Echinoderes parthenope, Cepeda & González-Casarrubios & Sánchez & Spedicato & Michaud & Zeppilli, 2022
|
publication ID |
https://doi.org/ 10.1016/j.jcz.2022.09.001 |
|
persistent identifier |
https://treatment.plazi.org/id/616487EB-FFFB-7755-FFF2-CEF3CA7BFD93 |
|
treatment provided by |
Felipe |
|
scientific name |
Echinoderes parthenope |
| status |
sp. nov. |
3.2. Echinoderes parthenope View in CoL sp. nov
Zoobank code: urn:lsid:zoobank.org:act:4E6BFE9E-D25F-4A91-
99F0E5D47E37BA91.
3.2.1. Material examined
Holotype, adult male, collected on October 6th, 2018, at Chirongui Bay , Grande-Terre Island, Mayotte Archipelago, SW Indian Ocean:
12 Ǫ 55 ′ 17.5 ′′ S, 45 Ǫ 09 ′ 10.4 ′′ E 12 Ǫ 55 ′ 22.8 ′′ S, 45 Ǫ 09 ′ 09.1 ′′ E in black organic mud at the intertidal zone; mounted in Fluoromount G ®, deposited at MNHN under catalogue number: 613 Ma. Paratypes, five adult males and one adult female, same collecting data as holotype; mounted in Fluoromount G ®, deposited at MNHN under catalogue numbers: 637 Ma, 641 Ma-643 Ma, 649 Ma and 651 Ma. Additional material, one adult male, same collecting data as type material; mounted for SEM, stored at the Meiofauna collection of the UCM; 10 juveniles, same collecting data as type material, deposited at MNHN under catalogue numbers: 638 Ma-640 Ma, 644 Ma-648 Ma, 650 Ma and 652 Ma .
3.2.2. Diagnosis
Echinoderes with spines that are short, poorly sclerotized, weakly articulated in middorsal position on segment 4, lateroventral position on segment 6, sublateral position on segment 7 and lateral accessory position on segment 8, plus large tubes in lateroventral position on segment 5, sublateral position on segment 8 and laterodorsal position on segment 10 (only in males). Minute, rounded type 2 glandular cell outlets in subdorsal position on segments 5–6, in laterodorsal position on segment 9, in midlateral position on segment 4, in sublateral position on segment 7, and in lateral accessory position on segment 6. Enlarged, triangular sieve plates in sublateral position on segment 9, consisting of a slightly convex region bearing numerous pores followed by a small, slightly concave area bearing a single, enlarged pore. Males with tergal plate of segment 10 forming a pair of cuticular, rectangular extensions in subdorsal position, with straight, abruptly tapering, slightly curved tips; females with tufts of hairs instead. Males with tergal plate of segment 11 forming a middorsal, triangular, bulged process that extends slightly beyond the tergal extensions.
3.2.3. Etymology
The species is dedicated to the Greek, mythological mermaid Parthenope (from the Ancient Greek Παρθενόπη). Parthenope gave name to the first Greek settlement now part of the Italian city of Naples. According to legend, Parthenope despaired after failing to lure Odysseus to her island, cast herself into the sea and drowned, her body washed ashore near Naples. The mermaid is usually represented in art with two tails, which resembles in shape the characteristic segment 11 tergal plate of the species.
3.2.4. Description
See Table 4 for measurements of selected morphological traits and dimensions, and Table 5 for summary of acicular spine, tube, nephridiopore, glandular cell outlet, sensory spot, hair tuft and cuticular extension locations.
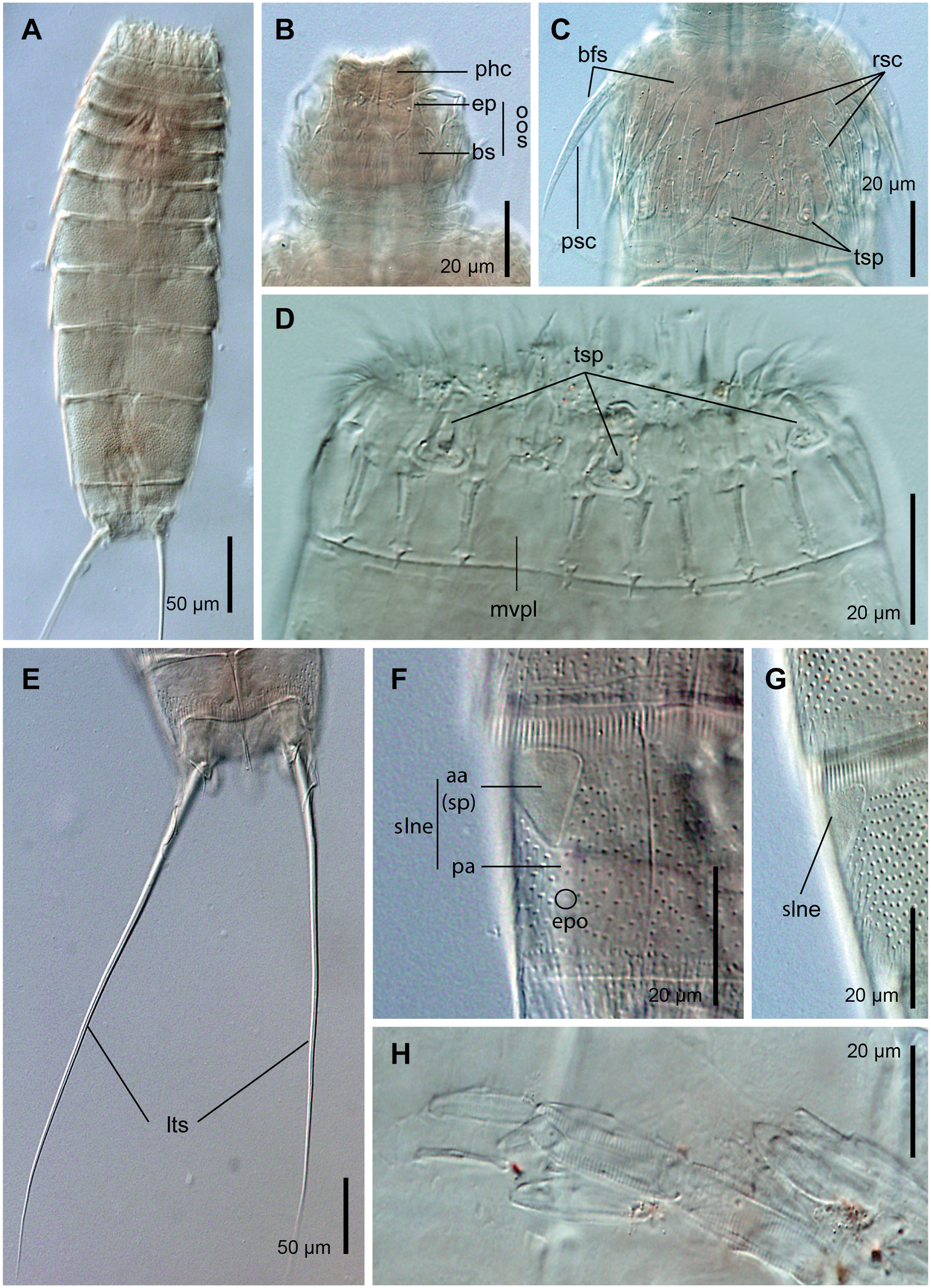
Head: a single specimen for LM was found with a completely everted head, hence only a few details in some structures can be provided. Ring 00 of mouth cone with nine outer oral styles alternating in size between slightly larger and smaller ones. Outer oral styles composed of two jointed subunits: a rectangular, basal piece with two proximal fringes located at their lateral edges with a couple of tips each one; and a triangular, hooked, distally pointed and curved end-piece ( Fig. 6B View Fig ). Triangular, poorly sclerotized cuticular thickenings flanking the outer oral styles. Outer oral styles located anterior to each introvert sector, except in the middorsal sector 6.
Introvert with six rings of scalids (one ring of primary spinoscalids and five rings of regular-sized scalids) and 10 longitudinal sectors delimited by the arrangement of the primary spinoscalids. Ring 01 of introvert with 10 primary spinoscalids, larger than remaining ones, laterally compressed, composed of a medially fringed basal sheath and a distal, elongated, flexible, distally pointed end-piece. Remaining rings of introvert with scalids variable in size but always smaller than the primary spinoscalids, morphologically similar to the latter ( Fig. 6C View Fig ). Scalids tend to collapse when mounted for LM, and specimens for SEM with fully everted heads were not available, so details on the scalid arrangement are not provided.
Neck: 16 trapezoidal placids, slightly wider at base, closely adjacent, with distinct joint between the neck and first trunk segment. Midventral placid widest (ca. 13–15 μm wide at base), remaining ones narrower (ca. 8–10 μm wide at base). A ring of six long, superficially haired trichoscalids associated with the placids, attached to small, rounded trichoscalid plates ( Fig. 5A–B; 6 View Fig C-D; 7A-C; 9A-B).
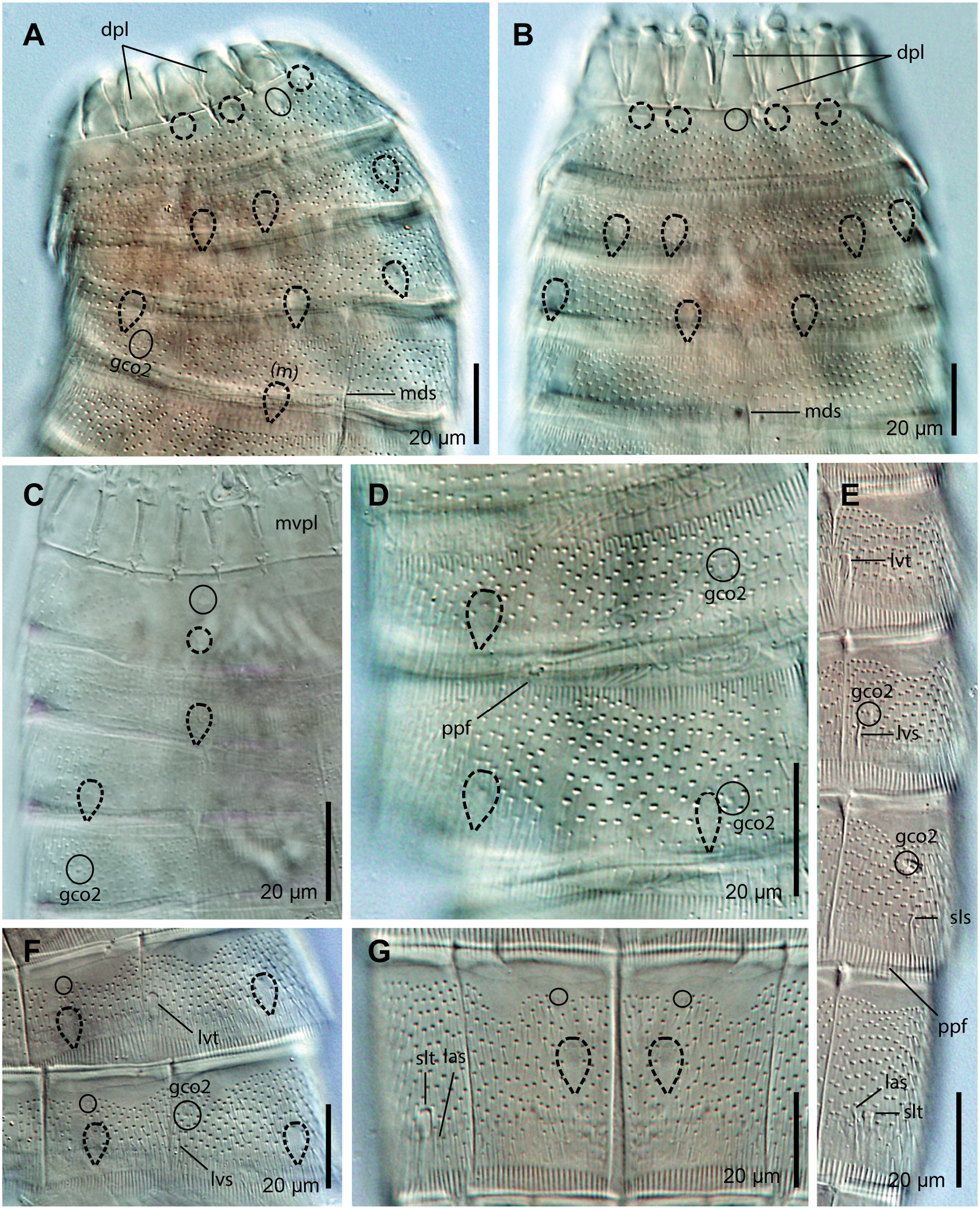
Trunk: fusiform to softly rectangular, composed of 11 segments, heart-shaped in cross-section. Segments 1–2 closed, ring-like cuticular plates, remaining ones with one tergal and two sternal cuticular plates ( Fig. 5A–D; 6A View Fig ; 9A). Maximum sternal width at segment 8, trunk progressively tapering toward anterior and posterior ends. Cuticular hairs throughout segments 1–10, acicular, non-bracteate, distally pointed, emerging from rounded to slightly oval perforation sites. Cuticular hairs arranged as 8–10 (dorsal) and 4–6 (ventral) straight, irregular, transversal rows densely covering the whole surface of segment 1 dorsally and the most posterior half of the segment ventrally; as 6–8 transversal rows densely covering the whole surface of segment 2; as 6–16 transversal rows, becoming wavy at the lateral and ventral areas, densely covering the whole surface of segments 3–9 except in the vicinity of the droplet-shaped sensory spots and the large, oval areas (muscular scars) in laterodorsal and paraventral positions (also of segment 10 in females); as 3–5 transversal rows densely covering the midlateral to ventrolateral region of segment 10 in males ( Fig. 5A–D; 6 View Fig E-G; 7A-G; 8A-B, G; 9B-L). Muscular scars in laterodorsal position on segments 3–9, with several spherical perforations ( Fig. 5A–B; 7 View Fig A-B, D; 8A; 9B, E, J). Posterior segment margins straight, with a strongly serrated primary pectinate fringe ( Fig. 5A–D; 6 View Fig F-G; 7A-G; 8A-C, E, G; 9A-B, D-F, H-J, L). Secondary pectinate fringes not detected.
Segment 1: type 1 glandular cell outlets in middorsal and lateroventral positions, the former near the anterior segment margin, the latter located at the anterior half of the segment ( Fig. 5A–B; 7 View Fig A-C); type 1 glandular cell outlets on this and following segments rounded, with a single pore. Rounded sensory spots (sensu Lundbye et al., 2011) in subdorsal, laterodorsal and lateroventral positions ( Fig. 5A–B; 7 View Fig A-C; 9B-C). Rounded sensory spots as circular, depressed areas with 5–6 rings of micropapillae surrounding a single, central pore with a short, emerging cilium ( Fig. 9B–C).
Segment 2: droplet-shaped sensory spots (sensu Lundbye et al., 2011) in subdorsal, laterodorsal and ventrolateral positions ( Fig. 5A–B; 7 View Fig A-C; 9B). Droplet-shaped sensory spots, on this and following segments, as oval areas with 7–8 rings of micropapillae with a single, anterior pore from which a long, thin cilium may emerge ( Fig. 9B, E-H; J-K).
Segment 3: droplet-shaped sensory spots in subdorsal and midlateral position ( Fig. 5B; 7 View Fig A-C; 9B).
Segment 4: acicular spine in middorsal position ( Fig. 5B; 7 View Fig A-B; 9D); acicular spines on this and following segments are short (ca. 3–8 μm long), poorly sclerotized and weakly articulated ( Fig. 5A–B; 7 View Fig E-G; 9D, H- I). Type 2 glandular cell outlets in midlateral position ( Fig. 5B; 7C View Fig ; 9E);
(caption on next page)
type 2 glandular cell outlets, on this and following segments, as minute, circular areas smaller than sensory spots bearing a single pore ( Fig. 9E, G-H, J). Droplet-shaped sensory spots in subdorsal position only in males ( Fig. 5B; 7A View Fig ).
Segment 5: tubes in lateroventral position ( Fig. 5A; 7 View Fig E-F; 9E). Type 2 glandular cell outlets in subdorsal position ( Fig. 5B; 7D View Fig ; 9E). Type 1 glandular cell outlets in ventromedial position ( Fig. 5A; 7F View Fig ). Droplet-shaped sensory spots in midlateral and ventromedial positions ( Fig. 5A–B; 7D, F View Fig ; 9 E-F).
Segment 6: acicular spines in lateroventral position ( Fig. 5A; 7 View Fig E-F). Type 2 glandular cell outlets in subdorsal and lateral accessory positions ( Fig. 5A–B; 7 View Fig D-F; 9E, G). Type 1 glandular cell outlets in ventromedial position ( Fig. 5A; 7F View Fig ). Droplet-shaped sensory spots in subdorsal, midlateral and ventromedial positions, the former located more lateral than the subdorsal type 2 glandular cell outlets but still in subdorsal position ( Fig. 5A–B; 7D, F View Fig ; 9 E-G).
Segment 7: acicular spines in sublateral position ( Fig. 5A; 7E View Fig ; 9H). Type 2 glandular cell outlets in sublateral position, slightly lateral or above the acicular spines ( Fig. 5A; 7E View Fig ; 9H). Type 1 glandular cell outlets in ventromedial position ( Fig. 5A). Droplet-shaped sensory spots in subdorsal, midlateral and ventromedial positions ( Fig. 5A–B; 9F, H).
Segment 8: acicular spines in lateral accessory position, and large tubes in sublateral position ( Fig. 5A; 7E, G View Fig ; 9I). Type 1 glandular cell outlets in ventromedial position ( Fig. 5A; 7G View Fig ). Droplet-shaped sensory spots in subdorsal and ventromedial positions ( Fig. 5A–B; 7G View Fig ; 8A View Fig ).
Segment 9: type 2 glandular cell outlets in laterodorsal position ( Fig. 5B; 8A View Fig ; 9J). Type 1 glandular cell outlets in ventromedial position ( Fig. 5A; 8B View Fig ). Droplet-shaped sensory spots in paradorsal, subdorsal, midlateral and ventrolateral positions ( Fig. 5A–B; 8 View Fig A-B; 9J-K). Nephridiopores in sublateral position as triangular, enlarged sieve plates consisting of a slightly convex area with numerous pores followed by a small posterior, slightly concave region with a single, enlarged pore ( Fig. 5A–B; 6 View Fig F-G; 9J).
Segment 10: retractable into segment 9 ( Fig. 5A–D; 6A View Fig ; 9A). Large tubes in laterodorsal position only in males ( Fig. 5B; 8 E View Fig ; 9 J, L). Two type 1 glandular cell outlets in middorsal position, longitudinally aligned ( Fig. 5B, D; 8E View Fig ). Tergal plate of males with a pair of cuticular extensions in subdorsal position, rectangular, with straight, abruptly tapering, slightly curved tips protruding into the cuticular surface of the following segment; tergal plates of females with tufts of hairs instead ( Fig. 5A–B, D; 8E View Fig ).
Segment 11: retractable into segments 9–10 ( Fig. 5A–D; 6A View Fig ; 9A). Relatively long lateral terminal spines ( LTS:TL average ratio = 52.9%), apparently well sclerotized but still flexible, distally pointed, with a hollow central cavity ( Fig. 5A–D; 6E View Fig ; 8D View Fig ; 9A, L). Males with three pairs of penile spines, dorsal and ventral pairs longer and slender, smooth and distally rounded, medial pair shorter and stouter, hairy and distally blunt ( Fig. 5A–B; 8C View Fig ; 9L); females with short ( LTAS: LTS value =10.1%), slender, lateral terminal accessory spines ( Fig. 5C–D; 8F View Fig ). Female gonopores near the anterolateral margins of sternal plates ( Fig. 8G View Fig ). Type 1 glandular cell outlet in middorsal position ( Fig. 5B, D; 8E View Fig ). Tergal plate of males with a middorsal, triangular, bulged process that extends slightly beyond the tergal extensions; blade-like tergal extensions, distally pointed ( Fig. 5A–D; 8 View Fig D-E; 9L). Sternal extensions short, distally rounded ( Fig. 5A, C; 8 View Fig F-G) .
3.2.5. Remarks
Several specimens were found with ingested clusters of diatoms in the hindgut (segments 8–10) ( Fig. 6H View Fig ). Interestingly, the diatoms seem to be unaltered despite they were swallowed by the animals.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.