Tanarctus ittanmomen, Fujimoto, 2018
|
publication ID |
https://doi.org/ 10.12782/specdiv.23.209 |
|
publication LSID |
lsid:zoobank.org:pub:CCA2E8E2-81F1-4559-995E-412EC65752F1 |
|
persistent identifier |
https://treatment.plazi.org/id/E6F48ECA-1DE3-42CE-8B8D-C707DE85DE27 |
|
taxon LSID |
lsid:zoobank.org:act:E6F48ECA-1DE3-42CE-8B8D-C707DE85DE27 |
|
treatment provided by |
Felipe |
|
scientific name |
Tanarctus ittanmomen |
| status |
sp. nov. |
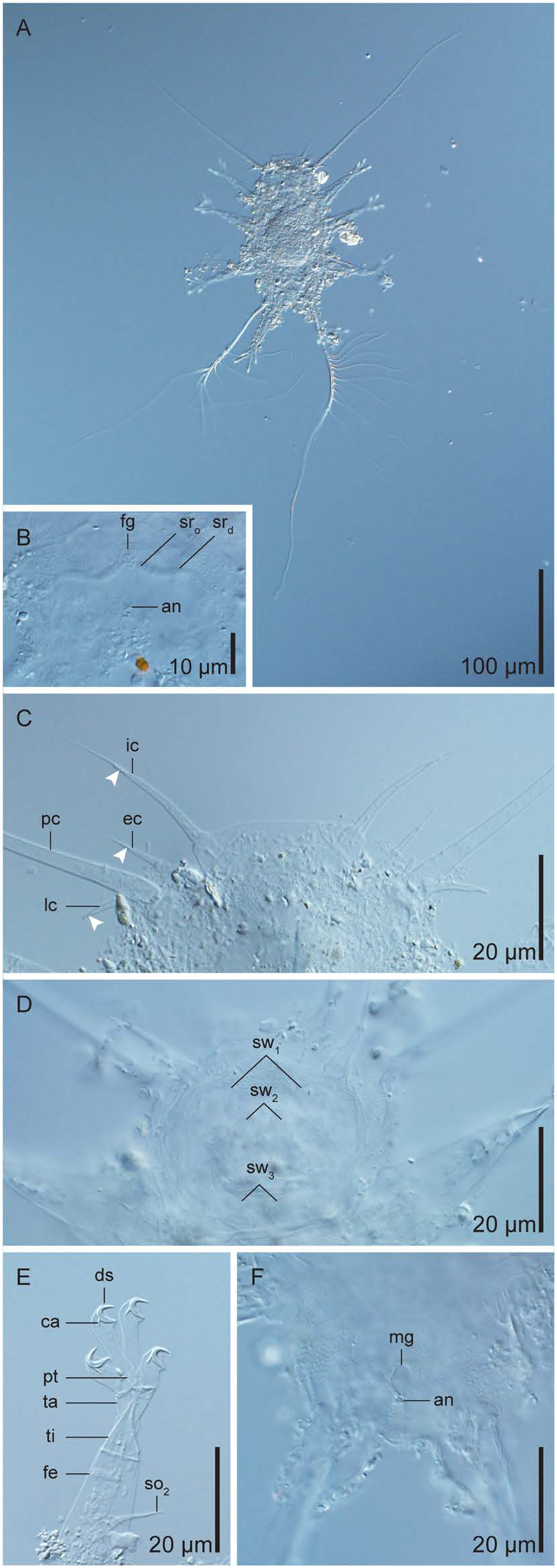
Tanarctus ittanmomen sp. nov. ( Figs 1 View Fig , 2 View Fig ; Table 1)
Diagnosis. Tanarctus with the scapi of the internal cirri embedded in the cuticle; smooth primary clavae; flat secondary clavae each with three internally directed swellings; leg IV appendages each consisting of a main branch exceeding double the body length with variable numbers of flexible, mostly long secondary branches; all branches with unmodified tips; seminal receptacles open overlapping gonopore and each terminates in a laterally situated round vesicle with a duct of approximately even width.
Type material. Holotype: KUZ Z1954 View Materials , adult female mounted in glycerol collected at Ohama Beach , Miyatojima, Oku-Matsushima, Japan, by the author on 20 November 2017 . Paratypes: KUZ Z1955 View Materials and Z1956, two adult females; KUZ Z1957 View Materials , juvenile moulting into adult female; KUZ Z1958 View Materials , adult male; and KUZ Z1959 View Materials , four-digit juvenile of undetermined sex . All collected with the holotype and mounted in glycerol.
Type locality. Ohama Beach , Miyato-jima, Oku- Matsushima, Japan (38°19′40.4″N, 141°09′46.0″E) GoogleMaps .
Etymology. The specific epithet refers to ‘Ittan-momen’ (e.g., Mizuki 1994). The appearance of the new species gives the impression that it may drift and twine like this yokai (a term for mysterious beings from Japanese folklore).
Description of holotype. Adult female ( Figs 1A View Fig , 2A, B View Fig ; Table 1). Dorsoventrally flattened body 145 µm long, 71 µm wide between legs II and III. Dorsal and ventral cuticles smooth with visible epicuticular pillars.
Cephalic region ( Figs 1A View Fig , 2A View Fig ) with unpaired median cirrus, paired internal cirri, paired external cirri, paired lateral cirri, paired primary clavae, and paired secondary clavae. Median cirrus (13 µm) inserted dorsally, 17 µm from anterior margin of cephalic region, without clear subdivision, but constricted tip. Internal cirrus (37 µm) inserted dorsally on anterior margin of cephalic region, consisting of scapus (6 µm), long tubular portion (22 µm), and flagellum (10 µm). Cuticle present between two scapi, more than half the length of the scapi embedded. External cirrus (22 µm) inserted externally to internal cirri on ventral side of cephalic region, consisting of scapus (11 µm), tubular portion (7 µm), and flagellum (3 µm). Lateral cirrus (16 µm) and primary clava (195 µm) inserted on short, common cirrophore at lateral margin of cephalic region. Lateral cirrus positioned dorsoposterior to primary clava, without clear subdivision, basally swollen and distally constricted. Primary clava long, smooth, with van der Land’s body at base. Dense, rod-like particles present near tips of median and lateral cirri and near tips of tubular portions of internal and external cirri. Mouth cone protrudes anteroventrally. Buccal apparatus partially recognised; pharyngeal bulb 9 µm long, 14 µm wide. Narrow buccal tube, with stylets and stylet supports. No furcae or placoids recognised. Secondary clava present as area without epicuticular pillars surrounding mouth. Thorough observation of this structure obstructed by protruding mouth cone. Seven amoebocytes distributed posteriorly in cephalic region. Possible disturbed amoebocytes present anteriorly in cephalic region (not drawn in Fig. 1A View Fig ).
Paired cirri E (30 µm) ( Fig. 1A View Fig ), with proximal annulation arising dorsally between legs III and IV.
Each leg consists of indistinct coxa, femur, lance-like tibia, conical tarsus, and four digits, each with claw. Leg I femur has tapering sensory organ (18 µm) consisting of proximal (12 µm) and distal (6 µm) portions. Femurs of legs II and III have sensory organs as tapering spines (21 and 24 µm, respectively) . Leg IV sensory organ modified into long, branching appendage arising from short process (5 µm) positioned dorsally at base of leg IV ( Figs 1A View Fig , 2A View Fig ) . Secondary branches (9 and 12 in number, respectively) of variable lengths (44–194 and 20–92 µm, respectively) grow on the main branches (170 and 301 µm, respectively; shorter appendage deformed [see Additional information from paratypes]). Secondary branches of longer appendage grow exteriorly on proximal half of main branch. Paired internal digits arise from common pretarsus; each terminates in crescent-shaped claws with strong calcar and minute, dorsal spur. Paired external digits each with basal cuticle fold, terminating in crescent-shaped claws with strong calcar. Internal digits always longer than external digits (legs I: 12 µm> 8 µm; II: 14 µm> 9 µm; III: 16 µm> 9 µm; IV: 17 µm> 10 µm) .
Female genital structure ( Figs 1A View Fig , 2B View Fig ) consists of rosettelike gonopore (7 µm in diameter), paired seminal receptacles with anus 8 µm posterior to gonopore. Seminal receptacle opens ventrally, overlapping gonopore, with duct of even width running laterally, terminating in round vesicle.
Additional information from paratypes. Dense, rodlike particles embedded in the cephalic cirri ( Fig. 2C View Fig ) and in the leg I sensory organ were confirmed in all of the paratypes. The complete contours of the flat secondary clavae were recognised in the old cuticle of KUZ Z1957 View Materials ( Figs 1B View Fig , 2D View Fig ) . The paired clavae each had two swellings anterior to the mouth and one posterior swelling. All swellings were directed internally, but never fused with their counterparts. Amoebocytes were distributed both anteriorly and posteriorly in the cephalic region in the paratypes (see Table 1 for number of amoebocytes). The dorsal spur on the internal claws and the well-developed calcar on all claws were best observed when KUZ Z1955 View Materials was squeezed in distilled water ( Fig. 2E View Fig ) . The male gonopore was observed in KUZ Z1958 View Materials ( Fig. 2F View Fig ) . The longitudinally long, oval gonopore (length 6 µm, width 5 µm) opens immediately anterior to the anus. As only one male was observed, sexual dimorphism in the quantitative traits was not investigated. The leg IV appendages of the paratypes resemble the longer appendage of the holotype, but exhibited variation in the number and length of the secondary branches ( Table 1) . Unlike the holotype, no apparent difference was observed between the lengths of the two main branches in the paratypes. The shorter leg IV appendage of the holotype is deemed to be deformed as no similar appendage was observed in the paratypes .
Remarks. The new species resembles Tanarctus arborspinosus Lindgren, 1971 , T. dendriticus Renaud-Mornant, 1980 , T. hirsutospinosus JØrgensen, Boesgaard, MØbjerg and Kristensen, 2014, and T. longisetosus Grimaldi de Zio, D’Addabbo Gallo, Morone De Lucia, Vaccarella and Grimaldi, 1982 , due to its branching leg IV appendages with unmodified tips ( Lindgren 1971; Renaud-Mornant 1980; Grimaldi de Zio et al. 1982; JØrgensen et al. 2014) [see JØrgensen and Kristensen (2001) for a discussion on the leg IV appendages]. However, the new species differs from T. arborspinosus and T. dendriticus in the lack of tertiary branches ( Lindgren 1971; Renaud-Mornant 1980). Unlike the new species, the other two species have only short secondary branches ( Grimaldi de Zio et al. 1982; JØrgensen et al. 2014). The new species could also be distinguished from T. arborspinosus , T. hirsutospinosus , and T. longisetosus by its smooth primary clavae (unknown in T. dendriticus ) ( Lindgren 1971; Renaud-Mornant 1980; Grimaldi de Zio et al. 1982; JØrgensen et al. 2014). Besides the prominent leg IV appendage and the primary clava used above for species identification, it is noteworthy that the large, flat secondary clavae similar to that of the new species have been report- ed in T. helleouetae Renaud-Mornant, 1984 and T. velatus McKirdy, Schmidt and McGinty-Bayly, 1976 , and also from confamilial Actinarctus lyrophorus Renaud-Mornant, 1979 , A. neretinus Grimaldi de Zio, D’Addabbo Gallo, Morone De Lucia, Vaccarella and Grimaldi, 1982 , and Zioella pavonina Renaud-Mornant, 1987 ( McKirdy et al. 1976; Renaud-Mornant 1979, 1984, 1987; Boesgaard and Kristensen 2001).
| KUZ |
Zoological Collection of the Kyoto University |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |