Jessethoa ausubeli, Gordon, 2020
Gordon, Dennis P., 2020, New Hippothoidae (Bryozoa) from Australasia, Zootaxa 4750 (4), pp. 451-476 : 461-464
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4750.4.1 |
|
publication LSID |
lsid:zoobank.org:pub:AE9FDD46-5471-44B3-97FB-11C4BD45C59B |
|
DOI |
https://doi.org/10.5281/zenodo.3717928 |
|
persistent identifier |
https://treatment.plazi.org/id/0BB77268-A5E5-45B9-B51C-9B51C5D79A1D |
|
taxon LSID |
lsid:zoobank.org:act:0BB77268-A5E5-45B9-B51C-9B51C5D79A1D |
|
treatment provided by |
Plazi |
|
scientific name |
Jessethoa ausubeli |
| status |
sp. nov. |
Jessethoa ausubeli n. sp.
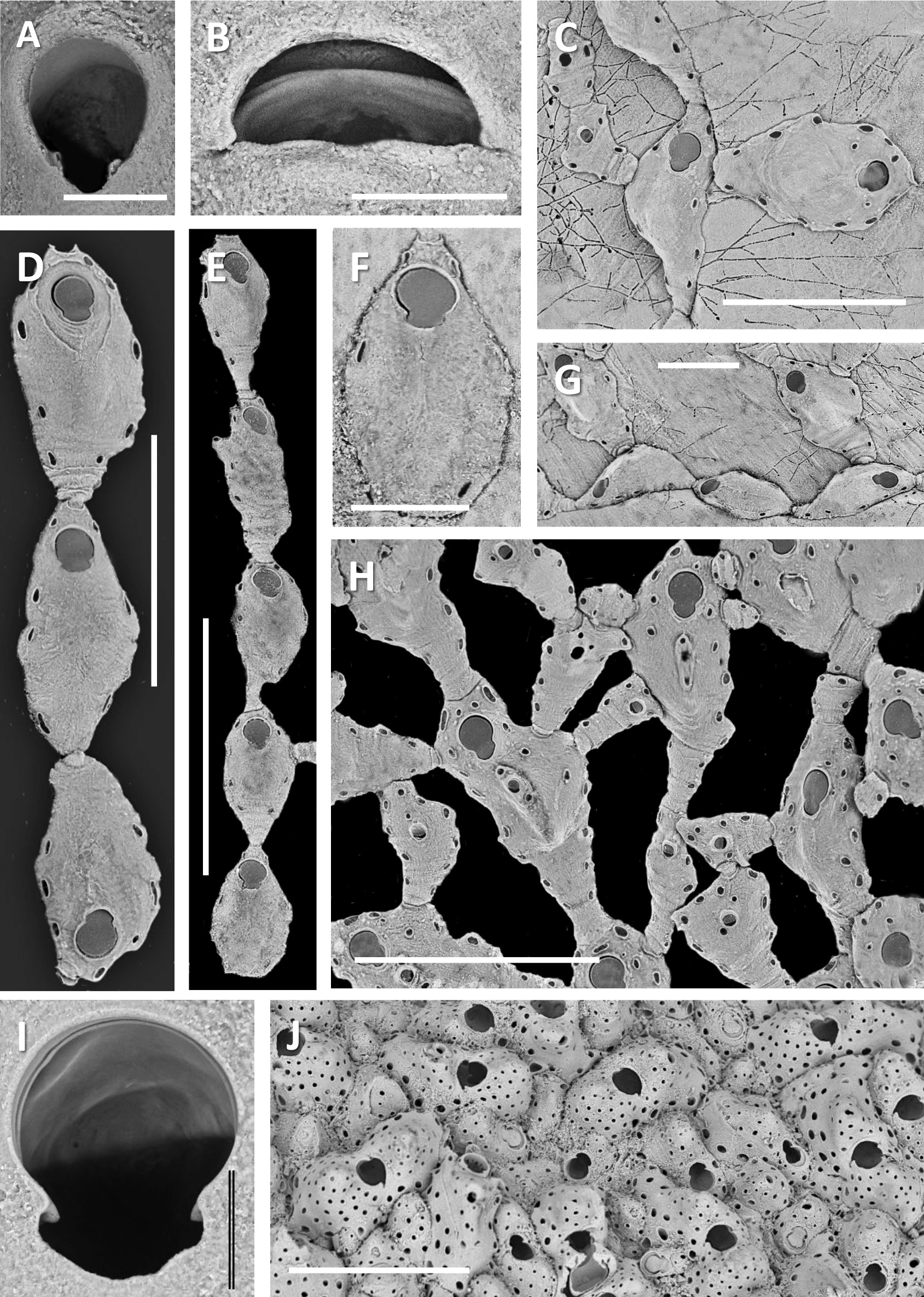
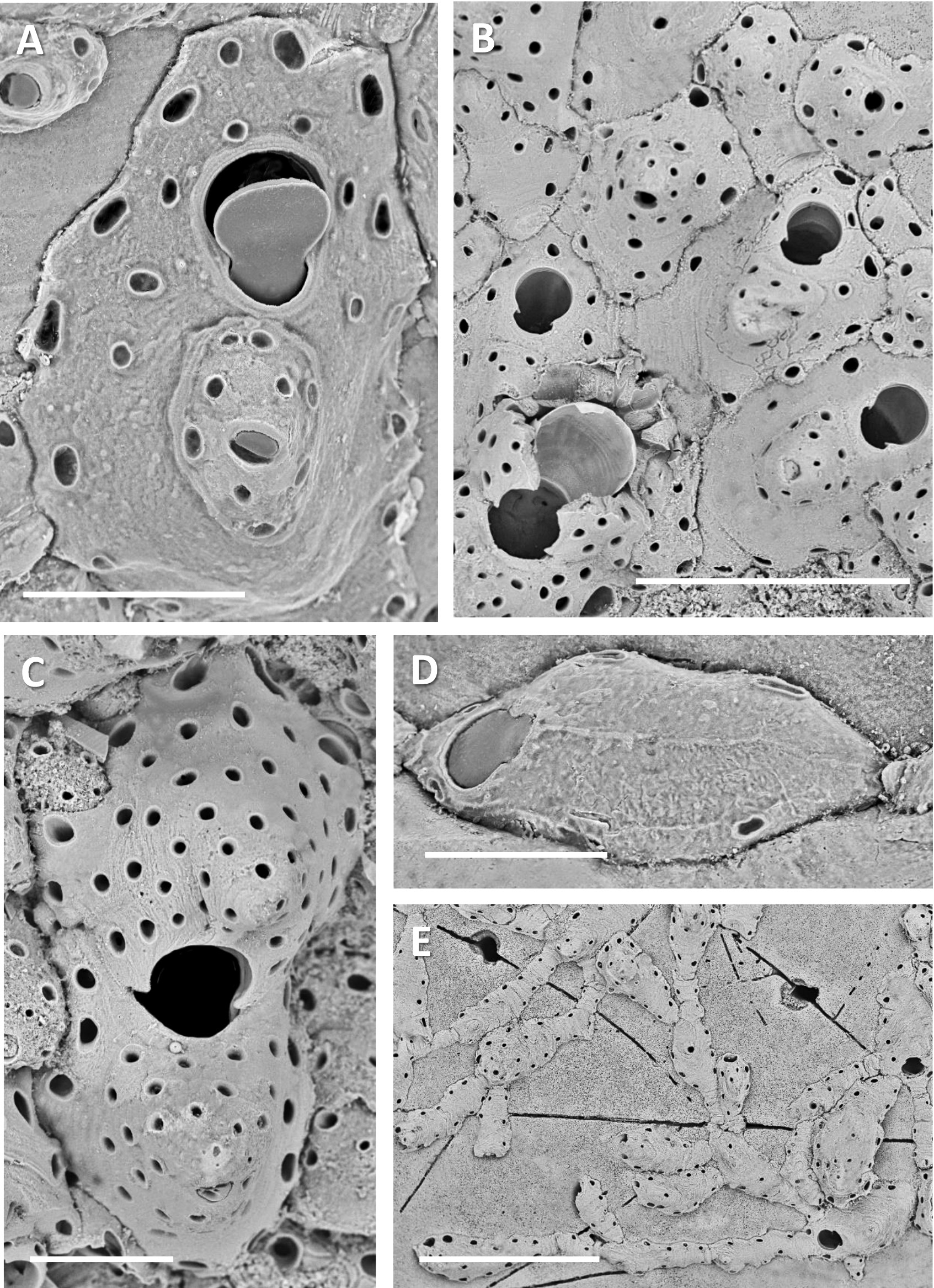
( Figs 6 View FIGURE 6 C–J; 7A–E)
Etymology. As above, honorific for Jesse H. Ausubel.
Material examined. Holotype: NIWA 132852, Stn Z9692, 34.3782° S, 172.8842° E, 52 m, 27 January 1999 on a species of Poirieria (Muricidae) . Paratype: NIWA 127695, same data as holotype, on a species of Pelicaria (Struthiolariidae) ; NIWA 132853, on a species of Amalda (Olividae) , 132854 (molluscan substratum indeterminate), Stn Z9711, 34.3208° S, 172.8687° E, 69 m, 29 January 1999. Other material: NIWA 132855, Stn Z9676, 34.3643° S, 172.8412° E, 57 m, 25 January 1999, on a species of Phenatoma (Turridae) . All samples collected by F. V. Ben Gunn NE of Spirits Bay, North Island, New Zealand.
Description. Colony encrusting, uniserial, initially open-branching and extensively ramifying, zooids becoming densely crowded and contiguous in mature state; maximum colony length or diameter c. 25 mm.
Autozooids initially all elongate-oval ( Fig. 6 View FIGURE 6 C–E), becoming more clavate with reasonably stout non-filiform cauda ( Fig. 6H View FIGURE 6 ), widest and highest in centre of dilatation, no carina. Typically 4–5 oval pore-chamber openings just inside lateral margins, facing frontally, variable in size, generally larger on dilatation. A few sparse gymnocystal pores more frontally spaced in gymnocyst. Zooids distal of ancestrula initially in linear uniserial chain before each zooid starts budding laterally from largest pore-chambers, one on each margin subjacent to orificial sinus, at right angles to axis of parent autozooid. At intermediate colony sizes autozooids becoming more variable in shape, ranging from more elongate, to more oval and less caudate, depending on availability of space for growth, in both cases having more pore-chamber openings (6–8 along each lateral margin), some of these relatively larger than the others, and second row of smaller pseudopores in an arc distal to the orifice. Autozooids leaving excavation in calcareous substratum. ZL 384±60, 274–482 (15); ZW 75±10, 61–89 (15).
Orifice longer than wide; anter evenly rounded, 2/3 length of entire orifice; sinus a wide, deep U shape about 1/3 orifice length ( Fig. 6I View FIGURE 6 ), with small, rounded condyles at junction of anter and poster, pointing downwards. Operculum relatively thick. OL 75±8, 61–89 (15); OW 58±10, 40–80 (15).
Female zooids same size as autozooids, bearing relatively large ovicells ( Figs 6J View FIGURE 6 ; 7C View FIGURE 7 ). Ovicell prominent, terminal, cleithral; ooecium formed by distal kenozooid. Ooecium and female zooid each with relatively large number of pseudopores (up to 40 in ooecium); large pore-chamber openings tend to occur around periphery of ooecial kenozooid, constituting loci where interzooidal communications may be established with other zooidal elements in crowded colonies. Dimorphic combined maternal aperture larger than autozooidal orifice, near-circular; proximal corners of ooecium encroaching over opening, giving false impression of broad sinus ( Fig. 7B View FIGURE 7 ). ♀ ZL 461±55, 361–525 (8); ♀ ZW 226±35, 175–275 (8); OoL 219±42, 157–283 (9); OoW 235±31, 191–268 (9); ♀ OrL 76±3, 71–81 (10); ♀ OrW 87±4, 79–95 (10).
Zooeciules developing once colony ramification underway; initially budded laterally in lieu of an autozooid, later, in mature reproducing colonies, becoming abundant and developing mid-distally from autozooids and female zooids, even forming linear chains of successive elongate zooeciules ( Fig. 7E View FIGURE 7 ), all with frontolateral pseudopores (2–6 along each side), and many connecting with pore-chamber openings of adjacent autozooids, zooeciules, female zooids and ooecia. In mature crowded parts of colonies a zooeciule can develop mid-frontally on an autozooid ( Fig. 7A View FIGURE 7 ), budded from one or a pair of frontal gymnocystal pores; these frontal zooeciules longitudinally elongate and linear (if from single pore) or wider and more oval (if from 2 pores). Each zooeciule having very small oval subterminal aperture (if elongate) or midfrontal aperture (if oval), aperture with tiny condyles near its midlength, with articulating operculum ( Fig. 7A View FIGURE 7 ). Some small, constricted zooeciules in crowded parts of colonies (secondarily?) lacking aperture ( Fig. 7B View FIGURE 7 ). Damaged frontal zooeciules can regenerate without forming aperture, their frontals with many pseudopores. One zooid with regenerated frontal zooeciule had a total of 33 pseudopores over entire frontalshield area, including in zooeciule. ZoL 221±92, 121–397 (15); ZoW 111±28, 62–182 (15); ZoOL 22±2, 20–27 (7); ZoOW 19±2, 17–22 (7); FZoL 118±25, 83–156 (8); FZoW 76±40, 34–159 (8); FZoOL 20±4, 16–25 (4); FZoOW 20±3, 15–24 (4).
Ancestrula like autozooid, sinusoid with frontal shield. Two forms seen, one oval, not budding proximally ( Fig. 6E, F View FIGURE 6 ); second form elongate-oval, non-clavate, but bipolar, budding proximally as well as distally ( Figs 6D, G View FIGURE 6 ; 7D View FIGURE 7 ); in both cases having distolateral pair of pseudopores closer to orifice than in later zooids and frontal gymnocyst more fibrous. AnL 276±24, 260–303 (3); AnW 141±18, 121–158 (3).
Remarks. Whereas neanic colonies consist of mostly uniserial branching runners, ephebic colony parts can be so dense and irregular, with adventitiously budded female zooids and zooeciules as to appear almost celleporiform ( Fig. 6J View FIGURE 6 ). The density of pseudopores in ooecia and frontal shields of female zooids is also exceptional among hippothoids.
Distribution. Endemic; known only from northeast of Spirits Bay, Northland, New Zealand, 52–69 m depth, on gastropod shells.
| NIWA |
National Institute of Water and Atmospheric Research |
| V |
Royal British Columbia Museum - Herbarium |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.