Mantidactylus katae, Scherz & Crottini & Hutter & Hildenbrand & Andreone & Fulgence & Köhler & Ndriantsoa & Ohler & Preick & Rakotoarison & Rancilhac & Raselimanana & Riemann & Rödel & Rosa & Streicher & Vieites & Köhler & Hofreiter & Glaw & Vences, 2022
|
publication ID |
https://doi.org/ 10.11646/megataxa.7.2.1 |
|
publication LSID |
lsid:zoobank.org:pub:2FD8C310-6486-4592-92F6-5EB894EBD6AC |
|
DOI |
https://doi.org/10.5281/zenodo.7504385 |
|
persistent identifier |
https://treatment.plazi.org/id/0D319103-28D7-406F-A88F-4FC1A5E28057 |
|
taxon LSID |
lsid:zoobank.org:act:0D319103-28D7-406F-A88F-4FC1A5E28057 |
|
treatment provided by |
Plazi |
|
scientific name |
Mantidactylus katae |
| status |
sp. nov. |
Mantidactylus katae sp. nov.
Identity and justification.— This lineage has been considered as confirmed candidate species M. sp. 28 by Vieites et al. (2009) and M. sp. Ca28 by Perl et al. (2014). It was reported as ‘ Mantidactylus sp. aff. betsileanus “slow calls”’ by Glaw and Vences (2007), and has previously been considered as M. multiplicatus (e.g. Poth et al. 2012, 2013); however, DNA barcodes obtained in the present study have shown that this assignment was wrong and that the holotype of M. multiplicatus is conspecific with M. betsileanus , and the nomen therefore a junior synonym of M. betsileanus (see account of that species). Mantidactylus katae is a widespread species with a unique call, characterized by a very low pulse repetition rate (or rather, consisting of a single-pulse note repeated regularly). It also is genetically distinct in mitochondrial DNA and does not appear to share Rag-1 haplotypes with other similar species, including the sympatric (and syntopic) M. betsileanus .
Unfortunately, due to failure of other samples, M. katae sp. nov. is only represented by two individuals from the South East of Madagascar in our phylogenomic tree, from where no reliable call recordings are available. The samples (FGZC 163 and ZCMV 14842) cluster closely with M. tripunctatus . However, these samples correspond to those alleles that also in the Rag-1 haplotype network cluster close to M. tripunctatus , and we cannot exclude that they represent individuals of M. tripunctatus with an introgressed M. katae mitochondrial genome. The exact phylogenetic position of M. katae therefore remains in need of confirmation; the phylogenetic tree of Wollenberg et al. (2011), based on multiple mitochondrial genes, placed it (as M. sp. 28) in a clade with M. noralottae and M. tripunctatus (as M. sp. 29), which would agree with the affinities suggested by our phylogenomic tree. Typical M. katae from the Southern Central East and Northern Central East of Madagascar differ from both M. noralottae and M. tripunctatus very strongly in advertisement call structure, by femoral gland morphology, and do not share Rag-1 haplotypes with these two species, thus leaving no doubt about the species status of this lineage.
Holotype.— ZSM 79/2002 ( FGMV 2001.1179 ), an adult male, collected by M. Vences on 1 December 2001 at Andasibe (ca 18.9333°S, ca 048.4167°E, 920 m a.s.l.), Alaotra-Mangoro Region , Madagascar. GoogleMaps A 16S barcode sequence of the holotype was obtained in this study and was included in the analysis.
Paratypes.— A total of 17 paratypes: ZSM 79– 81 ; 83/2002 ( FGMV 2001.1197 = 2002.G24; FGMV 2001.1180 = 2002.G25; FGMV 2001.1275 = 2002.G66; FGMV 2001.1173 = 2002.G18), two adult females and two adult males, collected by M. Vences on 1–5 December 2001 at Andasibe (ca 18.9333°S, 048.4167°E, 920 m a.s.l.) GoogleMaps ; ZSM 637/2003 ( FG / MV 2002.132), adult male, collected by F. Glaw, M. Puente, L. Raharivololoniaina, M. Teschke (née Thomas), and D.R. Vieites on 15 January 2003 beside a small brook in Ranomafana National Park (21.250°S, 047.450°E, 932 m a.s.l.) GoogleMaps ; ZSM 668/2003 ( FG / MV 2002.255 ), adult male, collected by F. Glaw, M. Puente, L. Raharivololoniaina, M. Teschke (née Thomas), and D.R. Vieites on 16 January 2003 in Ranomafana National Park (21.250°S, 047.450°E, 932 m a.s.l.) GoogleMaps ; ZSM 708/2003 ( FG / MV 2002.348 ), adult male, collected by F. Glaw, M. Puente, L. Raharivololoniaina, M. Teschke (née Thomas), and D.R. Vieites on 20 January 2003 at Kidonafo Bridge, Ranomafana (21.2262°S, 047.3696°E, 1152 m a.s.l.) GoogleMaps ; ZSM 196/2021 ( FAZC 15504 , extraction ACP 3659 , tissue ACZC 8591 ), ZSM 197/2021 ( FAZC 15507 , ACP 3662 , ACZC 8594 ), and MRSN A7043 ( FAZC 15523 , ACP 3662 , ACZC 8594 ), two males and one female collected at Maromizaha (18.9771°S, 048.4682°E, ca 1000 m a.s.l.) GoogleMaps , in January 2017 by E. Coppola; ZMB 81918 ( JCR 105 ), adult male collected on 16 March 2010 by J.C. Riemann, and S.H. Ndriantsoa at Andalangina, Ranomafana area (21.29844°S, 047.60343°E, 480 m a.s.l.) GoogleMaps ; ZMB 81920 (field NSH 1069 ; GenBank JCR 1069), adult female, collected on 12 May 2010 by J.C. Riemann, and S.H. Ndriantsoa at Ambatovory, Ranomafana area (21.23966°S, 047.42581°E, 953 m a.s.l.) GoogleMaps ; ZMB 81922 (field NSH 2577 ; GenBank JCR 2577), adult male, collected on 26 March 2012 by J.C. Riemann, and S.H. Ndriantsoa at Sahamalaotra, Ranomafana area (21.23688°S, 047.39887°E); GoogleMaps UADBA-A 43149 (JCR 106), adult male collected on 16 March 2010 by J.C. Riemann, and S.H. Ndriantsoa at Andalangina, Ranomafana area (21.29844°S, 047.60343°E, 480 m a.s.l.) GoogleMaps ; UADBA-A 62106 ( JCR 245 ), subadult collected on 21 April 2010 by J.C. Riemann, and S.H. Ndriantsoa atAmbolo , Ranomafana area (21.26386°S, 047.50862°E, 643 m a.s.l.) GoogleMaps ; UADBA-A 62104 ( JCR 320 ), adult female collected on 21 May 2010 by J.C. Riemann, and S.H. Ndriantsoa at Ambolo, Ranomafana area (21.26307°S, 047.50696°E, 660 m a.s.l.) GoogleMaps ; UADBA-A 62105 ( JCR 323 ), adult female collected on 21 May 2010 by J.C. Riemann, and S.H. Ndriantsoa at Ambolo, Ranomafana area (21.26307°S, 047.50696°E, 660 m a.s.l.) GoogleMaps .
Additional material.— The following specimens are assigned to M. katae sp. nov. based on morphology, but have not been DNA barcoded: ZMA 20232 ( ZCMV 236 ), adult male, collected by M. Vences and I. de la Riva on 24 January 2004 at Ranomafana National Park , Maharira base camp (21.3258°S, 047.4024°E, 1248 m a.s.l.); GoogleMaps ZFMK 62212 , adult female, collected by F. Glaw, D. Rakotomalala, and F. Ranaivojaona on 10 March 1996 at Andasibe ; GoogleMaps ZMA 6828-863 ; 6828-864 , adult male and female, collected on 23 September 1972, and ZMA 6886-641–643 , three adult males, collected by R.M.A. Blommers-Schl̂ sser on 4 April 1972 at Andasibe ; GoogleMaps ZMA 6833-149 and 6833-156–158 , two adult females and two adult males, collected by R.M.A. Blommers-Schl ̂sser on 1 July 1971 at Ranomafana . GoogleMaps Furthermore, the following specimen (included in our phylogenomic analysis) from the South of Madagascar agrees with M. katae sp. nov. in mitochondrial DNA but due to the absence of bioacoustic data from this population, its identity is in need of confirmation: ZSM 91/2004 ( FGZC 163 ), adult male, collected by F. Glaw, M. Puente, M. Thomas and R. Randrianiaina on 31 January 2004 at ‘Camp 1’ between Isaka and Eminiminy , Andohahela (24.7586°S, 046.8542°E, 247 m a.s.l.) GoogleMaps .
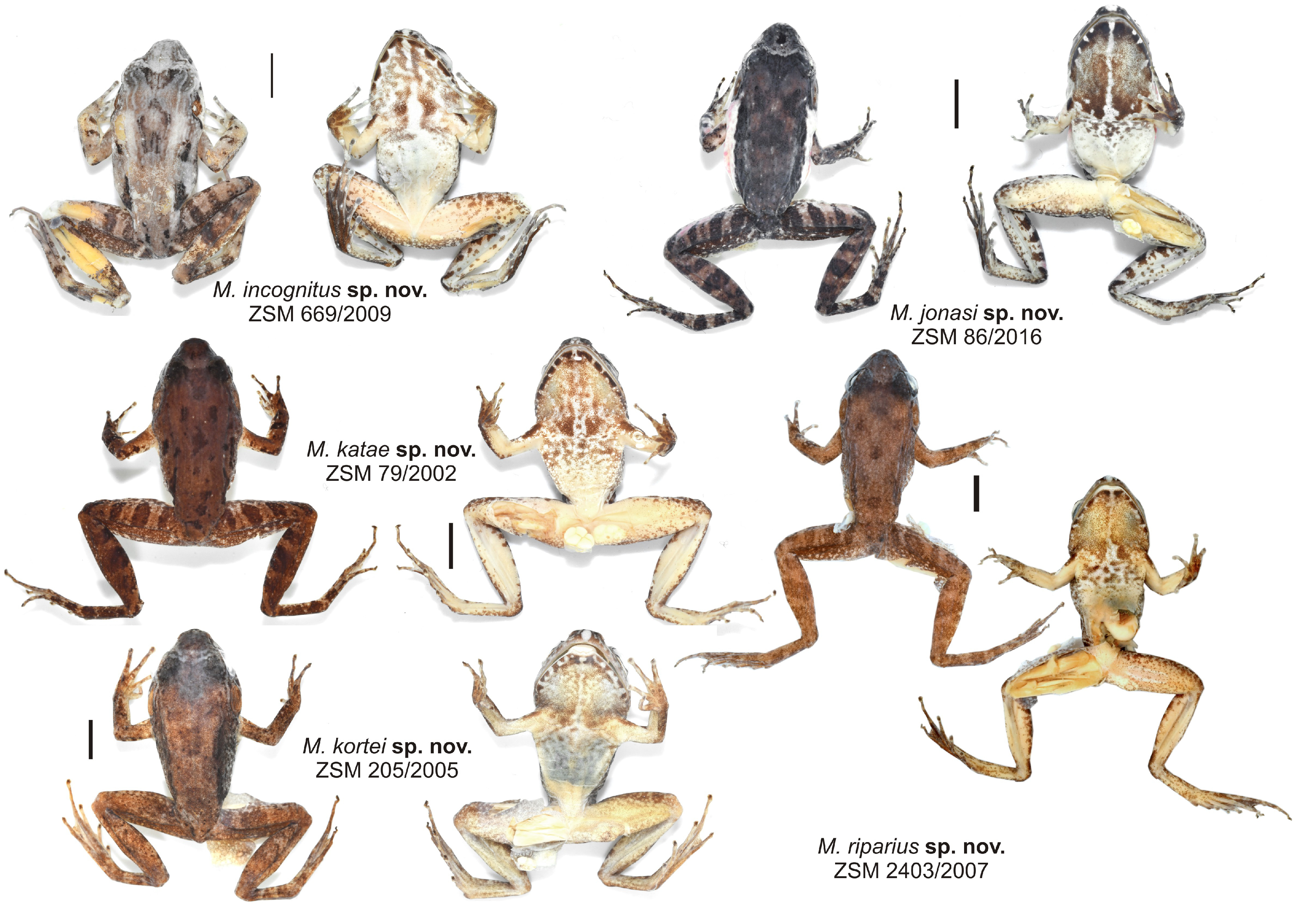
Diagnosis.— Mantidactylus katae sp. nov. is a member of the M. betsileanus clade, related to M. noralottae and M. tripunctatus . See Table 4 View TABLE 4 for a list of diagnostic morphological characters. The combination of a relatively small body size (male SVL 22–27 mm), slightly tubercular dorsal skin with distinct continuous dorsolateral ridges, absence of white spots on flanks, presence of a white marking on snout tip, large femoral glands (FGW up to 13% of SVL) that contact each other medially, and advertisement call consisting of a single pulse repeated at a slow rate of 10–16 calls per second distinguishes M. katae sp. nov. from species of all other clades.The only other species with a similar advertisement call structure is M. fergusoni sp. nov. which has a larger size and more tubercular dorsal skin (see account of that species below). Within the M. betsileanus clade, the new species can be distinguished from all other species by its unique call structure and larger femoral glands ( Table 4 View TABLE 4 ); furthermore from M. noralottae by smaller size of males and presence of a distinct white marking on snout. For a field diagnosis from the syntopic species M. betsileanus , according to our measurements, M. katae differs by a smaller dot on the snout tip (dot on the snout tip is 9–14% of head width vs 13–24%), a smaller distance between the femoral glands (distance between the femoral glands is 0– 14% of SVL vs 13–22%), larger femoral glands (femoral gland length is 19–31% of SVL vs 13–22%), and a higher number of granules per femoral gland (5–8 vs 1–5). For a detailed distinction from other new species described herein, see the respective species accounts. A full list of molecular diagnostic sites in the 16S gene of M. katae sp. nov. in pairwise comparisons to all other Brygoomantis species is provided as Supplementary appendix.
Description of the holotype.—Adult male in good state of preservation ( Fig. 33 View FIGURE 33 ). Some muscle tissue removed from right thigh. Femoral glands partly detached for examination in internal view. Body relatively slender.
Head slightly wider than body. Snout rounded in dorsal view, slightly truncate in lateral view. Nostrils directed laterally, slightly protuberant, nearer to tip of snout than to eye. Canthus rostralis weakly recognisable, slightly concave; loreal region slightly concave. Tympanum distinct, slightly wider than high, horizontal diameter of tympanum73% of horizontal eye diameter.Supratympanic fold rather distinct, running rather straight from behind eye and bending about 45° about midway towards forelimb insertion. Tongue ovoid, distinctly bifid. Maxillary teeth present. Vomerine teeth form two rounded aggregations, positioned posterolateral to choanae. Choanae rounded.
Subarticular tubercles single. Inner and outer metacarpal tubercles present. Fingers without webbing. Relative length of fingers: I<II<IV<III. Finger discs slightly enlarged. Nuptial pads absent. Foot slightly shorter than tibia (97%). Lateral metatarsalia separated. Inner metatarsal tubercle present. Outer metatarsal tubercle not clearly recognisable. Webbing formula: 1(1), 2i(1.5), 2e(1), 3i(2), 3e(1), 4i(2.25), 4e(2), 5(0.5). Relative length of toes: I<II<V<III<IV. Skin on the upper surface smooth with numerous longitudinal folds and ridges, granular on flanks. Ventral side smooth. Femoral glands large and distinct, very close to cloaca, with a distinct distal ulcerous macrogland; no clearly recognisable proximal granular gland field.
Colour in preservative: dorsally dark brown with some poorly contrasting, slightly darker markings and a slightly lighter band between eyes. Dark crossbands on limbs. Ventrally light beige with dark pattern on chest and throat, also extending on anterior belly. Throat with light colour medially, forming a very irregular and discontinuous broad medial stripe. Upper lip ventrally dark brown with white spots. Colouration of holotype in life not documented.
Variation.—Variation in measurements is given in Table 7. See Fig. 42 View FIGURE 42 for colouration in life and its variation. There is moderate sexual size dimorphism (confirmed male SVL 22.1–27.2 mm [n = 7] vs confirmed female SVL 25.6–35.9 mm [n = 3]). Males have a larger tympanum than females (HTD/ED ratio is 61–68% in females, 73– 100% in males). Femoral glands in males are large and very distinct, of a quite characteristic shape. They consist of a large-sized distal ulcerous macrogland located rather close to the insertion of the thighs and thus almost in contact with each other. Consequently, the proximal granular gland field (by definition located proximally from the ulcerous macrogland) is basically absent in these frogs. Glands are typically coloured yellowish in life.
Natural history.—Occurs along running water bodies in rainforest, where specimens can often be seen calling at night from muddy banks of somewhat larger, slow-moving streams. It often occurs in syntopy with M. betsileanus in rainforest, including forest fragments ( Riemann et al. 2015), and along streams with at least a narrow gallery forest band surrounded by degraded or unforested areas. However, in contrast to M. betsileanus it is not found in rice fields or other plantations ( Ndriantsoa et al. 2017). Usually sitting in shallow water or along the shore, hiding in the leaf litter or perched on low vegetation up to 0.5 m hight in the vicinity of a stream. Found at an elevational range between 450–1142 m a.s.l. in Ranomafana and surrounds. Females with visible eggs (transparent abdominal wall) were observed in May 2010 and from January to June in 2011 in the Ranomafana area.
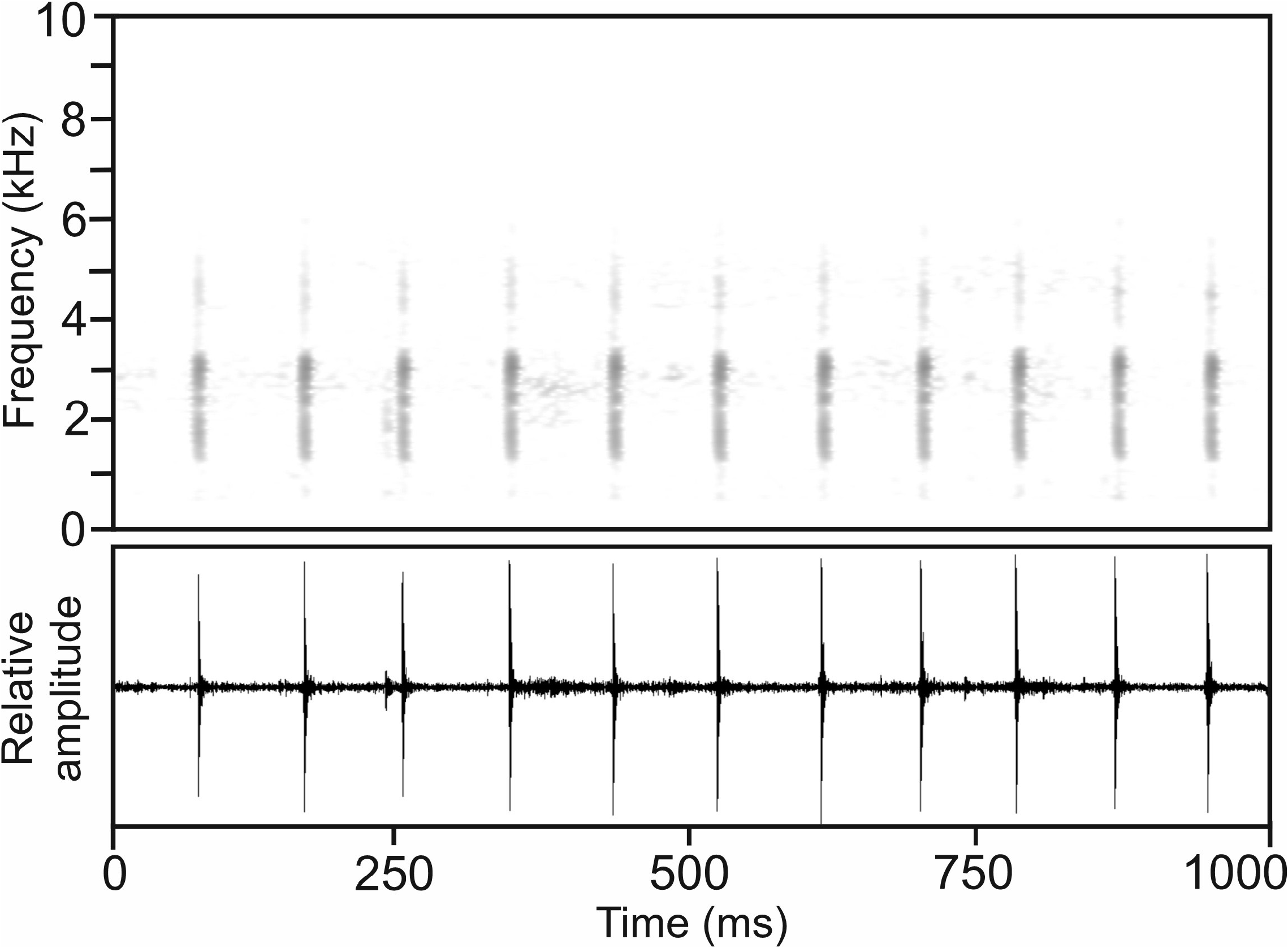
Calls.—The advertisement call of M. katae , recorded on 17 December 1994 at Andasibe, ca 20°C air temperature ( Vences et al. 2006: CD2, track 64, cut 2), consists of a simple, very short, single pulse ‘click’ note, emitted in long series at regular intervals and fast succession ( Fig. 43 View FIGURE 43 ). The duration of the call series analysed from Andasibe was 7568 ms. Numerical parameters of 47 analysed calls were as follows: call duration (= note duration) 2–5 ms (3.6 ± 0.8 ms); 1 pulse per note (1.0 ± 0.0); pulse duration = note duration = call duration; dominant frequency 3036–3165 Hz (3108 ± 46 Hz); prevalent bandwidth 1250–3540 Hz; call repetition rate (= note repetition rate = pulse repetition rate) within regular series 696–971 calls/min (840 ± 106 calls/min).
Calls recorded on 24 January 2004 at Maharira forest, Ranomafana National Park, 18.4°C air temperature ( Vences et al. 2006: CD2, track 64, cut 1) are in agreement with the calls from Andasibe in all spectral and temporal parameters, except for slightly lower dominant frequency ranging from 2713–2993 Hz (2833 ± 123 Hz), and slightly lower call repetition rate, ranging from 515–731 calls/min (626 ± 109 calls/min). Both slight differences can be explained by potentially larger body size of the calling male and lower temperature during recording and leave no doubt about the conspecificity of the calls from the two localities.
Another call recording from Andasibe, obtained on 20 March 1995, 23.4°C air temperature ( Vences et al. 2006: CD2, track 63) is similar to the calls described above, but differs by shorter call series (2226–2520 ms), slightly longer call duration (= note duration = pulse duration) of 8–10 ms, and lower dominant frequency of 1323–1433 Hz (1365 ± 52 Hz). However, the latter might be due to recording equipment and/or recording conditions, as the calls described above have a second frequency peak that roughly corresponds to the lower dominant frequency range described here. Call repetition rate (= note repetition rate = pulse repetition rate) is in the same range compared to the calls described above, ranging from 630–857 calls/ min (741 ± 90 calls/min).
Tadpoles.—The tadpole of M. katae was described by Knoll et al. (2007) under the name ‘ M. sp. aff. betsileanus “very slow calls”’.
Distribution.— Widespread in eastern Madagascar ( Fig. 7 View FIGURE 7 ). This species is known from Ambatomandondona, Ambohitsara,An’Ala,Andasibe,Andohahela,Andringitra, Bibiango, Ambatofotsy, Ifandiana, Mahatsara-Mantadia, Mariavatra, Mantady, Marolambo, Maromizaha, Pic d’Ivohibe,Ranomafana(varioussites,includingValohoaka, Ambatolahidimy, Ambatolahy, Ambolo, Ampitavanana, Andalangina, Beremby, Bibiango, Kidonavo, Maharira, Ampangadiamesa, Andranovorimainty, Ambodiriana, Imaloka, Sahamalaotra, Talatakely, and Vohiparara), Sahamalotra, Sahateza, Torotorofotsy, and Vohidrazana. Elevation range: 247–1248 m a.s.l.
Etymology.—We dedicate this species to Katharina (‘Kat’) Wollenberg Valero, in recognition of her numerous contributions to the research on little brown frogs of Madagascar, and specifically on the behaviour of this species (under the name M. multiplicatus ) in the framework of studies of their femoral gland compounds ( Poth et al. 2012).
| MRSN |
Italy, Torino, Museo Regionale di Scienze Naturali |
| ZMB |
Germany, Berlin, Museum fuer Naturkunde der Humboldt-Universitaet |
| ZFMK |
Germany, Bonn, Zoologische Forschungsinstitut und Museum "Alexander Koenig" |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |