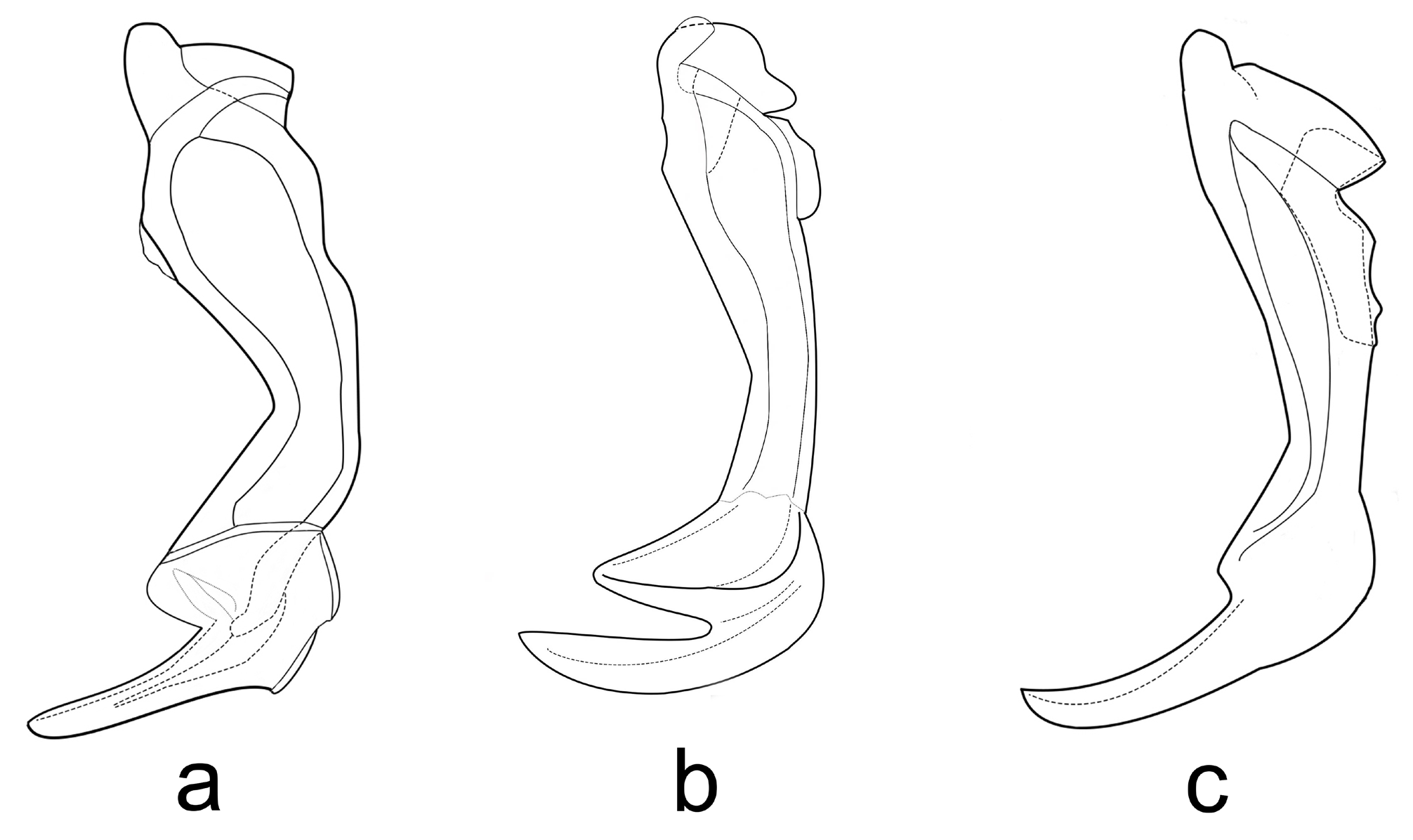
Diagnosis. Adults male. Penultimate flagellomere slightly longer than ultimate, or about equal in length. Flagellomeres pale or partly darkened, bases of whorls dark, giving annulated appearance. Scutellar stripes bare, orange brown–almost black, pleural sclerites pale yellow and with a varying degree of darker coloration. Legs straw yellow or grayish, apical tarsomeres may be somewhat darkened. Tergites yellowish brown or dark brown, bases of setae with a dark ring. Male epandrium either triangular or broadly rounded in shape, its length about 0.35–0.37 times the length of gonocoxite. Male paramere with a conspicuous apical claw, elongated, almost straight or curved.
Pupa. Outer rib of terminal process smooth, apical spines absent. Mid rib of terminal process usually darker than lateral ribs, margin of inner rib serrated along apical 2/3. Length/width ratio of 8 th segment ca. 0.36. Respiratory organ widest medially, constricted apically or not.
IV instar larva. Total length 7–15 mm (e.g. Parma 1971b). Labral blade elongated, degree of serration varied. Mandibular subordinate tooth (3) positioned at the vertex of tooth 2 and tooth 4. Mandibular lateral teeth either small and inconspicuous or larger and distinguishable. Dorsal process pointed.
Key to species of Chaoborus flavicans species complex
Note. All species key out as C. flavicans in the key by Saether (1972).
adult males
1 Gonocoxite and gonostylus stout in structure, length:width ratio 2.64 (2.4–3) and 9.43 (8–10.7), respectively ( Fig. 14a View FIGURE 14 ); apical claw of paramere relatively long ( Fig. 14 View FIGURE 14 c–e).............................................. C. posio Salmela sp. n.
- Gonocoxite and gonostylus slender, length:width ratio ca. 3–3.5 (2.37–3.9) and 12.5–13.7 (10.2–16), respectively ( Figs. 6a View FIGURE 6 , 11a View FIGURE 11 ); apical claw of paramere relatively short ( Figs. 6 View FIGURE 6 c–e, 11c–e)................................................ 2
2 Paramere medially bent and constricted; apical claw rather narrow, moderately curved ( Figs. 6 View FIGURE 6 , 8a View FIGURE 8 ).... C. flavicans (Meigen) View in CoL
- Paramere medially almost straight or gently curved; apical claw stout, curved ( Figs. 8b View FIGURE 8 , 11 View FIGURE 11 )...................................................................................................... C. albipes View in CoL (Johannsen stat. rev.)
pupae Separation of pond-dwelling C. flavicans and C. albipes pupae may be obscured by intraspecific variation
1 Respiratory organ club-shaped, lacking subapical constriction, relatively short (844 (770–930) µm, Fig. 9d................................................................................................ C View FIGURE 9 . posio Salmela sp. n.
- Respiratory organ with subapical constriction, either slender or voluminous ( Figs. 9 View FIGURE 9 a–c)............................. 2
2 Length of respiratory horn Ẑ 1000 µm (920–1360), either slender or voluminous ( Figs. 9 View FIGURE 9 a–b)......... C. flavicans (Meigen) View in CoL
- Length of respiratory organ mostly <1000 µm, may exceed 1000 µm (690–1050); slender ( Fig. 9c View FIGURE 9 )................................................................................................ C. albipes View in CoL (Johannsen stat. rev)
larvae Serration of the labral blade may vary in all species.
1 Mandibular lateral teeth inconspicuous; uppermost tooth shorter than subordinate tooth ( Figs. 10 View FIGURE 10 a–b); number of mandibular fan bristles ±16....................................................................... C. flavicans (Meigen) View in CoL
- Mandibular lateral teeth conspicuous; uppermost tooth about as long as subordinate tooth ( Figs. 10d,f View FIGURE 10 ); number on mandibular fan bristles almost always>16........................................................................... 2
2 Number of mandibular fan bristles>20, usually>22 (21–29) ( Fig. 10h View FIGURE 10 ); labral blade finely serrated, rather wide (length:width ratio 3,7 (3–5 Fig. 10g View FIGURE 10 )............................................................... C. posio Salmela sp. n.
- Number of mandibular fan bristles up to 25, usually 15–21; labral blade almost devoid of serration, slender (length:width ratio 5.59 (4.38–6.85, Fig. 10e View FIGURE 10 )..................................................... C. albipes View in CoL (Johannsen, stat. rev.)
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |