Labidocera rotunda Mori, 1929
|
publication ID |
https://doi.org/ 10.11646/zootaxa.3764.2.5 |
|
publication LSID |
lsid:zoobank.org:pub:F03537C1-2FCE-4E97-BE13-B6CE98E85C67 |
|
DOI |
https://doi.org/10.5281/zenodo.5621013 |
|
persistent identifier |
https://treatment.plazi.org/id/661287D1-B512-593A-FF45-8DE44AE2BB03 |
|
treatment provided by |
Plazi |
|
scientific name |
Labidocera rotunda Mori, 1929 |
| status |
|
( Figs. 1–4 View FIGURE 1 View FIGURE 2 View FIGURE 3 View FIGURE 4 )
Labidocera rotunda Mori, 1929: 177 , pl. 10, figs. 1–8 (type locality: Pusan, Korea); Mori, 1964: 35; Fleminger et al., 1982: 264, figs. 4m –n, 5f, 6k–l, 7f–g, 8f.
Labidocera bipinnata Tanaka, 1936: 31 , pl. 2, figs. 1–10, pl. 3, figs. 1–7; Tanaka, 1964: 25; Mori, 1964: 94, pl. 43, figs. 1–8; Brodsky, 1950, p. 410, fig. 291; Shen and Bai, 1956: 191, pl. 5, figs. 36–41; Shen and Lee, 1963: 581; Chen and Zhang, 1965: 97, pl. 39, figs. 10–13, pl. 40, figs. 1–5; Silas and Pillai, 1973: 814; Kim, 1985: 118, pl. 38, figs. e–g, pl. 39, figs. a, b; Yoo, 1995: 200, pl. 145.
Labidocera rotundata Mori, 1964: 52 (misspelling of specific name).
Material examined. Twenty females and twenty males were collected from the northeastern area of the East China Sea on 30 May (34° 10’ N 127° 49’ E), 24 July (34° 39’ N 127° 52’ E) and 23 October 2002 (34° 23’ N 128° 7’ E), of which ten females and five males were dissected and closely examined.
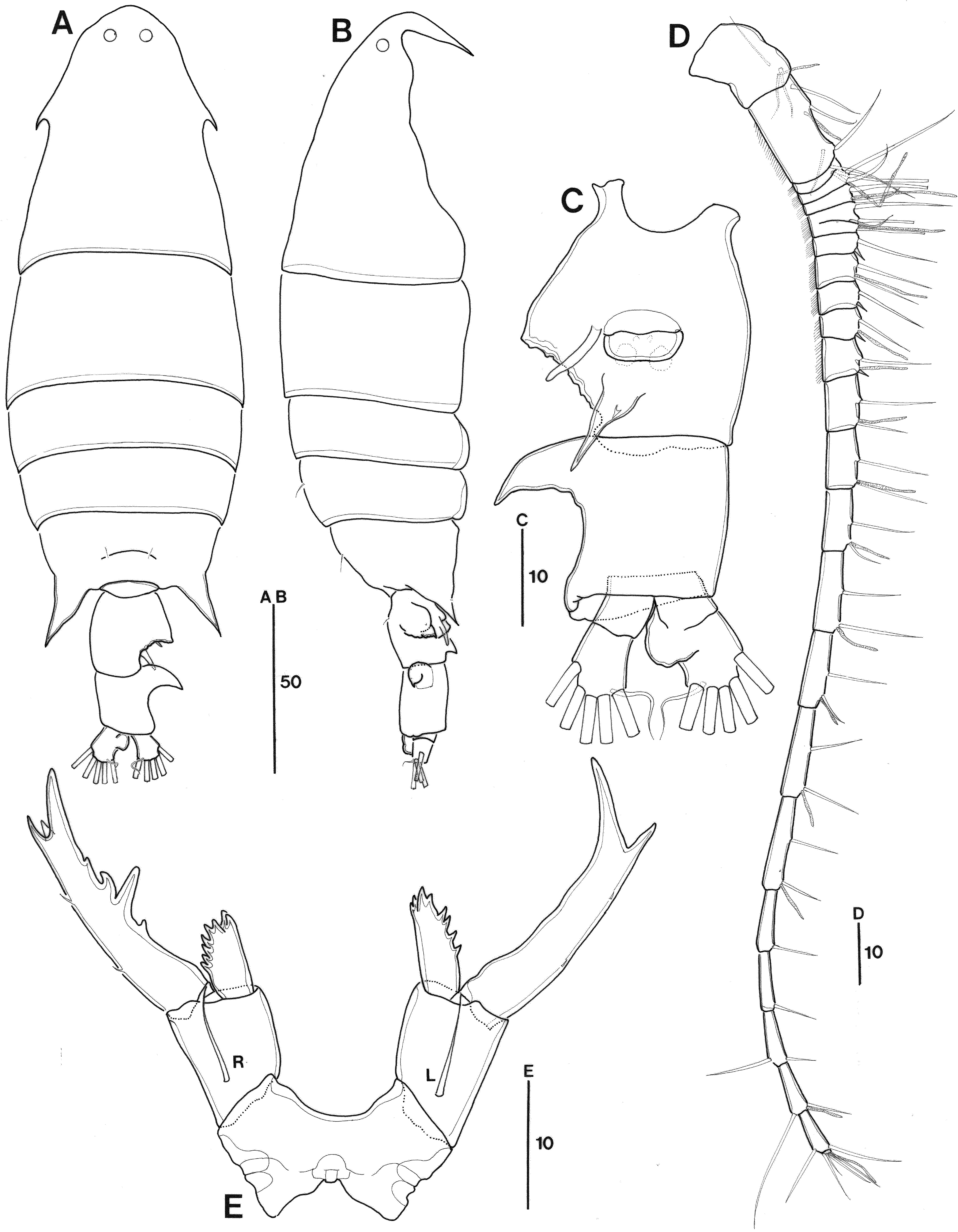
Female. Body length 1.78–2.57 mm (n=20). Prosome cylindrical: fourth and fifth pedigerous somites incompletely fused; posterior corners of prosome with pointed spiniform process extending to two-thirds of the genital double-somite ( Fig. 1 View FIGURE 1 A, B). Cephalosome with lateral hooks and pair of dorsal lenses; rostrum bifid, gap between rostral rami narrow. Urosome with 3 free somites: genital double-somite with wide spur on right mediolaterally and two spiniform processes ventro-laterally ( Figs. 1 View FIGURE 1 C, 2C, 4A–D); genital operculum concaved on central part of tip ( Fig. 4 View FIGURE 4 B, D); second urosomite with well developed spur on right anterior-laterally; anal somite and caudal rami partly fused; caudal rami asymmetrical, left ramus with blunt process along inner margin ( Fig. 1 View FIGURE 1 C).
Antennule ( Fig. 1 View FIGURE 1 D) 24-segmented, posterior margin of second to 12th segments fringed with fine hairs; ancestral segment II to IV, XXVII to XXVIII completely fused, and VIII and IX incompletely separated. Fusion pattern and setal formula as follows: I–3 +ae (aesthetasc), II– IV–4 +ae, V–2 +ae, VI–2, VII–2 +ae, VIII– IX–4 +ae, X– 2, XI–2 +ae, XII–2, XIII–2+ae, XIV–2+ae, XV–2+ae, XVI–2+ae, XVII–2+ae, XVII–2+ae, XVIII–2+ae, XIX– 2+ae, XX–2+ae, XXI–2+ae, XXII–1, XXIII–1, XXIV–1+1, XXV–1+1+ae, XXVI–1+1, XXVII–XXVIII–4+ae.
Fifth leg ( Fig. 1 View FIGURE 1 E) asymmetrical; coxa and intercoxal sclerite completely fused; basis with outer seta; right exopodal segment with small intercalary denticle between unequal apical processes, two to five unequal denticles along inner margin and 2 outer setae; left exopodal segment with 2 unequal processes distally and 2 outer setae; both endopods with nine denticles or, sometimes, reductions.
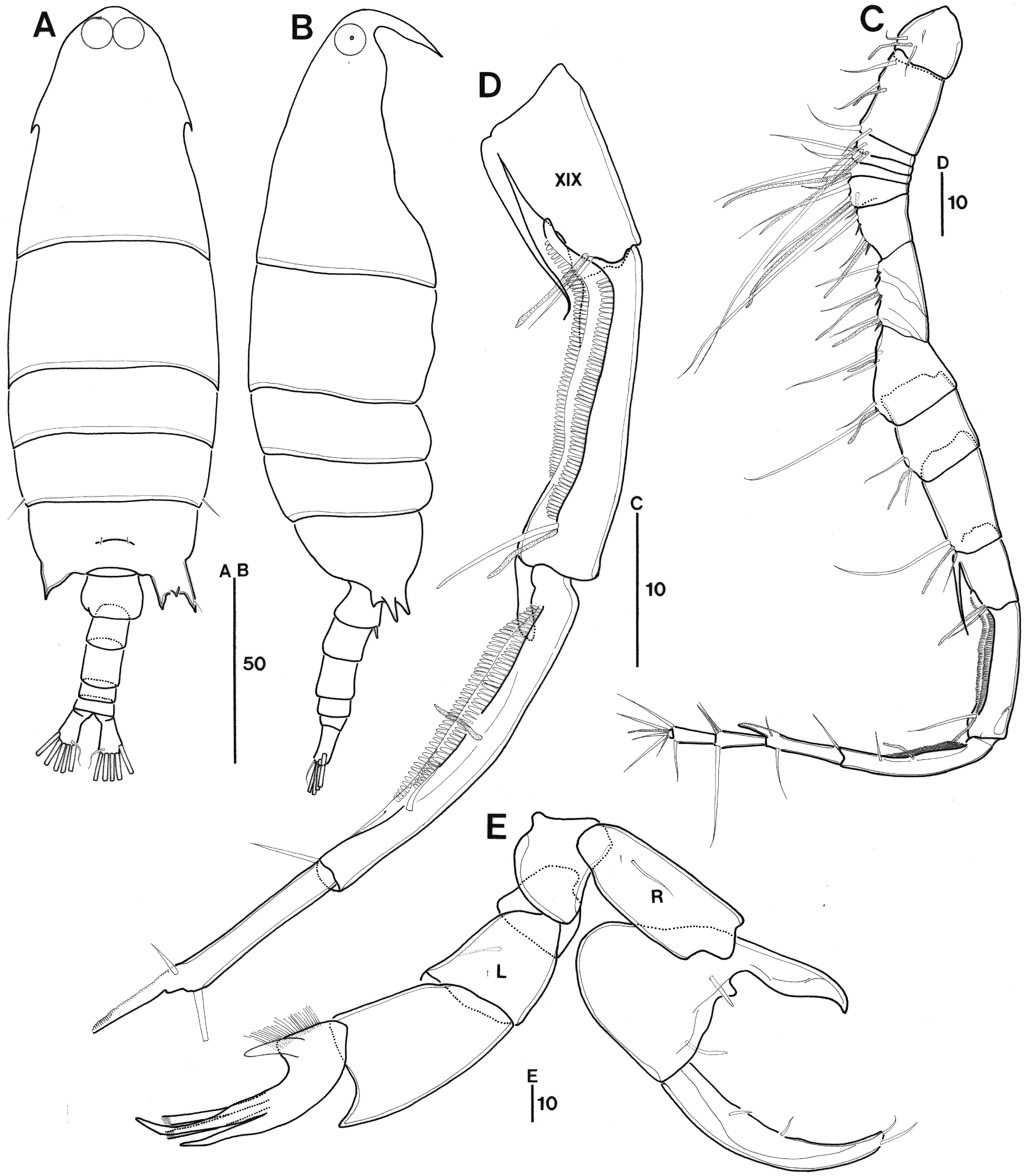
Male: Body length 1.62–2.31 mm (n=20). Prosome more compact than in female with well-developed dorsal lenses: posterior corners of prosome asymmetrical, right corner bifurcate with minute denticles between processes ( Fig. 2 View FIGURE 2 A, B). Urosome of 5 free somites: genital somite wider than long, with spine ventrally; anal somite and caudal rami partly fused.
Antennule ( Fig. 2 View FIGURE 2 C) geniculate on right side only, left one resembling that of females: right one indistinctly 13- segmented; segments XII–XIV with arthrodial membranes incompletely formed, segments II–IV, XV and XVI, XXI–XXIII completely fused. Fusion pattern and setal formula as follows: I–3 +ae, II– IV–4 +ae, V– XI–14 +4 aes (aesthetascs), XII–XIV–6+2 aes, XV–XVI–4+2 aes, XVII–2+ae, XVIII–2+ae, XIX–1+p (hooked process)+ae, XX– 1+p+ae, XXI–XXIII–2+p+ae, XXIV–1+1+p, XXV–1+1+ae, XXVI–XXVIII–6+ae. Segment XIX with anterior setiform process, segment XX and compound segment XXI–XXIII with toothed ridge provided with serrated denticles, respectively, and segment XXIV with spur-like process distally, expanding to middle of next segment ( Fig. 2 View FIGURE 2 D).
Fifth leg ( Fig. 2 View FIGURE 2 E) uniramous and asymmetrical: coxa of left exopod coalescent with intercoxal sclerite; right exopod 2-segmented, first segment with conical process, 2 spines and thumb-like process; second segment elongated, curved inwards with 4 spines on the concave surface; left exopod 2-segmented, first segment with bluntly triangular process postero-laterally, second segment with spine-like process proximally, 2 unequal spiniform processes, 2 unequal setiform processes distally, and base of processes proximal to spine-like process apex.
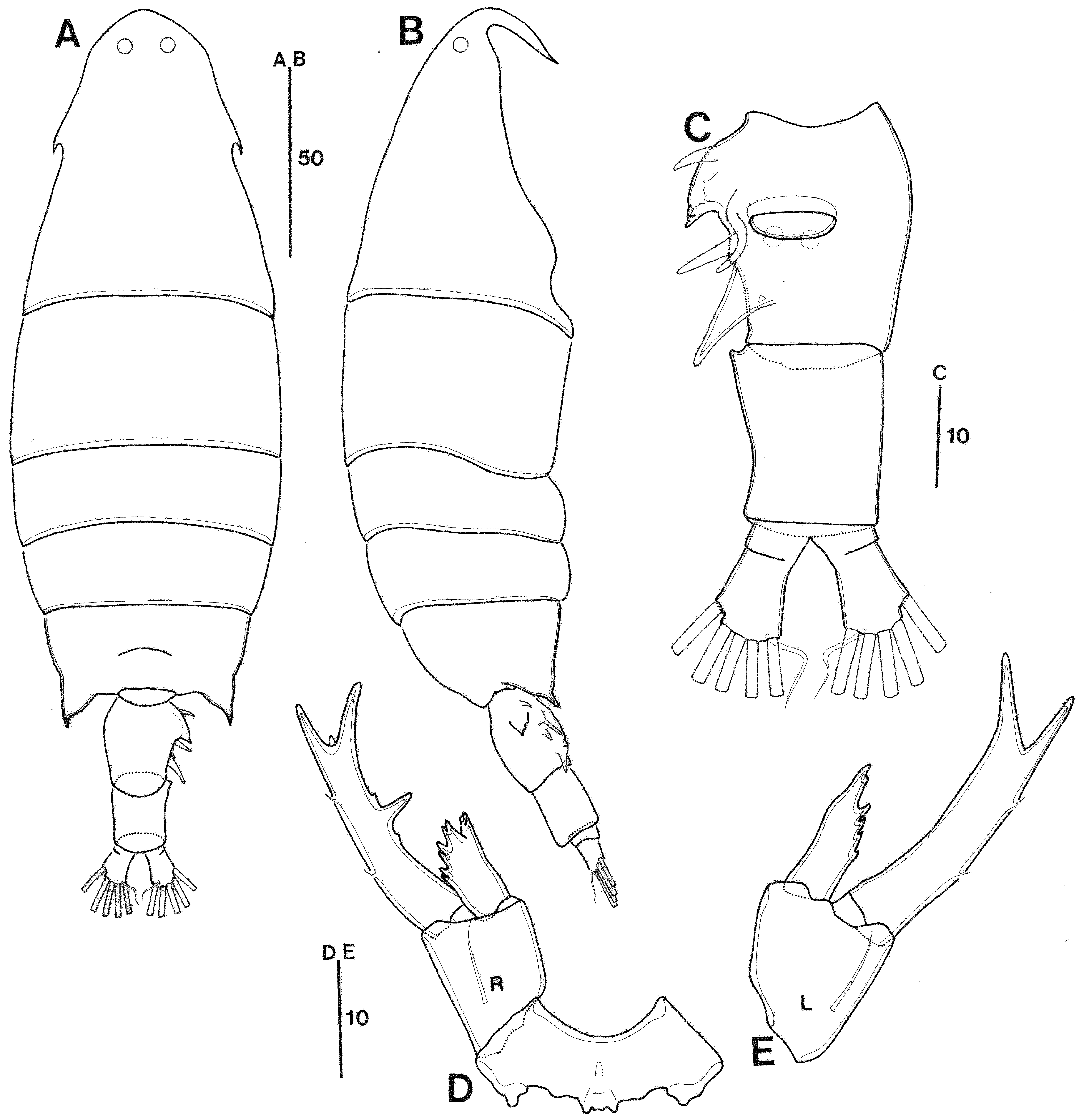
Remarks. In Korean waters, some female specimens of L. rotunda exhibited morphological differences such as: 1) posterior corners of prosome with triangular processes are extending to one-third of the genital doublesomite ( Fig. 3 View FIGURE 3 A, B), 2) the genital double-somite is having four spiniform processes ventro-laterally ( Fig. 3 View FIGURE 3 C), 3) the second urosomite is having a small knob on right antero-lateral surface ( Fig. 3 View FIGURE 3 C), 4) the caudal rami is symmetrical ( Fig. 3 View FIGURE 3 A, C), and 5) the length is similar between the apical processes on the exopod of the fifth leg ( Fig. 3 View FIGURE 3 D, E). Chen and Zhang (1965) described females having the aforementioned features as a variety, together with the typical form of L. rotunda from the East China Sea. Fleminger et al. (1982) and Othman and Toda (2006) also found some discrepancies in the individuals of female L. rotunda .
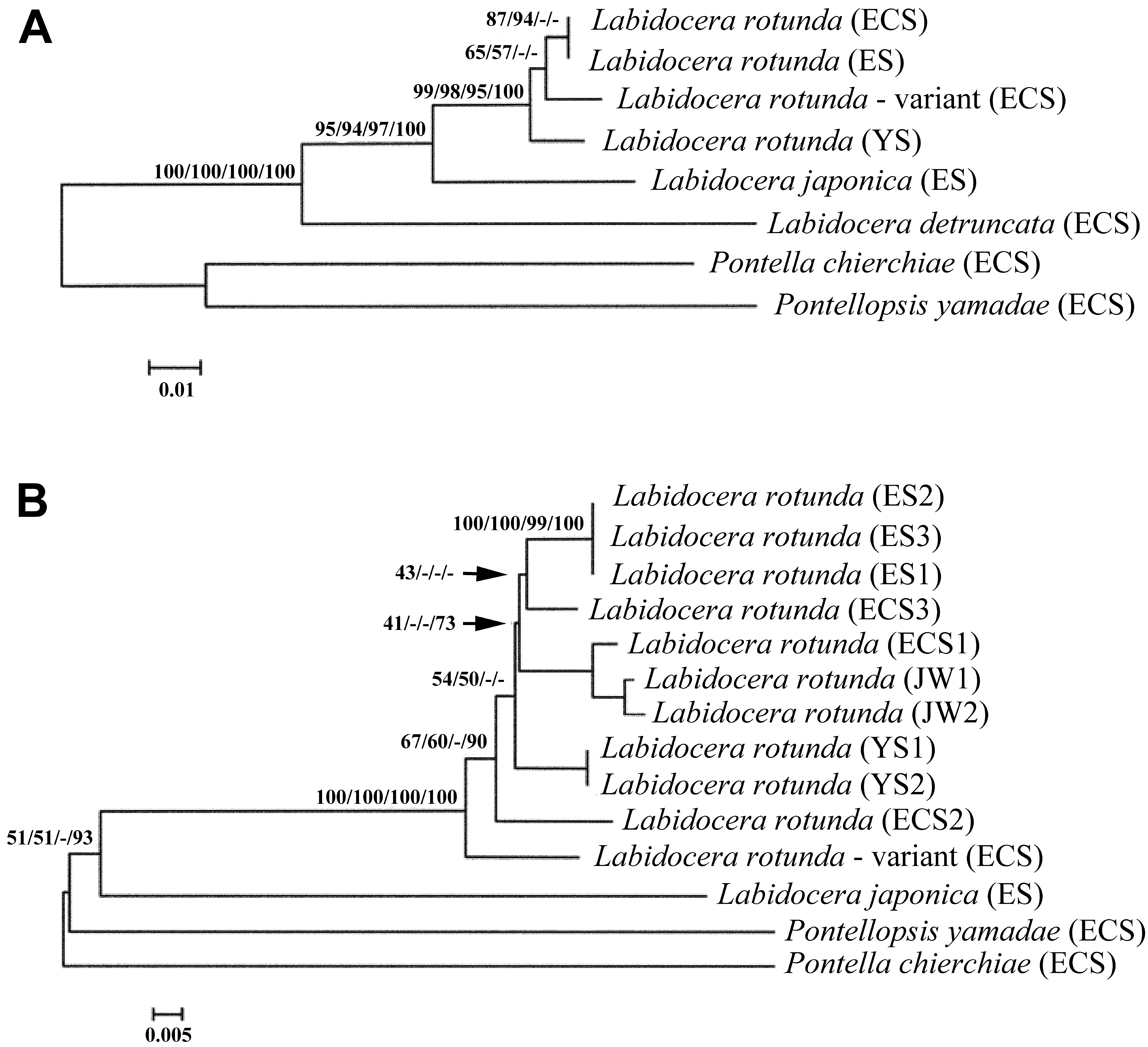
Molecular results. The genetic analysis was obtained for 5 pontellid species ( Table 1 View TABLE 1 ). The sequences of mt16S rRNA and mtCOI differed by 1.4%–2.9% among a 286 base-pair region and by 3.7%–4.7% among a 616 base-pair region between the typical form of L. rotunda and its variant, respectively ( Tables 2 View TABLE 2 , 3). The cladograms obtained indicate a monophyletic clade, suggesting a lack of divergence at the species level despite considerable morphological differences ( Fig. 5 View FIGURE 5 ). On the other hand, intraspecific differences were observed for the mt16S rRNA and mtCOI of L. japonica , which is morphologically very similar to L. rotunda . The differences were between 6.1%–7.2 % for a 286 base-pair region of 16S rRNA and 15.1%–16.7% for a 616 base-pair region of mtCOI. The cladograms also demonstrated separation at the species level ( Fig. 5 View FIGURE 5 ).
Pontellid species show a considerable intraspecific variation in complex, sexually modified structures and, as a result, display different phenotypes ( Chen & Zhang 1965; Fleminger et al. 1967; Zheng et al. 1982). Of these species, L. rotunda showed some extreme morphological variability in its female genital double-somite, second urosomite, caudal rami, and fifth legs. Such morphological differences, in particular, in the structures that function in mating might be enough to establish a new candidate species, as in similar cases of other centropagoid species ( Fleminger 1975; Blade & Youngbluth 1979; Barthlélémy et al. 1998, 1999; Soh et al. 2001, 2012; Walter et al. 2002; Jeong et al. 2009a, 2009b; Sakaguch & Ueda 2010). Recently, however, molecular analysis has provided a useful tool in identifying cryptic species as well as extensive intraspecific morphological variants, and in elucidating evolutionary relationships ( Bucklin et al. 1999, 2003; Goetze 2003; Ueda & Bucklin 2006; Sakaguchi & Ueda 2010).
In the present study, sequence data from two highly variable regions of DNA showed that differences between the typical form of L. rotunda and its variants were only 1.4%–2.9% for 16S rRNA and 3.9%–4.7% for mtCOI sequences, despite their pronounced morphological differences. Considering that in calanoid copepods, 16S rRNA and mtCOI genes usually vary by over 7% at the species level ( Bucklin et al. 1995, 2003), these data suggested that the variants belong to the same species. Similarly, the sequence differences between the two male morphs of Pseudodiaptomus koreanus Soh, Kwon, Lee & Yoon, 2012 is low (1.2% for ITS1 region and 3.8% for mtCOI), and they represent variants within a single species ( Soh et al. 2012).
Nevertheless, it is very interesting to note that the morphological and genetic differences are pronounced between the typical form and its variant; the local populations also comprise an independent clade within mtCOI analysis, except for the northeastern population of the East China Sea ( Fig. 5 View FIGURE 5 ). On the other hand, the genetic differences between L. rotunda and L. japonica were 6.1%–7.2% for mt16S rRNA and 15.1%–16.7% for mtCOI sequences. Although the differences for 16S rRNA are relatively low for species level separation in calanoid copepods, it is sufficient to separate L. japonica from L. rotunda , as the mtCOI and morphological characteristics are clearly distinguished. Labidocera rotunda and L. japonica are placed in the pectinata group, and display the following diagnostic characteristics: in female, the fifth leg is asymmetrical, the left exopod is long and strongly curved and the endopod is denticulated at apex; in male, the second exopodal segment of left leg is bearing hirsute and short lobiform process medially, and two or three spiniform processes and two setiform aesthete-like processes are located distally ( Fleminger et al. 1982; Mulyadi 1997). The distribution of these species overlaps in the East Sea (Sea of Japan) and off northeastern Japan, but the distribution of L. rotunda extends to the continental coast south to the Malay Archipelago, off northeastern Sumatra and the Andaman Sea off the southern Myanmar coast ( Razouls et al. 2013). The presence of L. rotunda in the Andaman Sea places it in proximity of the easternmost populations of L. pectinata (see Fleminger et al. 1982). The latter authors also suggested that L. rotunda might have extended northward in recent postglacial times via the Strait of Malacca, while L. japonica may have undergone a more recent regional speciation.
TABLE 2. Pairwise percent differences for 16 S rRNA sequences between individual females of Labidocera rotunda and L. japonica.
| 1 | 2 | 3 | 4 |
|---|---|---|---|
| 1 L. japonica -ES ( JQ740744 View Materials ) | |||
| 2 L. rotunda -ES ( JQ740741 View Materials ) 6.1 | |||
| 3 L. rotunda -YS ( JQ740742 View Materials ) 6.5 | 1.4 | ||
| 4 L. rotunda -ECS ( JQ740740 View Materials ) 6.1 | 0 | 1.4 | |
| 5 L. rotunda variant-ECS ( JQ740743 View Materials ) 7.2 | 1.4 | 1.4 | 2.9 |
| TABLE 3. Pairwise percent differences for mtCOI japonica . | sequences between individual | females | of Labidocera rotunda and L. |
| 1 2 | 3 4 5 6 | 7 | 8 9 10 11 |
| 1 L. japonica -ES ( JQ714069 View Materials ) | |||
| 2 L. rotunda -ES1 ( JQ714061 View Materials ) 16.7 | |||
| 3 L. rotunda –ES2 ( JQ714062 View Materials ) 16.7 0 | |||
| 4 L. rotunda –ES3 ( JQ714063 View Materials ) 16.7 0 | 0 | ||
| 5 L. rotunda -YS1 ( JQ714065 View Materials ) 16.4 2.3 | 2.3 2.3 | ||
| 6 L. rotunda –YS2 ( JQ714066 View Materials ) 16.4 2.3 | 2.3 2.3 0 | ||
| 7 L. rotunda –ECS1 ( JQ714058 View Materials ) 16.1 2.9 | 2.9 2.9 3.1 3.1 | ||
| 8 L. rotunda –ECS2 ( JQ714059 View Materials ) 16.4 4.6 | 3.4 3.4 3.4 3.1 | 3.1 | |
| 9 L. rotunda –ECS3 ( JQ714060 View Materials ) 15.9 2.8 | 2.0 2.0 2.0 2.6 | 2.6 | 3.6 |
| 10 L. rotunda –JW1 ( JQ714067 View Materials ) 16.4 1.1 | 3.1 3.1 3.1 3.3 | 3.3 | 4.4 2.9 |
| 11 L. rotunda –JW2 ( JQ714068 View Materials ) 16.6 1.3 | 3.3 3.3 3.3 3.4 | 3.4 | 4.6 3.1 0.5 |
| 12 L. rotunda variant-ECS 15.1 4.4 ( JQ714064 View Materials ) | 4.2 4.2 4.2 3.9 | 3.9 | 3.9 3.7 4.6 4.7 |
| Discussion |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Labidocera rotunda Mori, 1929
| Jeong, Hyeon Gyeong, Soh, Ho Young & Suh, Hae-Lip 2014 |
Labidocera bipinnata
| Kim 1985: 118 |
| Chen 1965: 97 |
| Shen 1963: 581 |
| Tanaka 1936: 31 |
Labidocera rotunda
| Fleminger 1982: 264 |
| Mori 1929: 177 |