Munidopsis geyeri Pequegnat & Pequegnat, 1970
|
publication ID |
https://doi.org/ 10.11646/zootaxa.5213.4.1 |
|
publication LSID |
lsid:zoobank.org:pub:61C83B69-29F4-456C-996C-AA42AFDAF930 |
|
DOI |
https://doi.org/10.5281/zenodo.7383519 |
|
persistent identifier |
https://treatment.plazi.org/id/6A4F4946-FFAD-FF84-FF22-1092FBFAFAF3 |
|
treatment provided by |
Plazi |
|
scientific name |
Munidopsis geyeri Pequegnat & Pequegnat, 1970 |
| status |
|
Munidopsis geyeri Pequegnat & Pequegnat, 1970
( Figs 4 View FIGURE 4 , 5B View FIGURE 5 , 6 View FIGURE 6 )
Munidopsis subsquamosa .— Ambler, 1980: 25–26.
Munidopsis cf. subsquamosa .— Turnipseed et al., 2004: 123.
Munidopsis geyeri .— Wicksten & Packard, 2005: 1761 (not Munidopsis subsquamosa synonym).
Munidopsis sp. — Olu et al., 1996: 371 (table), 372.— Olu et al., 1997: 209, 834 (table).— Cordes et al., 2007: 643, 647 (table), 649.
Munidopsis sp. A .— Coykendall et al., 2017: 267–270, tabs 1, 4, fig. 4 (phylogeny).
Munidopsis geyeri Pequegnat & Pequegnat, 1970: 139 , 149–151, figs 5–1, 5–2, 5–9, 5–10, (key, description and table); 1971: 5 (key), 19 (distribution remarks).— Mayo, 1974: 28, 431 (table), 38 (key), 144–154, figs 20–21 (description and ecology).— Gore, 1983: 202 (table), 208 (taxonomic remarks, discussion), 213 (zoogeography).— Navas et al., 2003: 201, 217 (key and list).— Baba, 2005: 163, fig. 76 (taxonomic remarks and distribution).— Macpherson & Segonzac, 2005: 25–26 View Cited Treatment , fig. 6 (taxonomic remarks, distribution and ecology). — Baba et al., 2008: 142 View Cited Treatment (list of taxa).— Felder et al., 2009a: 1066, 1094 (checklist).— Gaytán-Caballero, 2009: 1–146 (taxonomy, biology and ecology).— Olu et al., 2009: 2386, 2390, 2391, tabs 2, 6, 7, (ecology, local distribution, density and diet).— Olu et al., 2010: 4, 5, 7, tab. 3 (biogeography).— Navas et al., 2013: 3505, 3507 (biogeography).— Kilgour & Shirley, 2014: 406 View Cited Treatment , tab. 4, fig. 12H (reproductive biology).— Vázquez-Bader & Gracia, 2016: 23 (list).
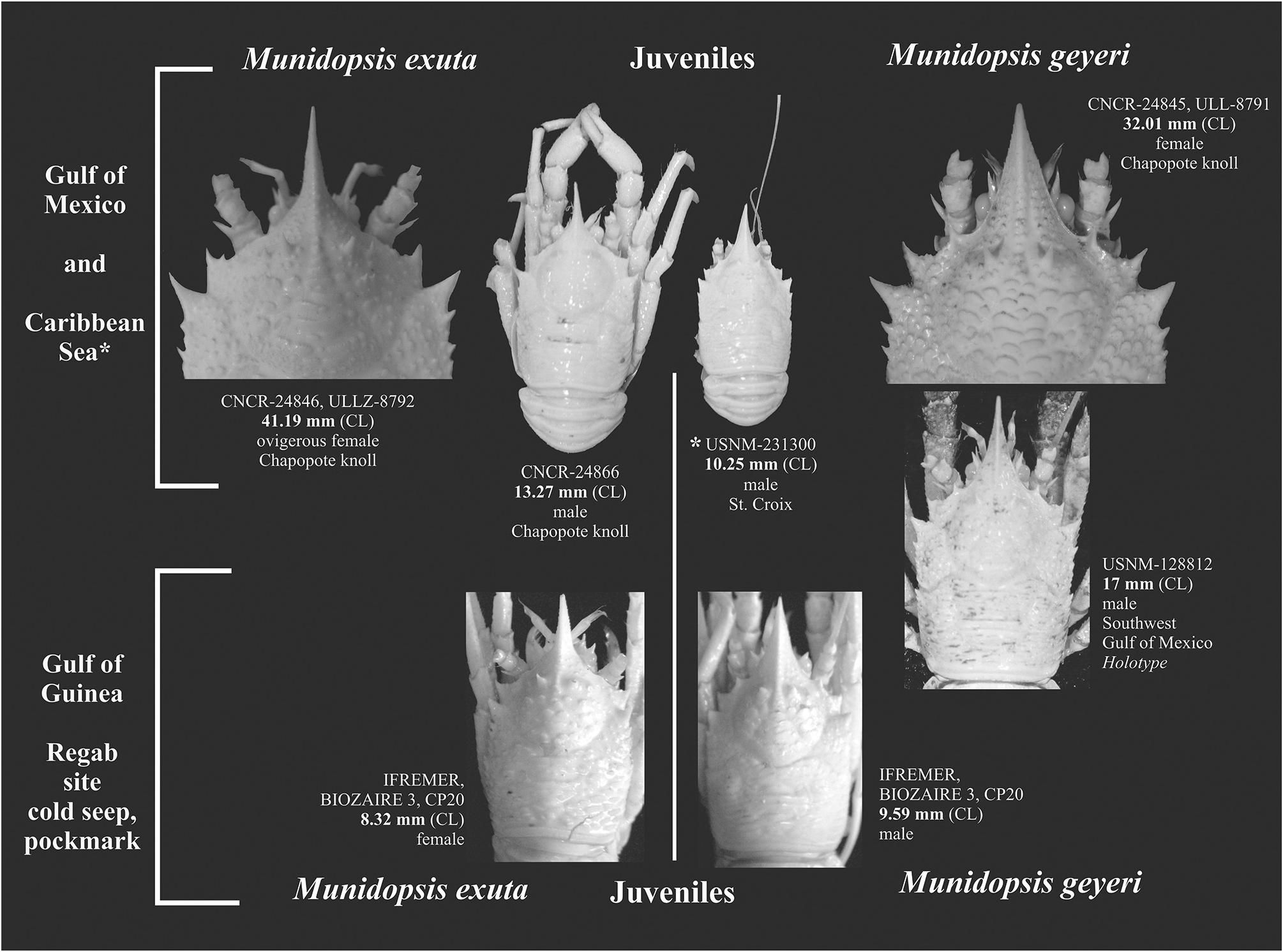
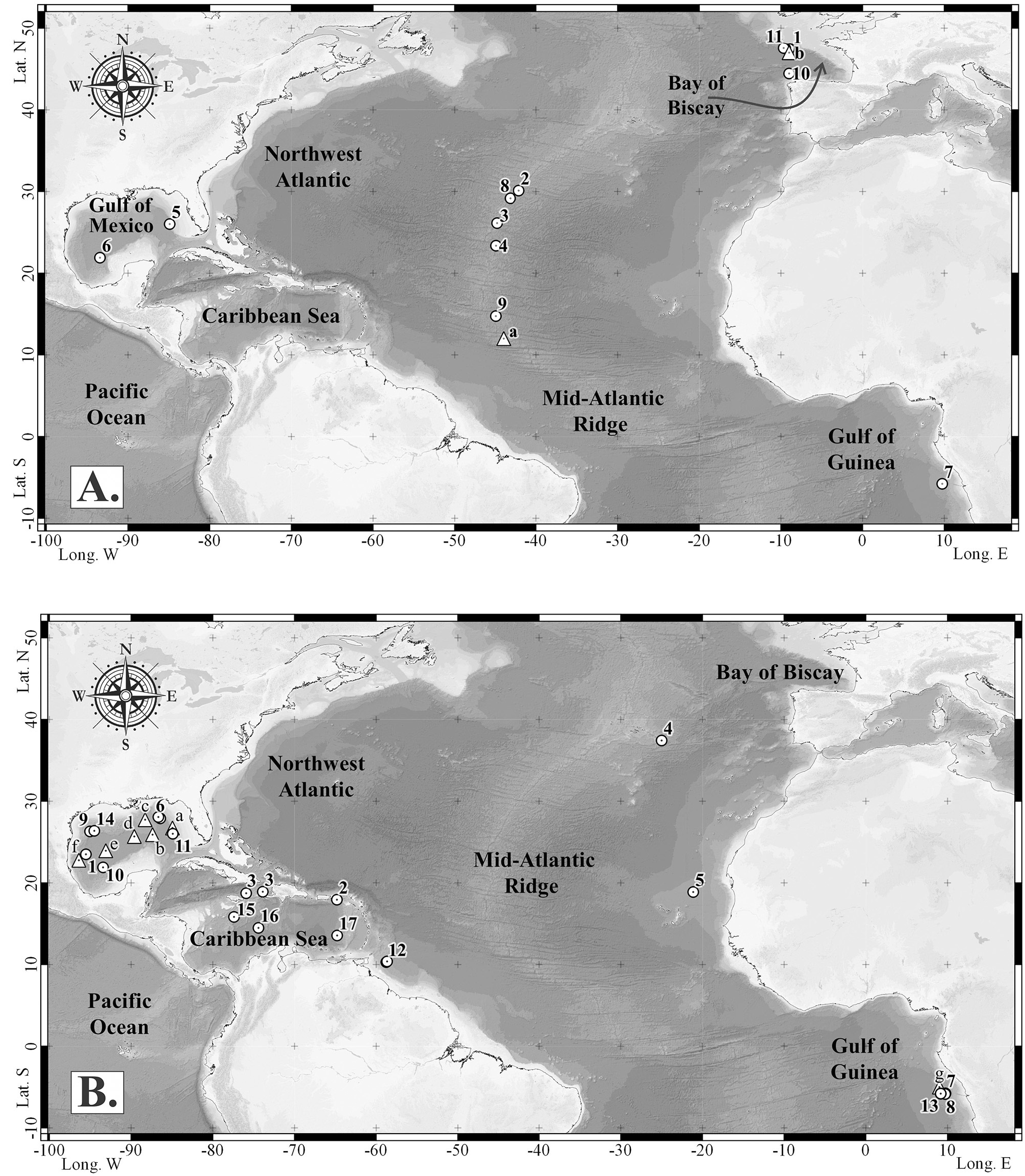
Type material examined. Holotype: juv. male, 17 mm ( USNM 128812 View Materials ), southwestern Gulf of Mexico, Stn 69-A- 11-92, 2330′N, 9532′W, 2926–2999 m, 27.08.1969.
Additional material examined. Soft abyssal substrate ecosystems: Caribbean Sea, San Croix, Alvin DSR/ V Stn 1078, 1794′N, 6481′W, 4000 m, 05.12.1980: 4 juv. female, 2 juv. male, 2.97–10.25 mm ( USNM 231300 View Materials = ULLZ 8923 View Materials ) . Gonave Bay , Haiti, Stn P-1180, 1855′–1844.4′ N, 7353′–7355′W, 3111–3496 m, 01.07.1970: 1 female, 27.1 mm, 1 male, 21.8 mm, 1 juv. male, 18.9 mm ( UMML 32 View Materials : 5246). Azores Islands, BIOACORES Stn 202, 3726.5′N, 2500′W, 2900 m, 06.11.1971: 1 male, 36.9 mm ( MNHN Ga 1182). Off Mauritania, Africa, EUMELI 4 Stn CPH-15, 1853′N, 2108′W, 3124 m, 01.06.1992: 1 juv. male, 11.2 mm, 1 male, 21.2 mm ( MNHN Ga 6525). Northeastern Gulf of Mexico, Gyre DGoMB, Stn S-41, 2754.23´N–2804.33´N, 8626.08´W–8640.65´W, 2930– 3030 m, 09.06.2000: 1 male, 23.2 mm ( USNM 310877 View Materials = ULLZ 8784 View Materials ) . Cold seep ecosystems: Gulf of Guinea, Regab Pockmark site, BIOZAIRE 3, Stn CP 20, 0546.89′S, 0944.66′E, 3113 m, 02.01.2004, 1 juv. female, 12.9 mm; 3 juv. females 17.68–19.49 mm, 3 ovig. females, 33.5–43.3 mm, 21 females, 20.1–38.5 mm, 3 juv. males, 17.6–19.6 mm, 19 males, 24.5–38.8 mm (five broken; MNHN Ga 5662 = MNHN IU 2008 13352); 1 female, 28.8 mm, 3 juv. males, 8.3–19.0 mm, 2 males, 28.8–31.1 mm ( CEAB CRU 2004-07 , Fig. 6A View FIGURE 6 ; ULLZ 8863 View Materials , ULLZ 8862 View Materials ). Off Gabon, BIOZ-RECUP Mac10 (160), (158), (154), (152), 0551′S, 0941.98′E, 3155 m, 01– 06.02.2003: 5 juv., 3.2–6.4 mm ( MNHN Ga 6521– MNHN Ga 6524). Northwestern Gulf of Mexico , Alaminos Canyon ( AC818 ) , ATLANTIS, MPB Stn J2-282, 2618′N, 9462′W, 2744.8 m, 01.07.2007: 1 male, 27.9 mm ( USNM 1178677 View Materials = ULLZ 8912 View Materials ) . Southwestern Gulf of Mexico , Chapopote , Dive 82 Stn GeoB10619-19, 2153.922′N, 9326.166′ W, 2875 m, 12.04.2006: 7 ovig. females, 31.0– 47.4 mm ( CNCR 24847 , CNCR 24848 = ULLZ 8794 View Materials , CNCR 24849 – CNCR 24852 , CNCR 24853 = ULLZ 8799 View Materials ) , 3 females, 27.0–32.0 mm ( CNCR 24845 = ULLZ 8791 View Materials , CNCR 24855 , CNCR 24856 , Fig. 6B View FIGURE 6 ), 9 males, 24.2–38.5 mm ( CNCR 24854 , CNCR 24858 – CNCR 24865 ) .
Diagnosis. Carapace slightly longer than broad (length-width ratio>1.2), 2 well-developed epigastric spines bearing spine-like tubercles and non-sharp tubercles through carapace, as well as ridges (setiferous scale-like on anterior half, and longer interrupted transverse ridges on posterior half). Anterior margin with antennal spine subequal to anterolateral (first spine on lateral margin) and this one directed slightly forward; second spine of lateral margin well-developed, bigger than anterolateral spine. Rostrum form as isosceles triangle (broad at base, distally somewhat narrowed), dorsally well carinated and lateral margins bearing small teeth. Cornea relatively reduced (moderately broader than the eyestalk) having distomesial eye-spine. Basal article of antennular peduncle bearing well developed dentate process, ending in a distomesial tubercle, and 2 spines in distolateral and distodorsal position. Absence of denticulate carina on mesial margin of P1. P2 over-reaching P1. P2–4 dactyli strongly curved distally. Epipods present only on P1. Pleon spineless, posteromedian margin of pleonite 6 weakly convex, not produced.
Redescription. Carapace slightly longer than broad (length-width ratio>1.2), pentagonal, wide at distal region; lateral margins slightly convex; cervical groove well-defined, gastric region with two well-developed conical epigastric spines, anterior branchial region with some spine-like tubercles (one to eight surrounding epigastric spines) and scale-like ridges elsewhere; posterior part of carapace with interrupted, elevated ridges; short setae across carapace ( Fig. 6C, 6D View FIGURE 6 ). Anterior margin of carapace oblique, bearing sharp antennal spine similar in size to anteriorly directed anterolateral (first spine on lateral margin) ( Fig. 6E View FIGURE 6 ). Second spine of lateral margin well developed, larger than anterolateral, originating just behind anterior branch of cervical groove. Usually 2 or 3 more spines (up to 6 spines on lateral margin of carapace). Posterior margin of carapace with a continuous double ridge along with setae ( Fig. 6D View FIGURE 6 ). Rostrum forming isosceles triangle (broad at base, distally somewhat narrowed, Fig. 6F View FIGURE 6 ), strongly upturned distally, proximal half somewhat straight. Median carina well defined on dorsal region, ridges scale-like and small tubercles that increase in number proximally, ventral region smooth. Lateral margins bearing small teeth. Rostrum usually one-half carapace length, sometimes less (in smaller specimens).
Pleon weakly tuberculate, tubercles apparent on pleura ( Fig. 6C View FIGURE 6 ), with some isolated setae; pleonites 2–4 bearing 2 elevated transverse ridges; pleonite 5 and pleonite 6 with scattered scale-like tubercles, anterior edge with row of rounded teeth and continuous line of short setae ( Fig. 6B, D View FIGURE 6 ). Pleonite 6 with well-defined posterolateral lobes, posteromedian margin weakly convex, not produced or overhanging the posterolateral lobes ( Fig. 6G View FIGURE 6 ). Protopod of uropod with posterolateral margin in 3 lobes bearing scale-like tubercles and setae; posterior lobe with group of denticles and sharp teeth separated by notch. Telson wider than long (wide/length ratio usually 1.43, range 1.28–1.69), composed of 8 to 10 plates (usually 8), armed with scale-like tubercles with setae scattered over surface ( Fig. 6G View FIGURE 6 ). Several short, calcified setae on margin (except proximal one).
Thoracic sternum bearing transversal depressions between each sternite, anterior margins between coxae of P1 serrate; small scale-like tubercles with setae along forward edge. Sternite 3 complete, slightly narrow forming apposed lobe bearing anteromedian process at either side of slightly deep median groove ( Fig. 6H View FIGURE 6 ).
Ocular peduncles slightly movable, wide at proximal region, bearing small tubercles and setae. Cornea relatively reduced (moderately broader than the eyestalk), armed with an elongate and sharp distomesial eye-spine directed slightly forward (sometimes directed anterolaterally) and covering less than one-half of the ocular (cornea, Fig. 6E View FIGURE 6 ).
Antennule basal article somewhat tuberculate, inflated laterally; dentate process well developed, ending in a distomesial tubercle (sometimes as small spine) and 2 sharp spines, distolateral and distodorsal, of almost equal size ( Fig. 6I View FIGURE 6 ). Setae of extended flagellum barely reaching tip of rostrum.
Antennal peduncle bearing small tubercles, usually decreasing in number by article 4. Article 1 broad, armed with two triangular teeth, often ventromesial tooth sharper and longer, while lateral tooth is broad. Article 2 with distomesial tubercle and elongated distolateral spine barely reaching one-half of article 3; dorsal region bearing a tooth on proximal margin. Article 3 with small tubercles or spines around distal margin, usually larger denticle in lateral and mesial margins armed with smaller teeth ( Fig. 6J View FIGURE 6 ). Flagellum long, approximately 4 times carapace length.
Third maxilliped articles bearing small tubercles and some setae. Basis with mesial ridge armed with 2–7 (usually 5 teeth), ischium with mesial denticulate carina (range 15–27 teeth) and with small spine on extensor distal margin. Merus armed with distal spine on extensor margin, sometimes also with tubercles or small spines. Flexor margin with (1–6) usually 4 well developed spines and smaller spines or tubercles also. Carpus usually unarmed, setae thicker in mesial region, as well as in propodus and dactylus ( Fig. 6K View FIGURE 6 ).
P1 (cheliped) with epipod, article surfaces armed with tubercles and spines associated with setae. Ischium armed with dorsolateral spine and one smaller mesial spine, as well as scattered tubercles ( Fig. 6L View FIGURE 6 ). Merus smaller than chela, armed with scale-like tubercles more apparent in dorsal region, 4 distal spines: distomesial, distodorsal, distolateral, and distoventral, and middorsal spines (range 2–8), decreasing in length proximally region. Carpus slightly narrow (length:width ratio 1.1–1.6) armed with tubercles on dorsal region and variable distal ornamentation (usually 4 spines: lateral, dorsolateral, adjacent to propodus joint and mesial, Fig. 6M View FIGURE 6 ). Chela narrow, not slender (width:length ratio usually 0.28; range 0.24–0.45), dorsoventrally compressed in distal region and somewhat inflated in proximal region, armed with scale-like tubercles bearing row of rounded teeth and continuous short setae along forward edge. Propodus pollex (fixed finger) without denticulate carina on mesial margin. Tips of dactylus (movable finger) and pollex spooned, margins dentate.
P2–P5 lacking epipods. P2–P4 similar in shape, with tubercles and spines associated with setae, slender and slightly narrow (measured on P2: ratio of total pereiopod length/merus width 9– 13), armed with tubercles, spines, and setae. Ischium bearing dorsal blunt teeth and projections. P2 usually over-reaching P1 or nearly same size. Merus slightly longer than propodus, with well-developed distal spines in extensor and flexor margins, dorsomesial ridge usually with 6 spines decreasing in length proximally region (range 0–11), along with tubercles and setae arranged in lines. Carpus bearing enlarged spines at dorsomesial angle of distal margin, usually followed by row of 3 smaller spines and ridge of tubercles; tubercles scattered through carpus, decreasing on flexor surface. Propodus bearing two mobile spines on distal flexor region and with several raised longitudinal rows of small tubercles on flexor surface and more apparent ridges on extensor surface; variable number of spines (range 0–8) along with setae. Dactylus moderately slender (ratio of dactylus width measured at mid-length/total dactylus length: range 0.17–0.26, usually 0.21–0.22), length approximately 3/4 propodus length, strongly curved at the end of extensor margin, and ending in a brown claw. Flexor margin with a row of proximally diminishing low dactylus teeth (range 12–17, usually 14), each with corneous spinule projecting from anterior edge. Distal tooth remote from the terminal claw (end of dactylus) and much closer to penultimate tooth ( Fig. 6N View FIGURE 6 ). P5 armed with small tubercles on lateral margin, small and blunt teeth on ventral margin. Propodus and dactylus distal region bearing elongated setae. Male pleopods as Figure 6O View FIGURE 6 (gonopod 1, G1) and Figure 6P View FIGURE 6 (gonopod 2, G2).
Color. White with golden setae, cornea orange.
Size. Maximum CL 47.41 mm ovigerous female (CNCR 24848) from Chapopote Knoll, southwestern Gulf of Mexico, CL 38.51 mm female and CL 38.79 mm male (IFREMER) from Regab Pockmark, Gulf of Guinea. Juveniles (gonopods immature) present a CL between 2.17 mm and 20.00 mm.
Remarks. The morphological similarity between M. geyeri and M. subsquamosa was noted since the first description of M. geyeri, Pequegnat & Pequegnat (1970) recognized the following differences: M. subsquamosa with a mobile eyestalk, pleon armed with granules and three denticulate spines on merus of third maxilliped. Those differences were considered insufficient to support the specific difference between species and fell into synonymy (e.g. Ambler 1980; Wicksten & Packard 2005). Mayo (1974) and Gore (1983) compared the species and the original description by Henderson (1888) along with M. pallida Alcock, 1894 (as M. subsquamosa var. pallida Alcock & Anderson, 1894 ), with emphasis on the carapace spine-like tubercles (more than 2 epigastric spines in M. subsquamosa ), the appearance of the cardiac region (less apparent), the length and orientation of the rostrum (usually 1/3 of the carapace length, almost straight), and the direction of the second spine of the carapace margin (anterolateral). Baba (2005) reviewed M. pallida in detail and compared it with the holotype of M. geyeri , concluding that the principal difference between the species lays in the strong distal curvature on P2–P4 dactyli and the greater proximity of the ultimate flexor marginal process to the penultimate than to the tip of the article in M. geyeri . Similarly, five species from the Pacific Ocean ( M. abyssicola Baba, 2005 , M. panamae Baba, 2005 , M. petila Baba, 2005 , M. producta Baba, 2005 and M. recta ) resemble M. geyeri morphologically. These can easily be distinguished by the P2–P4 dactylus curvature and dactylus/propodus length ratio ( M. panamae and M. recta by a slightly curved dactylus, ratio 0.68–0.75, M. petila with a ratio of 0.71–0.72, M. producta 0.64, and M. abyssicola 0.55) as well as shape and direction of the rostrum. Munidopsis bracteosa and M. scotti ( Jones & Macpherson 2007) are morphologically similar to M. geyeri . These species can be differentiated by a produced posteromedian lobe in pleonite 6 on M. bracteosa , whereas M. scotti has a carapace armed with more than two gastric spines, rostrum weakly carinate and almost straight. Other Atlantic Ocean species are similar to M. geyeri : M. crassa differs by>20 spines on the carapace and>4 spines on the lateral margins, and a produced posteromedian lobe on pleonite 6 (as pointed out by Mayo 1974). Munidopsis hirtella Macpherson & Segonzac, 2005 and M. exuta differ from M. geyeri by a spinelike rostrum, posterolateral lobes of pleonite 6 overreaching the transverse posteromedian margin, P2–P4 dactylus length similar to the propodus, a larger number of gastric spines in M. hirtella , and the absence of well-developed antennal spines in M. exuta ( Fig. 4 View FIGURE 4 ).
The morphological intraspecific variability of M. geyeri was recorded in 24 juveniles and 70 adults (35 females, 9 of which were ovigerous, and 35 males), and among individuals of the same size. More than half of the reviewed characters varied both in juveniles and adults. This variability led us to re-describe M. geyeri . The re-description follows Baba (2005), Gore (1983), Macpherson & Segonzac (2005), Pequegnat & Pequegnat (1970), Pequegnat & Pequegnat (1971) and Mayo (1974) description of three specimens (one female, one male and one juvenile) and the specimens analyzed herein.
Biology. Sexual dimorphism on lateral margin of telson, with more dense setae in males. Males and females are not significantly different in size in the Chapopote Knoll population (t-test, df = 22, t = 1.239, p = 0.2285, n = 24; Gaytán-Caballero 2009) and with those from the northern Gulf of Mexico (t-test, df = 9, t = -1.023, p = 0.333, n = 11; Kilgour & Shirley 2014). Ovigerous females were sampled form the Regab Pockmark site in January (Gulf of Guinea), and from Chapopote Knoll in April (Gulf of Mexico), no ovigerous females have been sampled from June through August, and December in the northern Gulf of Mexico ( Kilgour & Shirley 2014). Other isolated sampled specimens prevent recognition of the existence of ovigerous females form the sites. Ovigerous females from Chapopote Knoll, carry up to 140 embryonated eggs with an average size of 2.36 mm (2.36 + 0.22; n = 590). In the Regab Pockmark site embryonated eggs have an average size of 2.23 mm (2.23 + 0.14; n = 21). Based on the number and size of eggs in Munidopsis geyeri this study suggests that this species could have lecithotrophic larvae.
Ecology. Microscopic filamentous epizoans were recorded attached to the body surfaces, appendages and setae in specimens from Gonave Bay ( Mayo 1974). Parasites found on M. geyeri include the branchial bopyrid isopod (in female from the Venezuela Basin; Gore 1983) and Cirripedia (Superorder: Rhizocephala Müller, 1862) on the pleonal ventral region (six specimens from the Regab Pockmark and in one male form Chapopote Knoll: CNCR- 24854).
As mentioned above, M. geyeri was collected along with M. livida . It co-occurs with M. colombiana Pequegnat & Pequegnat, 1971 , M. crassa , M. aries (Milne Edwards, 1880) and M. reynoldsi (Milne Edwards, 1880) in the Colombia Basin ( Pequegnat & Pequegnat 1971). It co- occurs with M. bermudezi Chace, 1939 in the Venezuela Basin ( Gore 1983) and the Alaminos Canyon in the northeastern Gulf of Mexico ( Coykendall et al. 2017). It co-occurs with M. hirtella at the Regab Pockmark in the Gulf of Guinea. In Chapopote Knoll, M. geyeri was randomly distributed co-occurring with M. exuta and background fauna. The specimens were more abundant on the asphalt and the active seeping sites than in the background habitats. On the asphalt, the species coexists with typical seep biota including tube worms ( Escarpia laminata Jones, 1985 ), mussels ( Bathymodiolus heckerae Turner, Gustafson, Lutz & Vrijenhoek in Gustafson, Turner, Lutz & Vrijenhoek, 1998 and B. brooksi Gustafson, Turner, Lutz & Vrijenhoek, 1998 ), ophiuroids ( Ophioctenella acies ), caridean shrimps ( Alvinocaris muricola ), holothurians ( Chiridota heheva ), encrusting sponges, and demersal fishes ( Pachycara Zugmayer, 1911 ). Similarly, in the Regab Pockmark site M. geyeri specimens have been recorded in vesicomyid clusters empty shells on the periphery of the pockmark and cooccur with holothurids and other seep biota. The species is more abundant in active seeps ( Olu et al. 2009). Association with M. exuta in Regab Pockmark is difficult to confirm due to a single recorded juvenile female ( Table 1 View TABLE 1 ).
Geographical distribution. The known geographic distribution of Munidopsis geyeri is extended to the northwestern (NW) and to the south-southwestern (SSW) Gulf of Mexico ( Felder et al. 2009b), and off Mauritania, Africa ( Fig. 5B View FIGURE 5 ). Munidopsis geyeri displays an amphi-Atlantic distribution ( Navas et al. 2013; Schnabel et al. 2011). The species is known from the wider Caribbean Sea, the Gulf of Guinea, off Mauritania in the northwestern Africa, and the Azores Islands ( Fig. 5B View FIGURE 5 ) at depths of 1700 to 4151 m. Mayo (1974) calculated the occurrence of the species to a depth range of 2790–4151 m for three Caribbean Sea specimens (off Gonave, Haiti) based on the greatest depth of occurrence, at the shallowest station, to the least depth at the deepest station.
| V |
Royal British Columbia Museum - Herbarium |
| MNHN |
Museum National d'Histoire Naturelle |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Munidopsis geyeri Pequegnat & Pequegnat, 1970
| Gaytán-Caballero, Adriana, Escobar-Briones, Elva, Robles, Rafael & Macpherson, Enrique 2022 |
Munidopsis sp. A
| Coykendall, D. K. & Nizinski, M. S. & Morrison, C. L. 2017: 267 |
Munidopsis geyeri
| Wicksten, M. K. & Packard, J. M. 2005: 1761 |
Munidopsis cf. subsquamosa
| Turnipseed, M. & Jenkins, C. D. & Van Dover, C. L. 2004: 123 |
Munidopsis sp.
| Cordes, E. E. & Carney, S. L. & Hourdez, S. & Carney, R. S. & Brooks, J. M. & Fisher, C. R. 2007: 643 |
| Olu, K. & Lanc, S. & Sibuet, M. & Fiala-Medioni, A. & Dinetj, A. 1997: 209 |
| Olu, K. & Sibuet, M. & Harmegnies, F. & Foucher, J. P. & Fiala-Medioni, A. 1996: 371 |
Munidopsis subsquamosa
| Ambler, J. W. 1980: 25 |
Munidopsis geyeri Pequegnat & Pequegnat, 1970: 139
| Vazquez-Bader, A. R. & Gracia, A. 2016: 23 |
| Kilgour, M. J. & Shirley, T. C. 2014: 406 |
| Navas, G. R. & Bermudez, A. & Angel-Yunda, C. & Campos, N. 2013: 3505 |
| Olu, K. & Cordes, E. E. & Fisher, C. R. & Brooks, J. M. & Sibuet, M. & Desbruye, D. 2010: 4 |
| Felder, D. L. & Alvarez, F. & Goy, J. W. & Lemaitre, R. 2009: 1066 |
| Gaytan-Caballero, A. 2009: 1 |
| Olu, K. & Caprais, J. C. & Galeron, J. & Causse, R. & von Cosel, R. & Budzinski, H. & Menach, K. Le & Roux, C. Le & Levach, D. & Khripounoff, A. & Sibuet, M. 2009: 2386 |
| Baba, K. & Macpherson, E. & Poore, G. C. B. & Ahyong, S. T. & Bermudez, A. & Cabezas, P. & Lin, C. & Nizinski, M. & Rodrigues, C. & Schnabel, K. E. 2008: 142 |
| Baba, K. 2005: 163 |
| Macpherson, E. & Segonzac, M. 2005: 25 |
| Navas, G. R. & Bermudez, A. & Cruz, N. & Campos, N. H. 2003: 201 |
| Gore, R. H. 1983: 202 |
| Mayo, B. S. 1974: 28 |
| Pequegnat, L. H. & Pequegnat, W. E. 1970: 139 |