Escherichia coli (Migula, 1895) Castellani & Chalmers, 1919
|
publication ID |
https://doi.org/10.1016/j.phytochem.2021.112766 |
|
DOI |
https://doi.org/10.5281/zenodo.8264293 |
|
persistent identifier |
https://treatment.plazi.org/id/673BE418-D91E-B04B-FCC5-FB5FFAB36A1B |
|
treatment provided by |
Felipe |
|
scientific name |
Escherichia coli |
| status |
|
3.3. Heterologous expression of His-AmSOD in E. coli View in CoL View at ENA
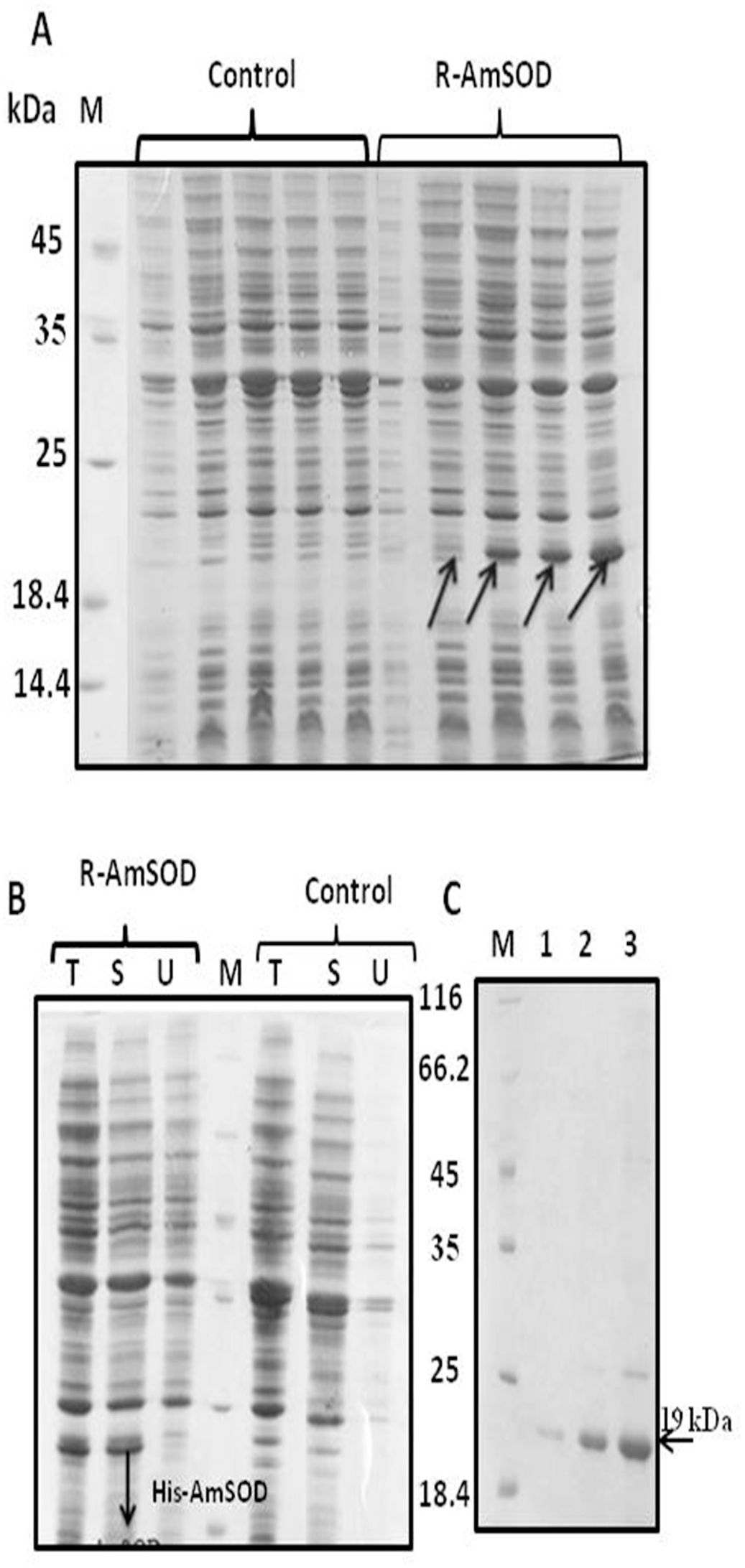
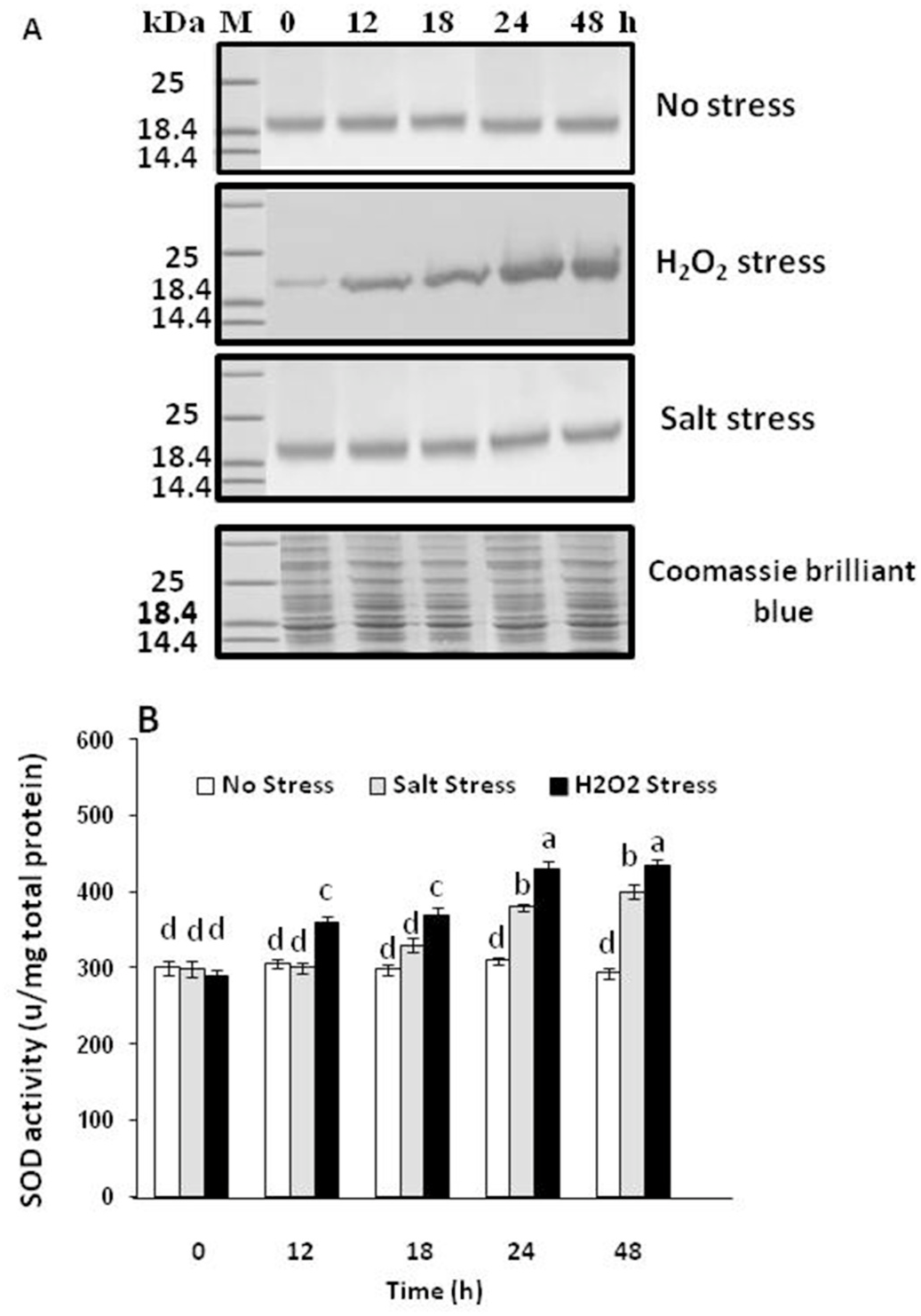
The gene encoding AmSOD1 contains 459 base pairs that encode a protein containing 152 amino acid. Since the gene was cloned in pET28a, the protein was expected to be produced as fusion protein with His.tag. In the present work the strain R-AmSOD1 (Rosetta stain carrying pET28a) was grown in the LB medium with kanamycin and chloramphenicol. The Rosetta strain containing empty pET28a was considered as control strain. Following induction with IPTG, the recombinant protein His-AmSOD1 was expressed in R-AmSOD1. The corresponding band was not observed in the control strain. The theoretical molecular weight for His-AmSOD1 was 19 kDa. SDS-PAGE of cell extracts showed a prominent polypeptide band of the expected molecular mass ( Fig. 3A View Fig ). Furthermore, the comparison between the intensity of band corresponding to His-AmSOD 1 in the soluble fraction and the intensity of His-AmSOD1 band in the total fraction indicated that the protein His-AmSOD1 has a high solubility ( Fig. 3B View Fig ). Whereas the addition of zinc in the medium was not affected on the solubility of His-AmSOD1, the solubility was increased in the presence of copper. The solubility of protein with supplementation of both zinc and copper was almost similar to solubility with supplementation of copper alone (Supplemental Fig. S2 View Fig ).
The recombinant His-AmSOD1 was purified from the soluble fraction using nickel affinity chromatography in yields of 30 mg /L. The quality of purification was assessed by SDS-PAGE analysis ( Fig. 3C View Fig ).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |