Mitocybe Cook and Loomis, 1928
|
publication ID |
https://doi.org/10.5281/zenodo.195299 |
|
DOI |
https://doi.org/10.5281/zenodo.6196603 |
|
persistent identifier |
https://treatment.plazi.org/id/5E2B325A-FFE6-7C7F-4E84-604D246EA4DF |
|
treatment provided by |
Plazi |
|
scientific name |
Mitocybe Cook and Loomis, 1928 |
| status |
|
Genus Mitocybe Cook and Loomis, 1928 View in CoL
Mitocybe Cook and Loomis, 1928:19 View in CoL . Chamberlin and Hoffman, 1958:184. Buckett, 1964:28. Jeekel, 1971:40. Gardner, 1975:17. Hoffman, 1980:118; 1999:188. Shelley, 2002:93.
Component and type species. M. auriportae Cook and Loomis, 1928 View in CoL , by original designation.
Diagnosis (for simplicity, podomeres are numbered proximal to distal as A/P1-6). A genus of long, thread-like Andrognathidae 18-25 times as long as wide. Head not pyriform, labrum broadly rounded. Dorsum without exoskeletal ornamentations, head and metaterga with dense, velveteen pubescence. Paranota short and blade-like, anterior and posterior margins continuous with those of metaterga; ozopores inconspicuous, not elevated on porosteles. Anterior gonopods with large, lobate, medially divided sternum; A6 moderately long and gently falcate, lying across anterior face of A5 and directed ventrolaterad, arising subterminally from latter.
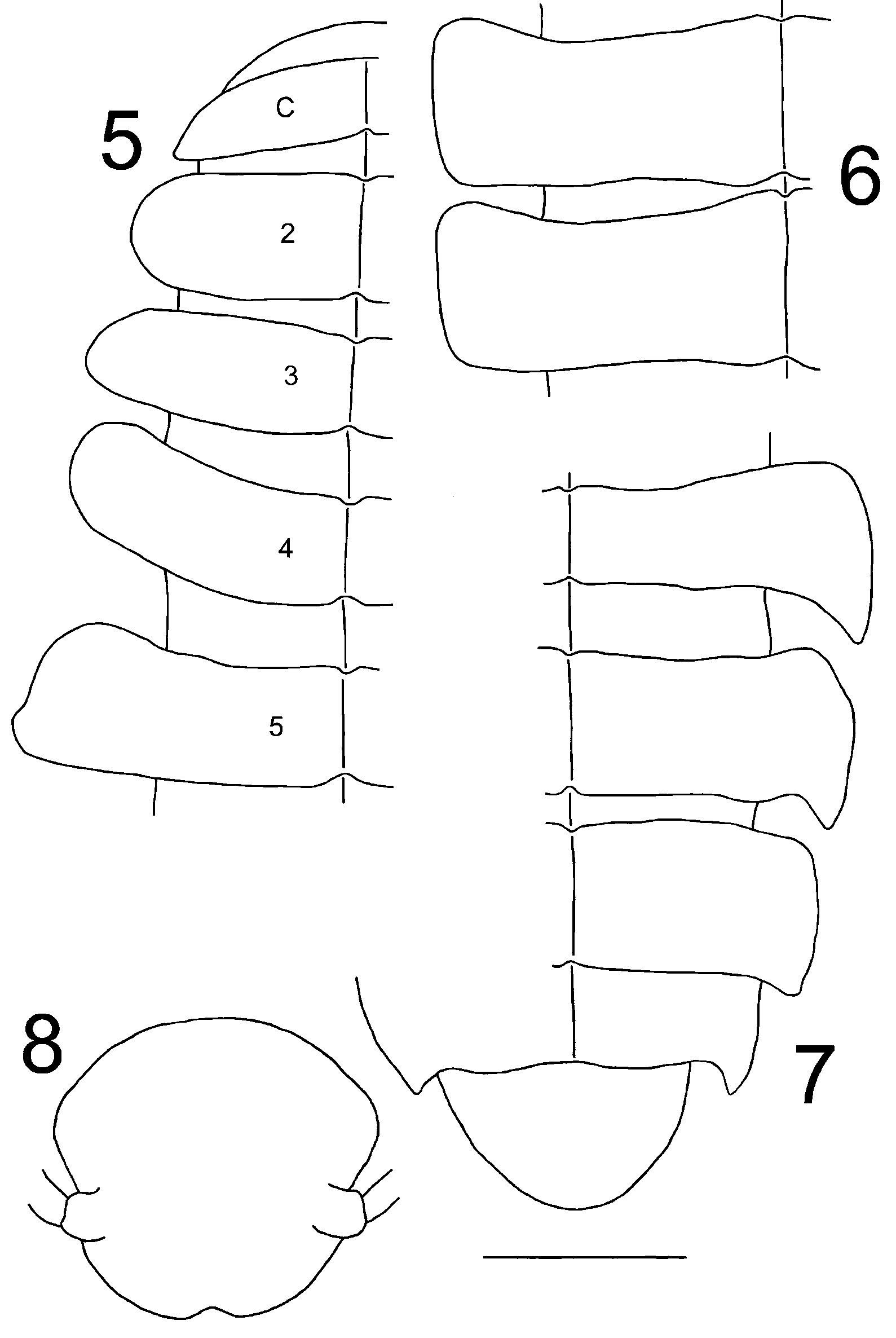
Description. Body dorsoventrally flattened; middorsal groove distinct. Prozona narrow and glabrous, head and metaterga covered with short pubescence; dorsal surface devoid of lobes, tubercles, pustules, or pimples ( Figs. 1-7 View FIGURES 1 – 4 View FIGURES 5 – 8 ). Paranota short and thin ( Figs. 5-7 View FIGURES 5 – 8 ), extending slightly beyond pleural margins, apically truncate, angled, or gently rounded; 4th paranota angling anteriad; 5th with suggestion of anterior lobe, caudolateral margin extending slightly laterad; caudolateral corners of caudalmost paranota prolonged, apically subacuminate. Head broad, ocelli absent ( Fig. 8 View FIGURES 5 – 8 ); antennae widely separated, short and densely pilose, extending caudad just beyond 3rd metatergite, 6th antennomere widest. Anterior end of body expanding through about segment 10; collum with anterior margin gently convex, not overhanging epicranium. Sterna narrow, with conspicuous longitudinal lobes in midline separating opposing coxae. Legs short and slender, absent from last two segments, not extending beyond sides of body, with moderately long hairs becoming longer distad, coxae expanding caudad into subrounded lobes; epiproct and preceding segment legless. Epiproct long and prominent ( Fig. 7 View FIGURES 5 – 8 ), overhanging and obscuring paraprocts in dorsal view, moderately pilose dorsad with noticeably longer hairs ( Fig. 3 View FIGURES 1 – 4 ), margin broadly rounded; margins of paraprocts not thickened, appearing slightly reentrant.
Gonopods ( Figs. 9–11 View FIGURES 9 – 11 ) minute and inconspicuous, upright in situ. Anterior gonopods dominated by broad, laminate, subtriangular, and lobate sternum, deeply but incompletely and narrowly divided in midline, extending ventrad to just beyond origin of A6. Latter arising subterminally on A5, moderately long and gently falcate, lying across anterior face of A5 and directed ventrolaterad, extending well beyond lateral gonopodal margin, tapering smoothly and continuously to acuminate tip; A5 apically excavated with detached, laminate, medial lobe; A3 largest podomere. Posterior gonopod bent sharply ventromediad at articulation of P1/2, latter largest podomere; P2–5 becoming progressively narrower; P6 long and acicular, arising from caudal side of P5, extending between anterior gonopods, curving ventrolaterad, and narrowing smoothly and continuously to acuminate tip, terminating around same level as A6.
Distribution. The San Francisco and Monterey Bay areas of coastal California from Marin to Santa Cruz cos. ( Fig. 12), a distance of approximately 65 mi (104 km).
Remarks. Hoffman (1980) cited two species in Mitocybe , the other undescribed from Guerrero, Mexico. Thirty years later, this species is still undescribed, and the substantial distance between Guerrero and Santa Cruz Co., ~ 1,950 mi (3,120 km), suggests that the Mexican form is not congeneric. Considerably closer to Guerrero is the type locality of Andrognathus hoffmani Shear and Marek, 2009 , in southern Nuevo León, ~ 500 mi (800 km) to the north, so assignment of the Guerrero form to Andrognathus Cope, 1869 , is more plausible geographically. Andrognathus hoffmani is dorsally pilose without ornamentations and the head is also broadly rounded, thereby differing from the subpyriform condition in the type species, A. corticarius Cope, 1869 ( Shear and Marek 2009, figs. 3, 12). As the bodies of species of both Andrognathus and Mitocybe are hairy and lack dorsal ornamentations, I contrast them in Table 1.
Head and metaterga covered with dense pubescence. Head and metaterga pilose, decidedly less hairy. Metaterga thin, coplanar with paranota. Metaterga thick and swollen dorsad, elevated above
paranota.
Metaterga not swollen or expanding caudad; caudal margins Metaterga swollen and expanding caudad; caudal continuous with those of paranota. margins discontinuous with, and caudal to, those of
paranota.
5th paranota with only slight anteriolateral expansions and 5th paranota angling anteriolaterad into distinct rounded suggestions of anterior lobes, caudolateral corners lobes, caudolateral corners flat or extending laterad into extending slightly laterad. distinct lobes.
Legs short, not extending beyond lateral margins of pleurae. Legs long, clearly extending beyond lateral margins of
both pleurae and paranota.
Ozopores inconspicuous, not elevated on porosteles. Ozopores conspicuous, elevated on porosteles. Epiproct broad and prominent, overhanging and obscuring Epiproct small and inconspicuous, not overhanging or paraprocts in dorsal view. obscuring paraprocts in dorsal view.
Hoffman (1980) provided a tentative classification of the order and family, assigning Mitocybe to the new tribe, Mitocybeini, in the subfamily Dolisteninae, while suggesting in commentary that the latter category could be abolished. Nineteen years later, he ( Hoffman 1999) listed andrognathid genera alphabetically, omitting both subfamilies and tribes because of the "unsatisfactory condition of the existing classification." While concurring with this statement, I cite all categories to record the full existing taxonomy that may require modification.
A definitive study of Platydesmida based on gonopodal details has never been conducted and is much to be desired. The two component families differ in the breadths of the sterna – broad with widely separated opposing coxae in Platydesmidae and narrow with nearly contiguous coxae in Andrognathidae . The nominate family, endemic to Mexico and Central America, includes only broad-bodied forms with long, alate paranota, but Andrognathidae presently encompasses this morphology plus species that are long and thread-like with short paranota. The two andrognathid forms, shown together for comparison by Shear and Marek (2009:150, fig. 1), occur sympatrically in North America and east Asia, but only long, slender ones occupy parts of Europe, North Africa, and the Middle East. Shelley et al. (2005) suggested restricting Andrognathidae to the latter components and elevating Bazillozoniinae Verhoeff, 1935, to family status for the broad ones. However, the American and east Asian genus Brachycybe , with its broad body, alate paranota, and narrow sterna, bridges the anatomical gap between platydesmids and narrow-bodied andrognathids; except for the sternal difference, species of Brachycybe more closely resemble platydesmids than andrognathids. Gonopodal factors will resolve the taxonomy, but from somatic features, two resolutions are evident: (1) formally elevate Bazillozoniinae to accommodate Brachycybe and Pseudodesmus Pocock, 1887 , or (2) combine all platydesmidans into a single family. Such a determination is beyond the scope of the present contribution, but a critical review of Platydesmida based on gonopods and addressing this question would constitute a worthy graduate student project.
Dissecting small gonopods, as in the subterclass Colobognatha (orders Platydesmida, Polyzoniida, Siphonocryptida , Siphonophorida (Shelley 2003)) and separating the anterior and posterior ones from each other is difficult and requires patience and a steady hand. A much easier course is to mount segments 7–8 upside down on a stub, examine the undissected gonopods in situ under SEM, and take photos at high magnifications that show more detail of the readily visible parts than light microscopy can possibly reveal. Recent SEM works on colobognaths by Marek and Bond (2006), on Illacme plenipes Cook and Loomis, 1928 ( Siphonophorida : Siphonorhinidae ), and Shear and Marek (2009), on Andrognathus spp., contain elegant SEM in situ gonopod photos (those of the former are provided in supplemental information at http:// www.nature.com/nature/journal/v441/n7094/suppinfo/441707a.html), but a significant drawback to this practice is that details of the anterior gonopod sternum are not shown. Since gonopods represent modified legs that are joined by a sclerotized sternum, the structure is a fundamental part of the "gonopod complex" whose presence, absence, size, and configuration may hold taxonomic utility at generic and supra-generic levels. Just as gonopods can be modified, enlarged, and reduced, so can the sternum that connects them; it can even be vestigial or lost altogether, as in the tribes Rhysodesmini and Apheloriini , respectively, of the Xystodesmidae (Polydesmida) ( Hoffman 1960, Shelley and Whitehead 1986). The condition of the sternum is thus important, but it is situated in the minute gap between the anterior gonopods and 8th legs in colobognaths and is invisible in situ. Marek and Bond provided 27 SEM gonopod photos of I. plenipes , but while a sclerotized connection between the anterior gonopods appears to exist, I could not perceive details even when enlarging them to near blurriness at 400%. In describing A. hoffmani, Shear and Marek characterized the sternum verbally, but it is not evident in the gonopod photos (figs. 18–19); additionally, the distal halves of the apophyses of A2 are shown, but the manner in which they arise is not. All gonopodal structures cannot be shown in any photo or drawing, but a complete view of colobognath sterna seems essential and can only come from dissected gonopods that are removed from the body. Having now studied M. auriportae , I cannot imagine an in situ SEM photo of its gonopods showing any part of the sternum, and such would therefore misrepresent the appendages despite the greater detail it would provide of other features. Though tedious, dissecting and mounting the "gonopod complex" for SEM examinations seems advisable because the platform with the stub can be rotated to show details of the sternum and basal podomeres. Phylogenetically significant traits likely involve the sternum; omitting this structure from illustrations and ignoring its features could result in flawed taxonomies within the Colobognatha.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
ParvClass |
Colobognatha |
|
Order |
|
|
Family |
Mitocybe Cook and Loomis, 1928
| Shelley, Rowland M. 2010 |
Mitocybe
| Shelley 2002: 93 |
| Hoffman 1980: 118 |
| Gardner 1975: 17 |
| Jeekel 1971: 40 |
| Buckett 1964: 28 |
| Cook 1928: 19 |