Bythotrephes cederströmii Schödler, 1877, Schodler, Karesuando, 1877
|
publication ID |
https://doi.org/10.11646/zootaxa.4379.3.2 |
|
publication LSID |
lsid:zoobank.org:pub:EEF541E1-F91C-411E-9B65-2C79ED2A2706 |
|
DOI |
https://doi.org/10.5281/zenodo.5962978 |
|
persistent identifier |
https://treatment.plazi.org/id/60573E65-D16B-FF94-6EC2-639DD0280521 |
|
treatment provided by |
Plazi |
|
scientific name |
Bythotrephes cederströmii Schödler, 1877 |
| status |
|
Bythotrephes cederströmii Schödler, 1877
( Figs. 8‒14 View FIGURE 8 View FIGURE 9 View FIGURE10 View FIGURE 11 View FIGURE 12 View FIGURE 13 View FIGURE 14 )
Bythotrephes Cederströmii Schödler, 1877 View in CoL : Schödler 1877: 233 ‒234; Lieder 1988: 125‒127, Figs. 3 View FIGURE3 , 4 View FIGURE 4 .
Bythotrephes borealis Sars, 1890 View in CoL : Sars 1890: 51; Ischreyt 1934a: 277.
Bythotrephes cederströmii var. cederströmii s.str.: Lilljeborg 1901: 619 ‒621, Tab. 82, fig. 11, Tab. 83, fig. 1, 2.
Bythotrephes cederströmii var. robustus: Lilljeborg 1901: 621 View in CoL ‒623, Tab. 83, fig. 3‒5; Tab. 84, fig. 1, 2.
Bythotrephes cederströmii var. connectens: Lilljeborg 1901: 623 View in CoL ‒625, Tab. 84, fig. 3‒7.
Bythotrephes cederströmii Schödler, 1877 : Ischreyt 1934a: 277, 1934b: 183‒202, Fig. 1 View FIGURE 1 , 2 View FIGURE 2 ; Rylov 1935a: 154 ‒155, Fig. 233 (partim); Flössner 2000: 377‒379, Abb. 137a-g; Litvinchuk 2002: 126 ‒127, Figs 32, 33, 2007: 190‒191; Korovchinsky 2015: 21‒29, Figs. 10‒11 View FIGURE10 View FIGURE 11 .
Bythotrephes longimanus cederströmii: Vekhov 1987: 28 ‒29, Fig. 2 View FIGURE 2 .
Material examined. Type material. This material was designated, enumerated and described in previous publication together with some additional, not type material (see Korovchinsky 2015).
Material examined in present study. Sweden: Lappland: 1) subsample (MEUU, old museum number 633a with specimens from Lilljeborg catalogue N 2669) labeled “ B. l. arcticus, Sw, Lpl, Karesuando , 30.7.1875, Lillj.”, some ad; 2) subsample (MEUU, old museum number N 635d with specimens from Lilljeborg catalogue N 2682) labeled “ B. c. robustus, Sw, Lpl., Karesuando , 31.7.1875, Lillj.”, numerous ad and gam; 3) a slide (MEUU, old museum number 635e with specimens from Lilljeborg catalogue N P2685) labeled “ B. c. robustus, Sw, Lpl., Karesuando , 31.7.1875, Lillj.”, 1 ad, 2 males; 4) a slide (MEUU, old museum number 635f with specimens from Lilljeborg catalogue N P2309) labeled “ Bythotrephes borealis n. sp., ♀♂, Sw, Lpl., Karesuando, 30/7 75, Lillj.”, 3 ad, 1 male; 5) a slide (MEUU, old museum number 635g with specimens from Lilljeborg catalogue N P2310) labeled “ Bythotrephes borealis Ƌ, Sw, Lpl., Karesuando, 31/7 75, Lillj.”, 2 males, dissected; 6) a slide (MEUU, old museum number 635h with specimens from Lilljeborg catalogue N P2311) labeled “ Bythotrephes borealis n. sp., Sw, Lpl, Karesuando, 30/7 75, Lillj.”, 2 ad, dissected and dryed; 7) bottle (MNB, without number) with two tubes, one of which labeled “ B. cederströmii Schödler, Karesuando , 31.7.1875, W. Lilljeborg”, 22 gam, 5 males, 1 juv.; 8) sample (MEUU, Lilljeborg catalogue N 2667) labeled “ Bythotrephes cederströmii var. robustus, Norbotten, Karesuando , 31.7.1875, W. Lilljeborg”, numerous ad, gam, males; 9) sample (MEUU, Lilljeborg catalogue N 2687) labeled “ B. ced . robustus, Sw, Lpl, Karesuando, 29.7.1875, Lillj.”, numerous ad and males; Norrbotten: 10) subsample (MEUU, old museum number N 635b with specimens from Lilljeborg catalogue N 2679) labeled “ B. c. robustus, Sw, Nb, Muoniovaara , 9.7.1875, Lillj.”, numerous ad; 11) sample (MEUU, Lilljeborg catalogue N 2661) labeled “ B. longimanus var., Sw, Nb, Ruskola, Onkijärvi, 3.8.1875, Lillj.”, 1 ad; Västmanland: 12) sample ( MEUU, Lilljeborg catalogue N 2690) labeled “ B. ced . robustus, Sw, Vstm, Grythyttan Sör-Algen, 26.7.1894, Trybom F.”, 4 ad, 1 juv.; Dalsland: 13) a slide (MEUU, old museum number 635i with specimens from Lilljeborg catalogue N P2312) labeled “ Bythotrephes borealis, Sw., Dsl, Lelången , 30/8 82, J. Kolthoff”, 3 ad, 1 male; 14) sample (MEUU, Lilljeborg catalogue N 2315) labeled “ Bythotrephes cederströmii, Sw, Dsl, Lelangen , 30.8.1882, Lillj.”, 2 ad, 2 males; 15) sample (MEUU, Lilljeborg catalogue N 2665) labeled “ Bythotrephes cederströmii var. robustus, Dalsland , sjön Lelangen, 30/8 1882, J. Kolthoff”, many ad and males; Västergötland: 16) sample (MEUU, Lilljeborg catalogue N 2680) labeled “ B. c. robustus minor, Sw, Vg, Mullsjön, 17.6.1892, C. Aurivillius”, some ad; 17) subsample (MEUU, old museum number 636a with specimens from Lilljeborg catalogue N 2684) labeled “ Bythotrephes cederströmii var. connectens & forma minor; Västergötland, nära Hjo, Mullsjön, 17/6 1892, Carl Aurivillus”, numerous ad.
Ireland: 1) sample (MNB, N 18918 View Materials ) labeled “ Bythotrephes cederströmii Schödler, Lake Erne , Irland, leg. Kane, det. Scourfield ”, 6 ad, 2 gam, 4 males .
Russia: Karelia: 1) bottle (MNB, without number) with two tubes, one of which labeled “Onega Lake, Aug. 189…, H. Linder, Bythotrephes Cederströmii Schödler ”, 5 ad, 4 males, 1 juv.; 2) bottle (ZIN, N 73‒915) with 17 tubes labeled “Collection of Bythotrephes by A.K. Linko”), probably all from Lake Onega, numerous ad and juv; 3) Lake Pozemskoye (65°90889 N; 34°45611 E), 7.8.2009, 22 ad, 1 gam, 1 male, coll. A.V. Tiunov; 4) Lake Nizhnee Nilm-ozero, 5.7.2015, numerous ad, coll. A.Yu. Sinev; Murmanskaya Province: 5) Lake Kanstojavr (68°87366 N; 34°12415 E), 2 ad; Arkhangelskaya Province: 6) seven slides (ZIN, without numbers), each labeled “ Bythotrephes ” or “ B. c. robustus ”, Tundra near the mouth of the River Pechora, Peltsam”, 7 ad, some strongly deformed; 7) canal Ul’yanov Shar in delta of the River Pechora (68°14.844 N; 53°51.734 E), 12.8.2016, some ad and juv., coll. E.B. Fefilova and O. Kononova; Komi Republic: 8) Lake Golovka (67°36.010 N; 62°55.476 E), 5.8.2012, 2 ad, leg. E.B. Fefilova; Vologodskaya Province: 9) Lake Kubenskoe, June 2011, 1 ad; 2.8.2013, 1 juv.; 9.6.2014, 2 ad, 1 juv, leg. E.V. Labunicheva; 10) Lake Kovzhozero, 12.7.2011, 6 ad, 5 juv., leg. E.V. Labunicheva; 11) Lake Vozhe, 18.9.2012, 1 ad; 20.8.2013 ‒ 12.8.2014, 11 ad, 7 juv., 6 juv. males, E.V. Labunicheva; 12) River Shima, 5.7.2012, 1 ad, E.V. Labunicheva; Finnish Gulf: 13) Nevskaya guba, 2.8.2006, 2 ad, 1 juv., coll. L.F. Litvinchuk; Tverskaya Province: 14) a slide (ZIN, without number) labeled “Bologoe, Bythotrephes longimanus, R. Mink ” and “ cederstroemii Schödler”, 1 ad; Kostromskaya Province: 15) sample (ZIN, N 89‒1962) labeled “Lake Tchernoye near Kostroma City, 11.VI.1925, coll. S. Smirnov”, 5 ad; Rjazanskaya Province: 16) Lake Ivanovskoe, 22.7.1979, 1 juv., coll. N.M. Korovchinsky; Yakutia (Eastern Siberia): 17) Lake Taiylaar, August 2008, 21 ad, 6 males, 3 juv., coll. I.G. Sobakina; 18) Lake Teburen, August 2008, 1 ad, deformed, coll. I.G. Sobakina; 19) Vilujskoe reservoir, 11.7.2007, 1 ad, coll. I.G. Sobakina.
Data on body and body parts measurements of specimens from five populations are presented in Table 2. Data used in the previous description ( Korovchinsky 2015) have been included.
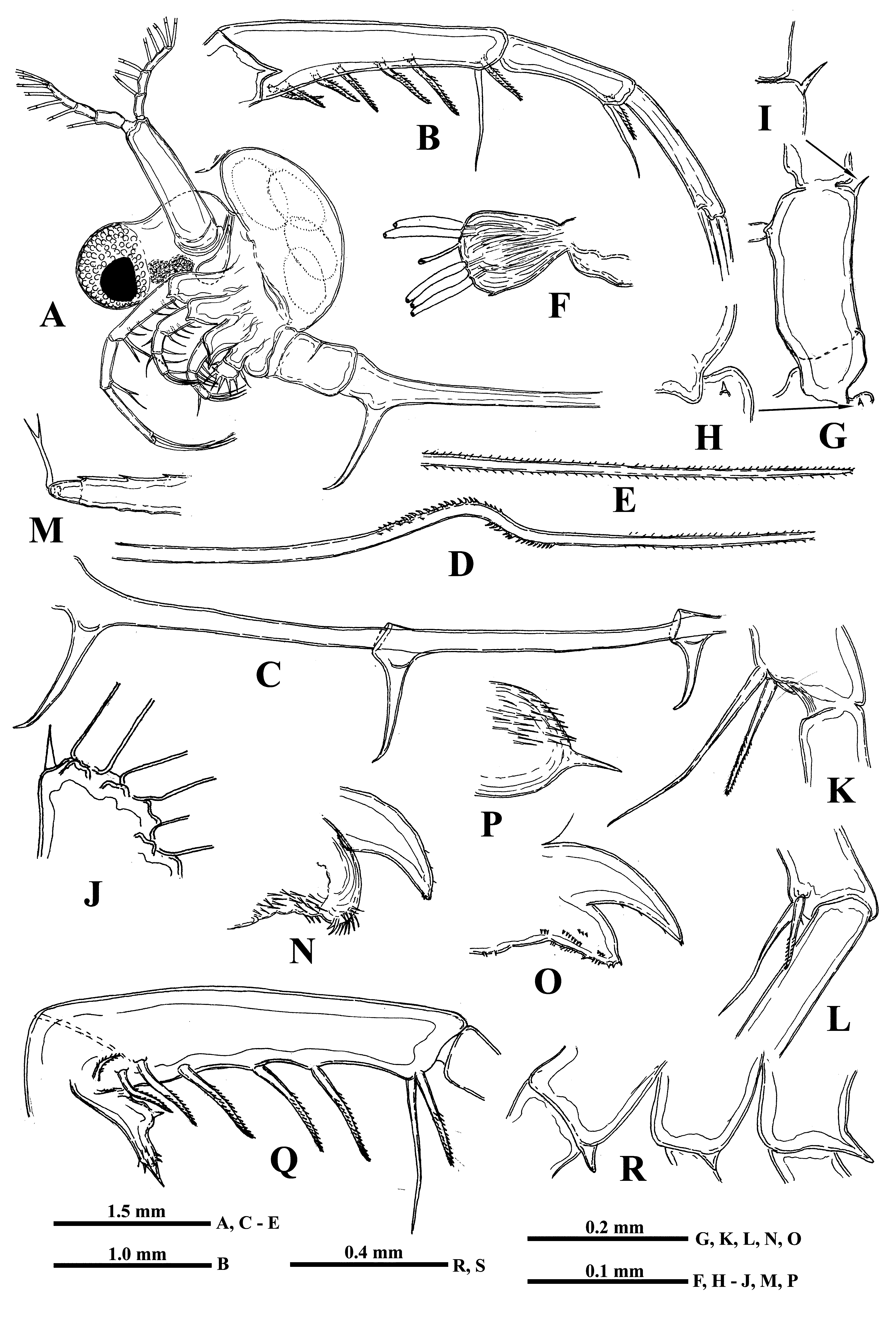
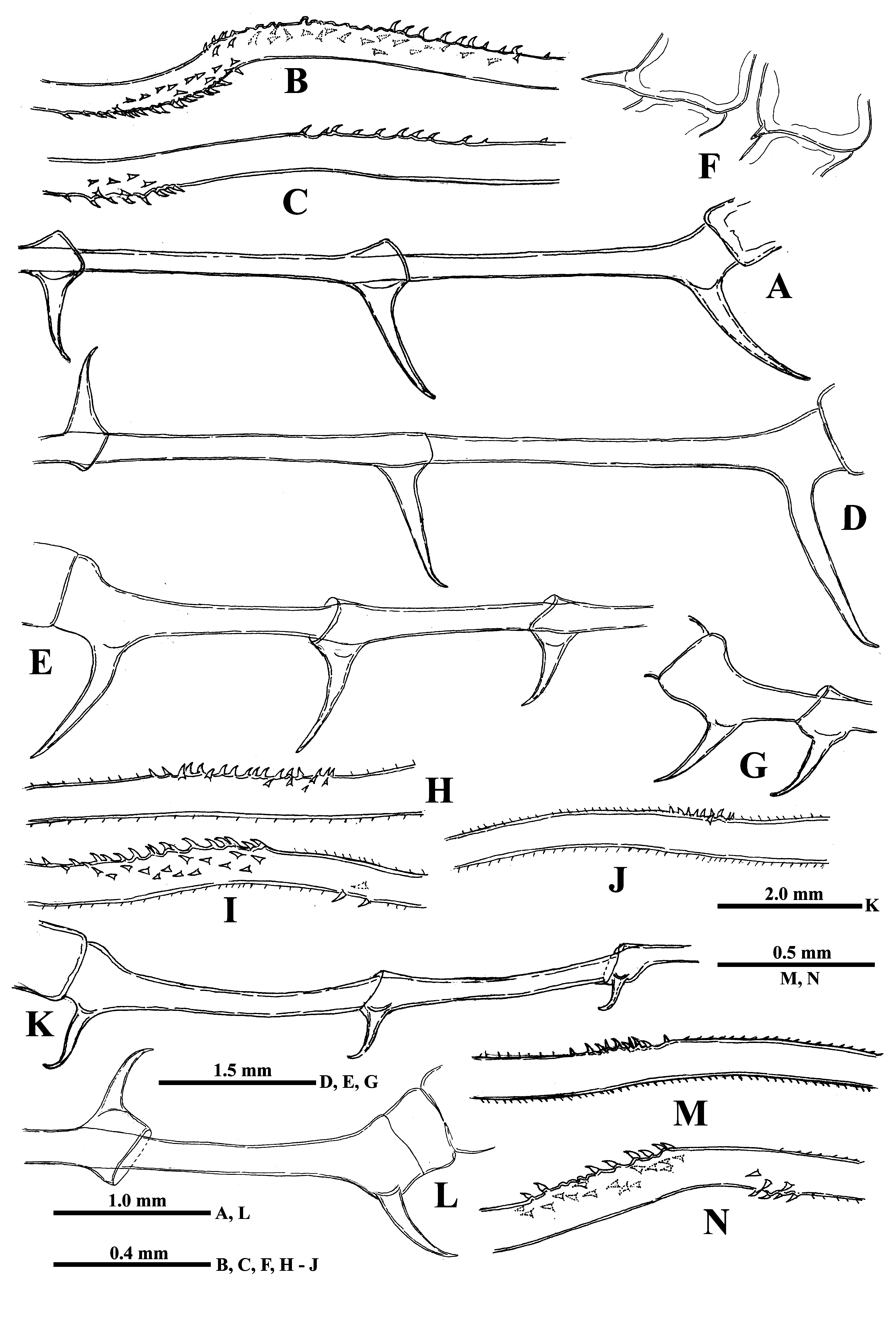
Description. Female. General body appearance and segmentation. General body shape as in B. longimanus and B. brevimanus ( Fig. 8A View FIGURE 8 ). Postabdomen bears ventrally a pair of massive and long claws curved anteriorly ( Fig. 8A, 8C View FIGURE 8 ). Caudal process is very long and thin, bearing similar but shorter claws situated distantly from the former ones and one from another and a conspicuous denticulated bend posteriorly (sometimes it may be either strongly reduced or absent) ( Figs. 8C, 8D, 8E View FIGURE 8 ). Body length of females (without caudal process) may reach 3.80 mm or slightly more (in the examined specimens it ranged from 1.01 to 3.81 mm), while the length of the caudal process exceeds the body length considerably.
Head. Comparatively very large (34.2‒45.2% of body length) and divided into two parts: a rounded anterior part mostly filled with a large compound eye and a posterior part, bearing dorsally a large saddle-shaped neck organ, swimming antennae and mouth parts.
Antennules. Small and situated on the ventral side of the anterior head part beneath the eye. They are bulbous ( Fig. 8F View FIGURE 8 ) and sit on the joined basis slightly split anteriorly. Terminally they bear five regular aesthetascs in two groups, in three and two in each one, and one shorter and thinner aesthetasc-like structure, situated in a group with two regular aesthetascs, and having a slightly widened apical end with dark granular structure inside.
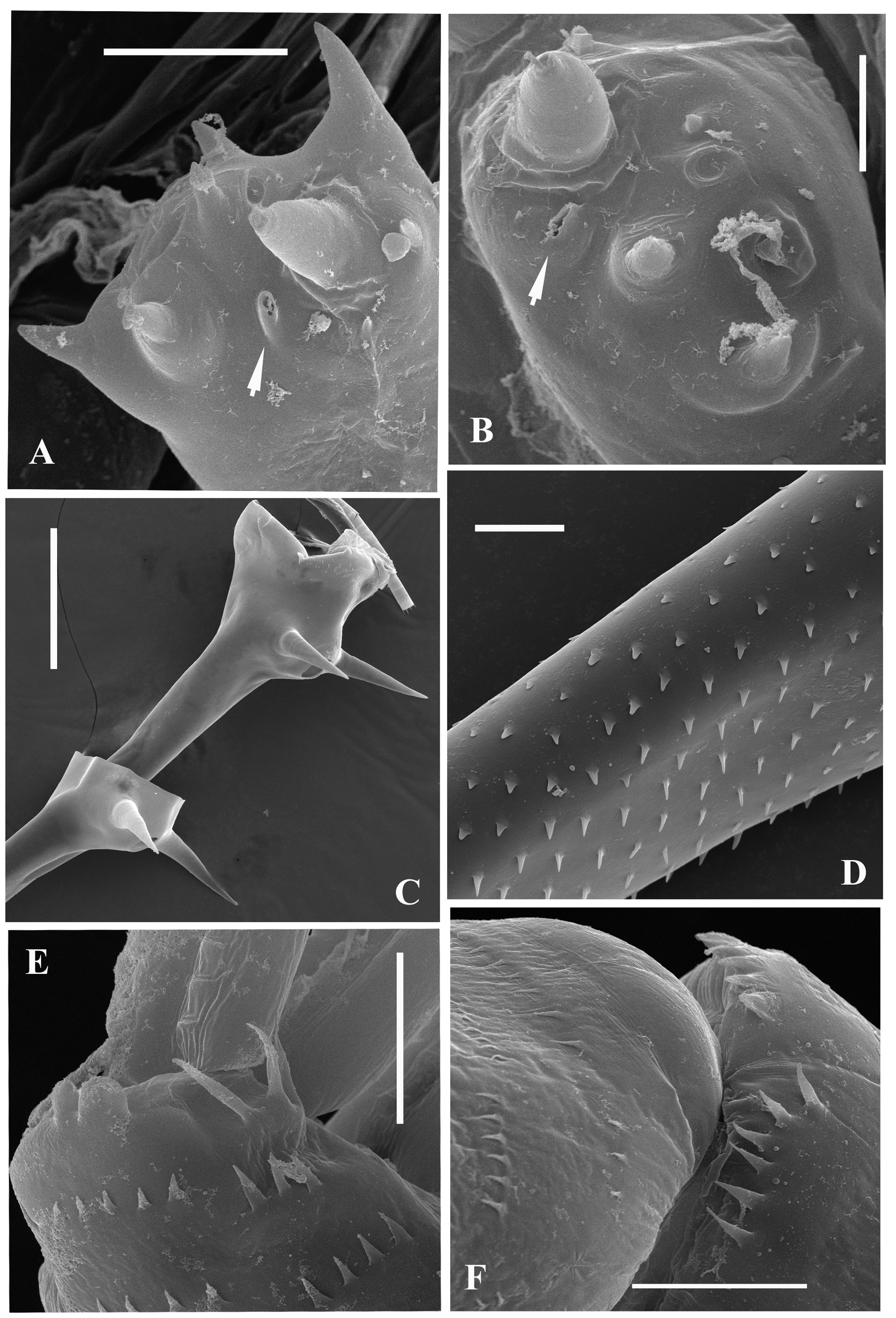
Swimming antennae. Comparatively long, with elongated cylindrical basipodite ( Fig. 8A View FIGURE 8 ) which has dorsal thin naked seta on its folded proximal part and tiny distal internal denticle located closer to base of upper branch ( Figs. 8G View FIGURE 8 , arrowed, 8H). Of two antennal branches, the lower three-segmented one (endopodite), sitting on the apical basipodital prominence, is slightly longer than upper branch. The upper branch is four-segmented and lower branch is three-segmented. Proximalmost segment of the upper branch is rudimentary and clearly visible only externally, all other segments of both branches are much more developed. Internally, two proximal segments seem fused rather firmly ( Fig. 8G View FIGURE 8 ) while distally their junction probably is more flexible. Integument of antennal basipodite and branches are covered by tiny spinules, which are situated in rows ( Figs. 5E, 5F View FIGURE 5 ). Small denticle with a saw-like row of surrounded minute prominences on the end of second segment of upper antennal branch ( Fig. 8I View FIGURE 8 ), similar prominences on the end of third segment of the same branch ( Fig. 5F View FIGURE 5 ), and small apical denticle on the distal segment of the same branch ( Fig. 8J View FIGURE 8 ). The distal segment of the lower branch is armed by two small apical spines and rounded prominences ( Fig. 5E View FIGURE 5 ). Small proximalmost segment of upper branch lacks setae, while other segments possess a row of two-segmented swimming setae of more or less similar size, except the distalmost which are shorter. All setae are bilaterally armed with rows of uniform thin setules. General formula of the antennal setae: 0‒1‒2‒5/ 1‒1‒5.
Mouth parts represented by upper lip (labrum), mandibles, and maxillules (maxilla I). The upper lip is composed of two parts: the posterior thick and slightly flattened triangular lobe and anterior large proboscis-like outgrowth ( Figs. 8N, 8O View FIGURE 8 ). The latter is separated from the former one by a deep indention of the cuticle. The triangular lobe bears numerous papillae along its posterior-interior (oral) margin ( Fig. 9A View FIGURE 9 ) while the outgrowth is armed with tiny spinules. Mandibles are bilobed and adapted for biting, with a toothed, blade-like posterior lobe and small anterior lobe (mandibular process) ( Fig. 9B View FIGURE 9 ) armed with a cluster of about 15 long outgrowths, slightly differing in size and bearing some prominences distally ( Figs. 9C, 9D View FIGURE 9 ). Posterior lobe is strongly sclerotized and divided in two tooth-shaped parts, the larger (posterior) of which has a small additional tooth about midway along its border ( Fig. 9B View FIGURE 9 ). Maxillules (mx I) look like two cylindrical structure situated posterior to mandibles. Distally, they bear short central seta and some spinules near it ( Fig. 8P View FIGURE 8 ). Maxillae (mx II) are absent; the opening of maxillar glands are situated near the bases of tl I laterally (see Olesen et al., 2003).
Carapace. It looks like a bag-like structure, strongly modified into closed brood pouch ( Fig. 8A View FIGURE 8 ) widely connected in its base with dorsal side of thorax. It may be often well developed and massive being filled by large embryos.
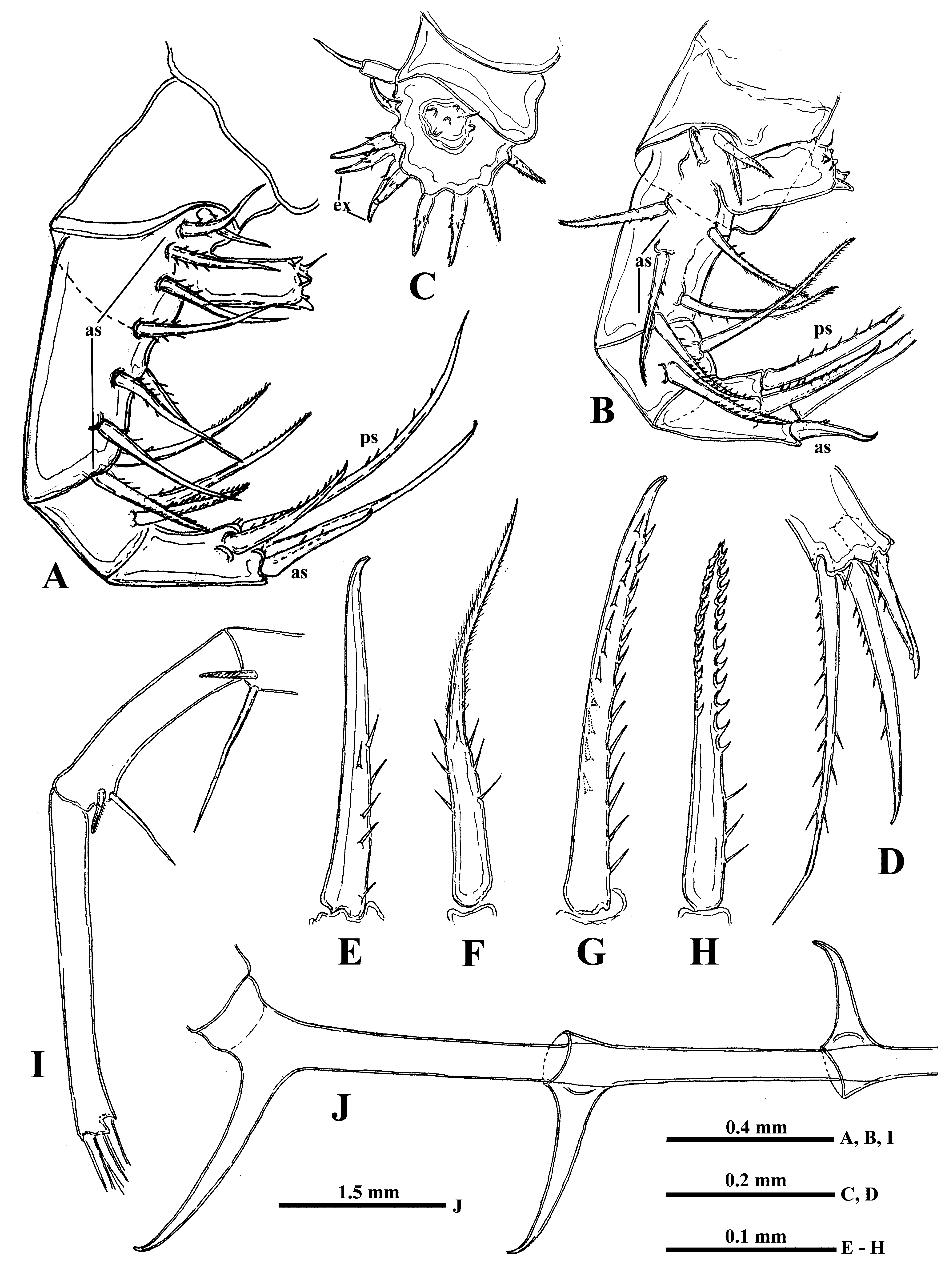
Thoracic limbs. Limbs of first pair (tl I) are especially long and strong (53.5‒88.7 % of body length) ( Figs. 8A, 8B View FIGURE 8 , 10I View FIGURE10 ). Terminally, the inner side of their protopodite bears a small triangular lobe, pseudognathobasic process (see the explanation of the term in Korovchinsky (2015)), armed laterally and distally with two outgrowths with apical setae and numerous spinules ( Figs. 8Q View FIGURE 8 , 9E View FIGURE 9 ). The external part of protopodite is longer than internal one and bears apically a small conical outgrowth ( Figs. 8B, 8R View FIGURE 8 ). The first segment of endopodite is long and bears 5‒7 anterior lateral setae ( Fig. 8Q View FIGURE 8 ). Distally, this segment bears rather long anterior seta ( Figs. 8K, 8Q View FIGURE 8 ) of type “H” (see Fig. 10H View FIGURE10 ) and longer posterior finely setulated seta ( Figs. 8Q View FIGURE 8 , 9F View FIGURE 9 ). Second segment of endopodite is conspicuously shorter and bears only two apical setae, comparatively long anterior seta of type “H” and longer posterior one ( Fig. 8L View FIGURE 8 ). The terminal, third segment of endopodite is also long but usually somewhat shorter than first proximal segment, however, sometimes it may be either of the same size or slightly longer (80.9‒102.2 % of the latter) and bears apically four long roughly spinulated setae, two of them sit terminally and two subterminally. Basally, these setae are armed with a row of smaller spines, while distally by larger lanceolate spines situated in two rows and directed terminally.
The limbs of second pair (tl II) are considerably shorter; their protopodite, again externally, is conspicuously longer and bears a conical outgrowth ( Figs. 8 View FIGURE 8 R-middle, 10A, 12F-right). The first, basal segment of their endopodite bears a row of 5‒8 (mostly 6‒7) rather long anterior lateral setae (their number also can vary in one individual) of type “F” ( Figs. 10A View FIGURE10 : as, 10F). Also there are 1‒3 posterior lateral seta of the same type on this segment ( Fig. 10A View FIGURE10 : ps). The terminal setae of the segment are different; the anterior one is shorter and roughly armored ( type “H”, see Fig. 10H View FIGURE10 ), while posterior one is longer and finely setulated. Internally, this segment bears stout cylindrical pseudognathobasic process ( Fig. 10A View FIGURE10 ), possessing some prominences of different size; one small, thin seta, and a pore apically ( Fig. 11A View FIGURE 11 ). The second segment of endopodite is short with only two setae, the anterior of which ( Fig. 10A View FIGURE10 , 11D View FIGURE 11 ) is similar to anterior terminal seta of previous segment, while the posterior seta is longer and finely setulated. The distal, third segment of endopodite of the limb bears four setae, two terminal and two subterminal ones ( Fig. 10A View FIGURE10 ). Of the latter, the anterior seta is comparatively short, thick and armed with a number of thin lateral denticles, while its distal end is naked and slightly hooked apically ( Fig.10G View FIGURE10 ). Its neighboring posterior subterminal seta is considerably longer and similar to subterminal and terminal setae of tl I, having similar spine armament and sharp apex. The anterior terminal seta is thick and comparatively short with longitudinal ribs, few thin lateral denticles, and slightly hooked apical end ( Fig. 10E View FIGURE10 ). The posterior terminal seta similar to the neighboring anterior one but longer having few lateral denticles and slightly hooked apical end.
The limbs of the third pair (tl III) ( Fig. 10B View FIGURE10 ) are generally similar to those of the previous ones, differing in some details. The external outgrowth of their protopodite is conspicuously larger ( Fig. 8 View FIGURE 8 R-right; 12 F-left) and lateral anterior and posterior setae (if present) of first segment of endopodite are fewer (5‒6 and 1, respectively) ( Fig. 10B View FIGURE10 ). Distal setae of the segment are similar to other ones. The pseudognathobasic process is also similar to that one of tl II ( Fig. 11B View FIGURE 11 ). Of setae of the second segment, the anterior one is similar to the respective one of tl II. Terminal and subterminal setae of third segment ( Fig. 10D View FIGURE10 ) are similar to those of tl II but slightly shorter and bear fewer denticles.
The limbs of the fourth pair (tl IV) ( Fig. 10C View FIGURE10 ) are considerably reduced; their protopodite bears slightly spinulated seta sited on a short cylindrical base. The only segment of endopodite has two rows of comparatively short spine-like setae. The external row (group) ( Fig. 10C View FIGURE10 : ex) always consists of two setae, and the internal row of 6‒8 setae, which differ in their appearance and armament. Almost the whole internal part of the endopodital segment is occupied by the reduced pseudognathobasic process, also having a pore and armed by some denticles and thin seta ( Fig. 11C View FIGURE 11 ).
Abdomen (metasome) ( Fig. 8A View FIGURE 8 ) is often deformed. It is inconspicuously delimited into two segments, short proximal and long distal with a prominent fold more or less in the middle on the dorsal side.
“ Postabdomen” consisting of two parts: the last small abdominal segment and postabdomen per se (see Korovchinsky (2015)) is comparatively small with the anal opening situated between postabdominal claws which flare outwards from the midline ( Figs. 8C View FIGURE 8 , 10J View FIGURE10 , 12A, 12D, 12E, 12G, 12K, 12L View FIGURE 12 ); the latter large and stout, quite varying in their size with apical ends curved forward (4.9‒51.0 %, usually 16.8‒41.2 % of body length).
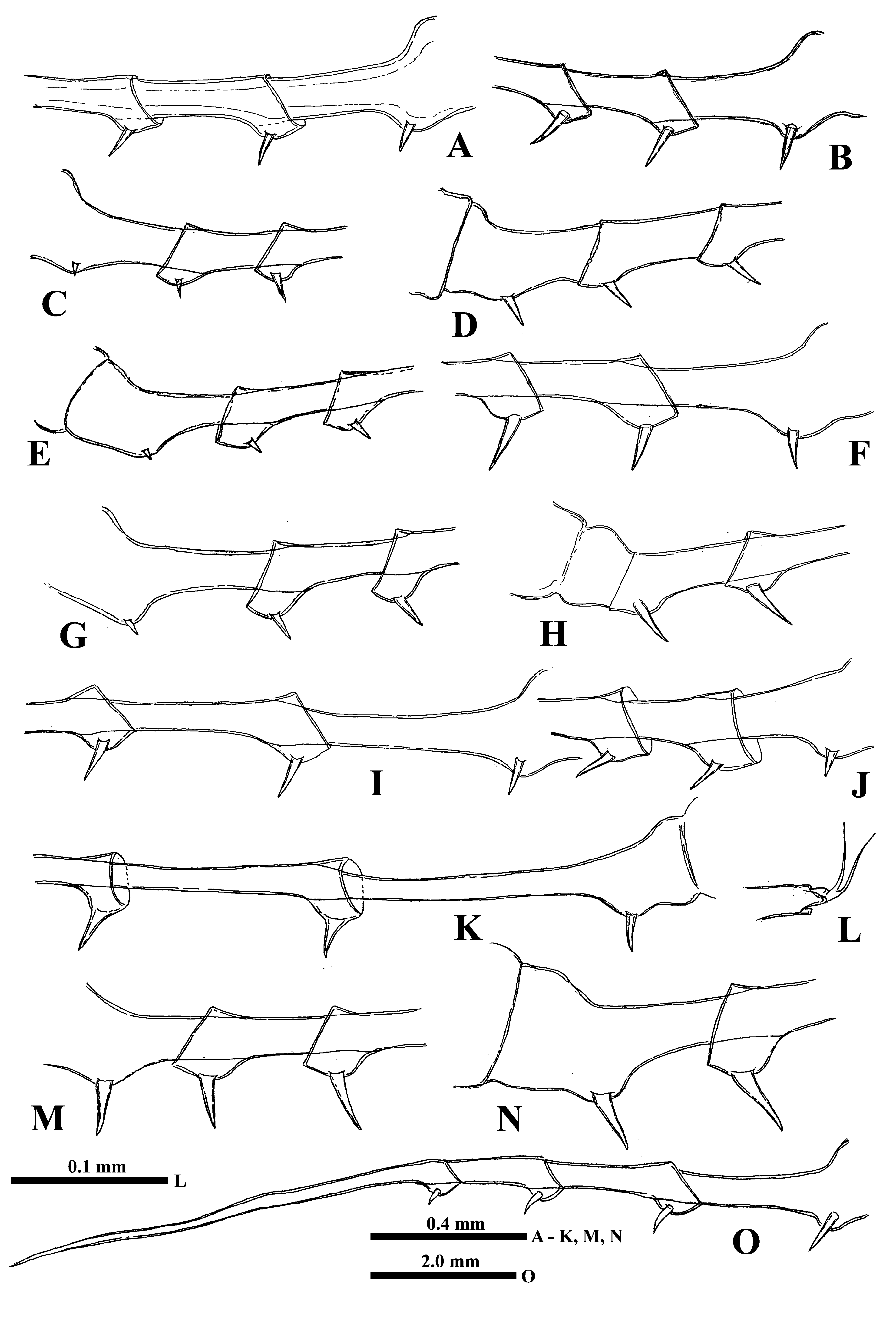
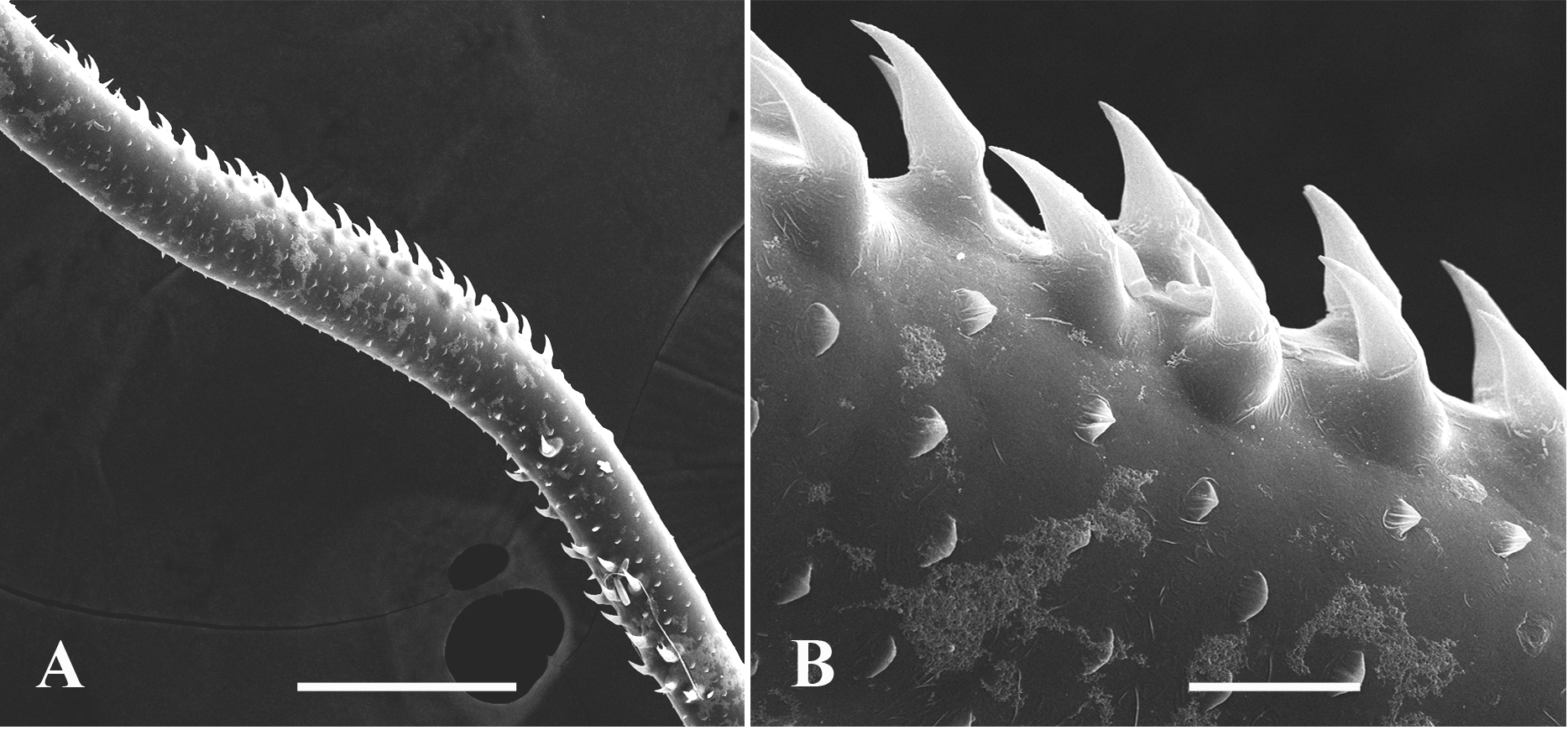
Caudal process is directly connected with postabdomen and then proceeds as a very long spine-like structure quite variable in its length (167.0‒545.5 %, usually 209.0‒512.0 %, of body length), thus surpassing body length in 1.6‒5.5 times ( Figs. 8A, 8C, 8D, 8E View FIGURE 8 ). It is comparatively thin; its thickness is also conspicuously variable (3.3‒7.8 % of body length). Infrequently, the caudal process possesses more sclerotized and darker colored portions. In its proximal half, it bears one or two pairs of claws similar to those on postabdomen but smaller (anterior pair 14.6‒ 32.7 % and posterior pair 5.3‒ 24.1 % of body length; in specimens from Lake Bolmen they are smaller: 6.9‒13.7 % of body length) ( Figs. 8C View FIGURE 8 , 10J View FIGURE10 , 12A, 12D, 12E, 12G, 12K, 12L View FIGURE 12 ). All pairs of claws usually sit quite distantly one from another, however, rarely they may be rather densely situated ( Fig. 12G View FIGURE 12 ) (9.2‒162.0, usually 27.4‒98.4% of body length), the posterior pair of claws is often turned upwards ( Figs. 10J View FIGURE10 , 12G, 12L View FIGURE 12 ). In its middle part or usually more distally, rarely more proximally (in 24.2‒71.7 % of length of caudal process), caudal process bears a prominent bend ( Figs. 8D View FIGURE 8 , 13A View FIGURE 13 ) covered by numerous small curved denticles directed forward, which sit on its dorsal and ventral sides ( Figs. 12B, 12N View FIGURE 12 , 13A, 13B View FIGURE 13 ). In specimens from different populations, and even in specimens of one population, this bend can be differently pronounced being either strongly or moderately developed ( Figs. 8D View FIGURE 8 , 12B, 12N View FIGURE 12 ) or underdeveloped ( Figs. 12C, 12I View FIGURE 12 ) and sometimes absent completely ( Figs.12H, 12J, 12M View FIGURE 12 ). In the latter case, the position of the bend is marked only by a few or even solitary large denticles. In isolated individuals, the bend and denticles can be absent at all, thus the caudal process has appeared to be straight, covered by numerous minute spinules only. Apically, the caudal process bears two minute setae arising from a common base ( Fig. 8M View FIGURE 8 ). Borders separating old molted integuments of caudal process with claws normally are quite conspicuous.
Size 1.01‒3.60 mm, up to 4.50 mm (“ B.c. var. robustus ”) according to Lilljeborg (1901).
Gamogenetic females differ from parthenogenetic ones only in presence of large yellow-brownish resting eggs ( 0.34‒0.46 mm in diameter) in their brood pouches. According to Lilljeborg (1901), large females (“ B.c. var. robustus ”) could carry up to six such eggs. Body length 1.98‒3.81 mm.
Males ( Fig. 14B View FIGURE 14 ), on average, are smaller than females (body length: 1.38‒3.04 mm, up to 3.60 mm (“ B.c. var. robustus ”) according to Lilljeborg (1901). Thoracic limbs of first pair (tl I) are of moderate size (61.6‒66.7 % of body length) as well as each endopodital segment of them, especially distal one (14.5‒15.9 % of body length), which is slightly swollen proximally and bears on its inner side a small strongly chitinized hook with two inner denticles; a circular area of tiny denticles with softer prominent cuticle in its center is situated under it ( Figs. 14C, 14D View FIGURE 14 , 11E, 11F View FIGURE 11 ). In juvenile males with one pair of postabdominal claws, this hook and copulatory appendages are undeveloped ( Figs. 14F, 14G View FIGURE 14 ). In adult specimens, the copulatory appendages are small (6.0‒12.7 % of body length) and armed with numerous minute spinules terminally ( Fig. 14E View FIGURE 14 ).
Juvenile females. Studied juvenile females differ from adults in slightly thicker caudal process and position of caudal bend situated closer to postabdomen (in 35.9‒37.4 % of length of caudal process) ( Korovchinsky 2015).
Intra- and interpopulation variability. The variability of some morphological structures of type specimens from Lake Saxen and specimens of other populations studied earlier ( Korovchinsky 2015) seem considerable. This mostly concerns length of tl I and their distal segment, apical setae of first and second segments of endopodite which might be slender or stouter with either more delicate or rough armament, length of caudal process and size of claws. Specimens from Lakes Bolmen and Store Le ( Sweden) possessed especially prominent bends on extremely long caudal processes (see Korovchinsky 2015). Those from the former locality have unusually small claws of postabdomen and caudal process. Subsequently, a similar feature was observed in some specimens from Lake Onega ( Russia) ( Fig. 12K View FIGURE 12 ).
Data on additional populations of the species ( Table 2) have also revealed considerable Intra- and interpopulation variability in body size, tl I size, and mostly in caudal process and claws. Specimens from Mullsj ӧn ( Sweden) (“ B. c. var. connectens ”) were especially variable (see also Lilljeborg (1901)), unusually small, and possessed unusually small claws whereas those from Lakes Pozemskoye, Nizhnee Nilm-ozero, and Taiylaar ( Russia) were conspicuously larger and generally less variable.
It is of special interest that specimens in some particular populations were quite variable with respect to the structure of the caudal process. Most of them possessed the process with either strongly or moderately developed denticulated bend, but in some others this bend could be almost or completely absent and its place was marked either by a group of denticles or solitary denticles ( Figs. 12H, 12J, 12M View FIGURE 12 ). Finally, a few specimens had a straight caudal process without any bend and denticles. At the same time, the presence of the characteristic large claws with apical ends curved forward unequivocally determines them as B. cederstr ӧmii (however, within typical specimens could occasionally be present single ones with abnormal claws (see Fig. 14A View FIGURE 14 )). For instance, six of 15 studied specimens from Mullsj ӧn ( Sweden) designated by Lilljeborg “ B. c. var. connectens & forma minor ” or “ B. c. robustus minor ” had a caudal process without the bend and only large denticles in its place. Specimens of “ B. c. robustus ” from Muoniovara ( Sweden) sometimes either had no bend but only large denticles in its place or had a straight caudal process without both bend and denticles. The same peculiarities were observed in populations of the species from the delta of the River Pechora, Lake Onega, Lake Tchernoye near Kostroma City ( Russia), and Lake Erne ( Ireland).
Remarks. A quite detailed description of the North American representatives of the species (their conspecificity should be checked further) was provided previously by Martin and Cash-Clark (1995). It fully coincides with the above description and partly supplements it with respect to SEM micrographs of some structures.
New material studied in the course of the present investigation permitted reevaluation of the diagnostic features of the species under consideration. It became clear that they were incorrectly understood being in fact much more variable. The type specimens and other ones studied previously (see Korovchinsky 2015) can be considered now as having very strongly developed morphological features. Moreover, their head and eye were deformed which prevented the adequate measurements of the individuals. The features of these specimens, i.e., very long caudal process, extremely large claws as well as unusually large interclaw distance, are clearly differentiated from those of the representatives of other populations. Only those from Lake Pozemskoye ( Karelia, Russia) approach the type specimens according to these parameters (see Table 2). All this has hindered the proper comparative evaluation of type and other specimens studied.
B. cederstr ӧmii can be viewed now as a quite morphologically variable species which includes a number of forms, “ B. c. var. robustus ”, “ B. c. var. connectens ”, and other unnamed ones. The representatives of the species may be of different body size having tl I, caudal process and claws of different size and structure. The caudal process usually possesses a more or less developed denticulated bend situated either closer to or farther from the claws. Infrequently, in some specimens of any population, the bend may be absent and only large denticles are present in its place. Finally, the bend and denticles may be absent at all, thus the distal part of caudal process has appeared to be straight and armed only with tiny spinules. Also the claws can be situated either more or less closely or distantly to each other. Normally, they are long and massive with their apical ends curved forward, but sometimes they may be either smaller or less curved.
Previously, it was concluded (see Korovchinsky 2015) that a mixture of typical B. cederstr ӧmii individuals with those having no denticulated bend comprised populations of hybrid forms. Also, hybrid status was assigned to specimens with rather densely situated claws of postabdomen and comparatively short caudal process. Now these statements must be corrected.
Differential diagnosis. B. cederströmii differs from all other species of the genus firstly in the presence of large, massive (rarely comparatively small) postabdominal claws, flared outwards, and apically curved forward, and caudal process (it is important to check a number of individuals from any particular population in respect of stability of the feature because the populations of the superficially similar hybrid individuals display great variability of size and shape of both caudal process and claws). The caudal process is long and comparatively thin, normally with denticulated bend; however, sometimes this bend may be absent and either only large denticles are present in its place or, even more rarely, both bend and denticles are absent altogether.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
SuperOrder |
Cladocera |
|
Order |
|
|
Family |
|
|
Genus |
Bythotrephes cederströmii Schödler, 1877
| Korovchinsky, Nikolai M. 2018 |
Bythotrephes Cederströmii Schödler, 1877
| Schödler 1877 : 233 |
Bythotrephes borealis
| Sars 1890 : 51 |
Bythotrephes cederströmii var. cederströmii
| Lilljeborg 1901 : 619 |
Bythotrephes cederströmii var. robustus :
| Lilljeborg 1901 : 621 |
Bythotrephes cederströmii var. connectens : Lilljeborg 1901 : 623
| Lilljeborg 1901 : 623 |
Bythotrephes cederströmii Schödler, 1877
| Rylov 1935a : 154 |
| Litvinchuk 2002 : 126 |
Bythotrephes longimanus cederströmii:
| Vekhov 1987 : 28 |