Tasmaniosoma armatum Verhoeff, 1936
|
publication ID |
https://doi.org/ 10.3897/zookeys.41.420 |
|
publication LSID |
lsid:zoobank.org:pub:FC5CFE57-05F9-4685-BC02-BB82AB9E4894 |
|
DOI |
https://doi.org/10.5281/zenodo.3788458 |
|
persistent identifier |
https://treatment.plazi.org/id/6B10878B-F811-A54E-FF34-FAF0FBDBFBD7 |
|
treatment provided by |
Plazi |
|
scientific name |
Tasmaniosoma armatum Verhoeff, 1936 |
| status |
|
Tasmaniosoma armatum Verhoeff, 1936 View in CoL
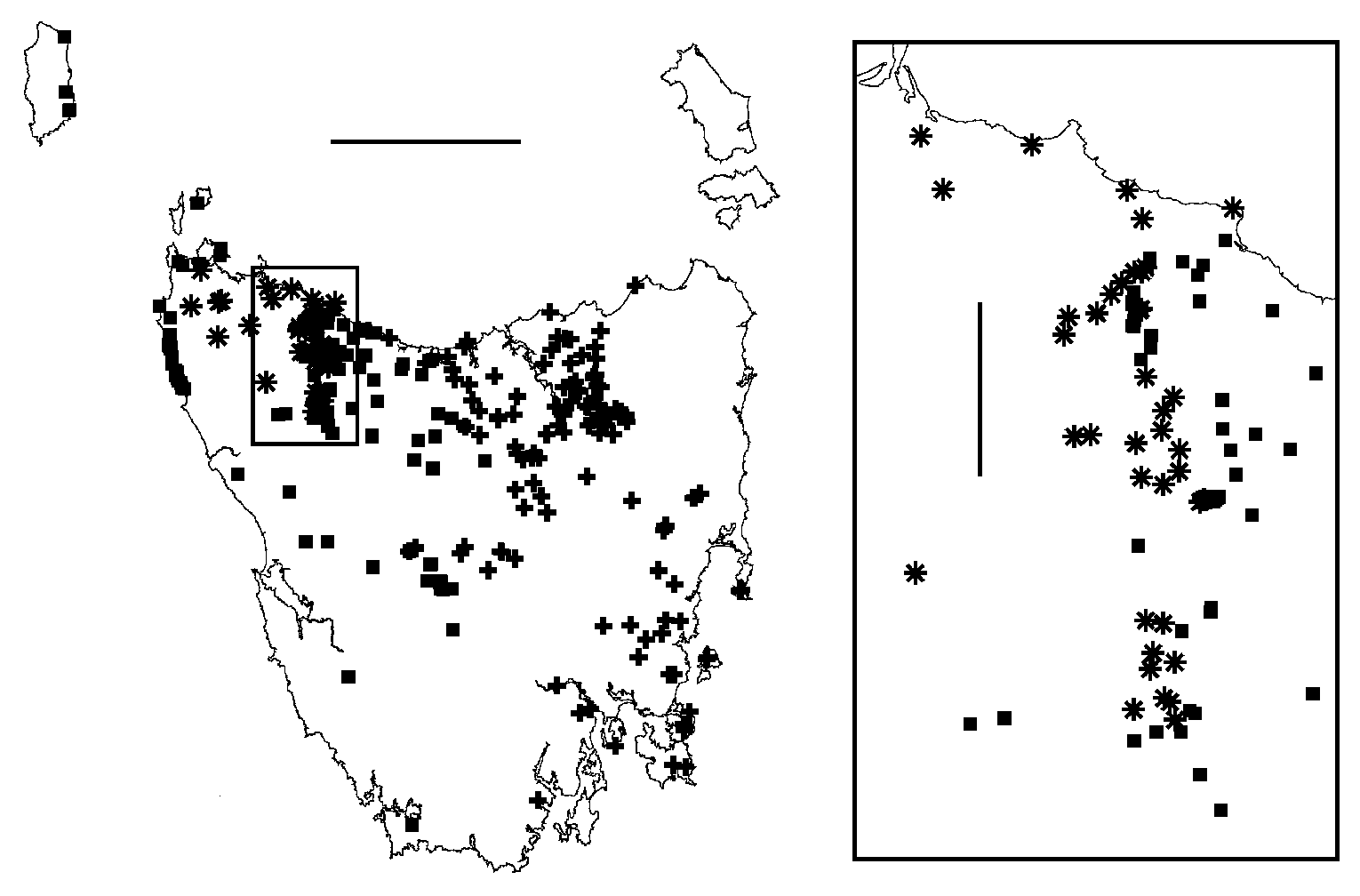
Figs 1A, 2, 3C, 4B, 5A, 6A, 6B, 7A; map Fig. 23 View Figure 23
Tasmaniosoma armatum Verhoeff, 1936:12 View in CoL , Figs 3–8 View Figure 3 View Figure 4 View Figure 5 View Figure 6 View Figure 7 View Figure 8 . Attems, 1940:443, Figs 630, 631. Jeekel, 1971:355; 1984:85.
Syntypes. Two males, Australia, Tasmania, Lake Leake, G.E. Nicholls, date not known, ZSM A20033578, A20033579, A 20052437 (see Remarks).
Figure |. A Living male Tasmaniosoma armatum Verhoeff, 1936 . Image by Hans Henderickx, used with permission. B–I Dorsal views of midbody rings and left lateral views of midbody ring of freshly killed T. clarksonorum sp. n. male, QVM 23:51683 (B, F); T. compitale sp. n. female, QVM 23:51680 (C, G); T. fasciculum sp. n. male, QVM 23:51665 (D, H); and T. hickmanorum sp. n. male, QVM 23:51681 (E, I). A–I not to same scale. Pigmentation of the four species in B–I fades progressively in alcohol and long-preserved specimens may be colourless. Th e whitish patch (p) below the paranotal margin in T. compitale sp. n. G and the whitish patches dorsally C change to light yellow in freshly preserved specimens (ca 1–10 weeks in alcohol).
Other material examined. 153 males, 128 females and 4 stadium VI males from 128 unique localities (see Appendix).
Diagnosis. Metatergites without tubercles; ring 6 sternite with discrete setal brushes on anterior margin; gonopod telopodite with stout, rod-like setae in longitudinal tract on posteromedial surface and two upright (distally directed), Y-shaped processes arising anterolaterally near apex.
Description. The following description is based on nine males and 10 females from the type locality (in QVM 23:46547, 23:46548 and 23:46567).
Male/female approximate measurements: length 14/ 14 mm, ring 12 paranota width 1.7/ 1.7 mm. Body, head and antennae uniformly dark reddish-brown in life (Fig. 1A), legs pinkish-red basally and darker distally; head and leg colour fades in alcohol, but body colour persists for many years.
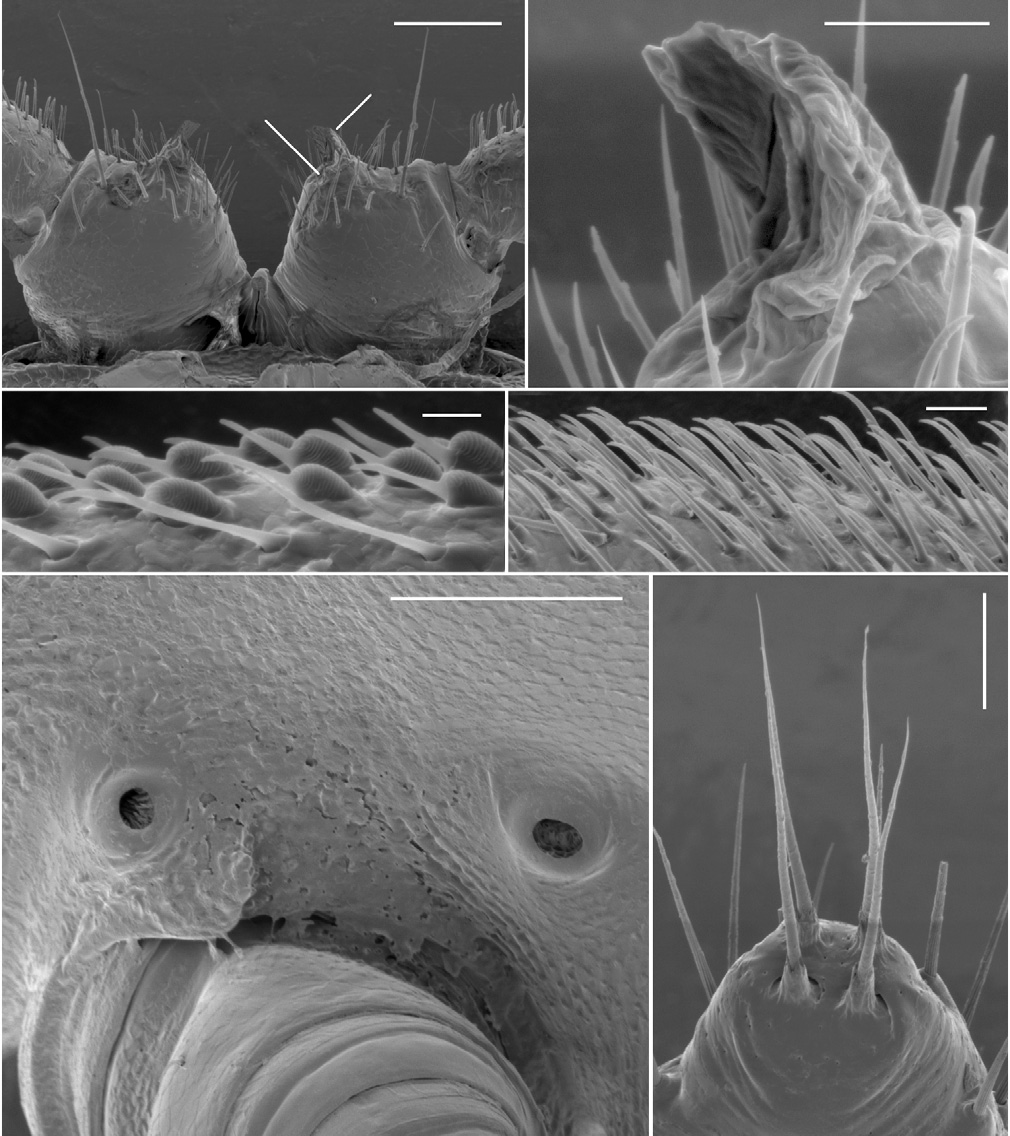
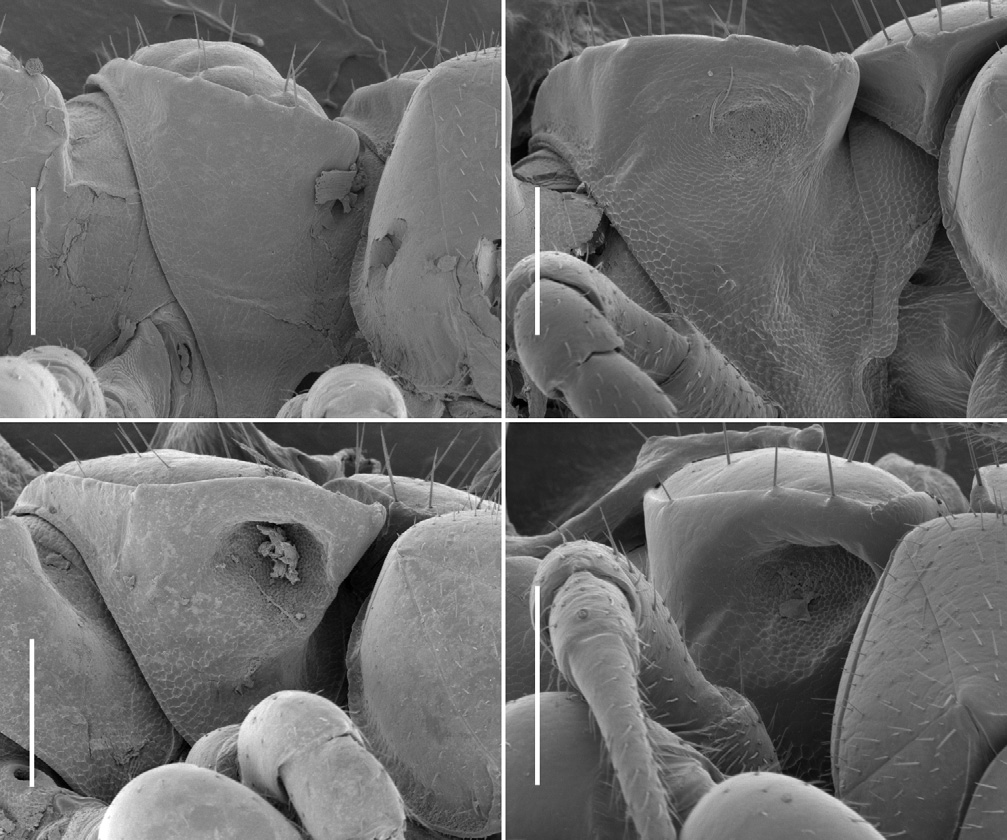
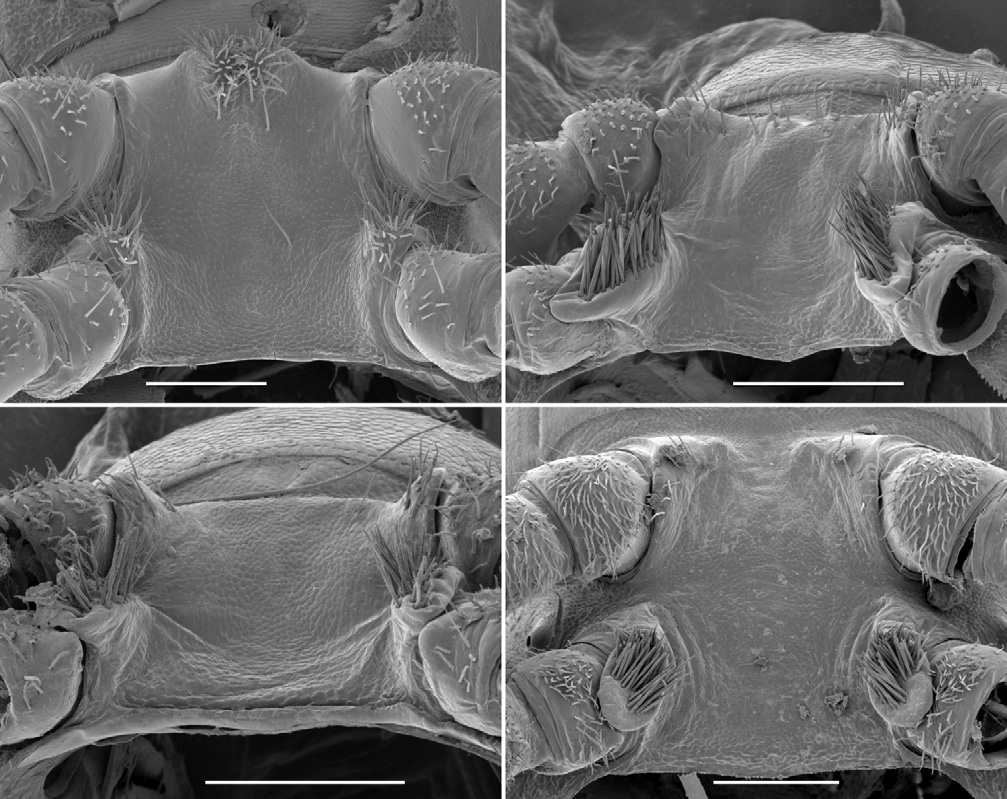
Male with head moderately setose anteriorly, vertex sparsely setose; sockets slightly impressed, separated by ca 2 × socket diameter; antennal groove deep laterally. Antenna slender, slightly clavate, when manipulated reaching back to ring 3; antennomere 6 widest, relative antennomere lengths (2,3)>6>(4,5). Collum from above reniform, convex anteriorly; corners rounded. Tergites 2–4 distinctly narrower than more posterior metatergites; overall widths tergite 6>5>4>(3,head)>2>collum; rings 6–15 about the same width. In lateral view, margin of ring 2 tergite slightly lower than margins of collum and ring 3 tergite. Ring 2 ventrally on either side with fairly shallow pit ( Fig. 4B View Figure 4 ), rim well defined anterolaterally but not medially or posteriorly. Ring suture and waist distinct on diplosegments, no longitudinal striations on waist; prozonites smooth; transverse furrow indistinct, metatergite smooth, not sculptured, with three transverse rows of ca 12 small setae: two rows anterior to transverse furrow, one close to posterior margin of metatergite; posterior metatergal margin slightly emarginate medially. Limbus composed of flat tabs, irregularly notched distally. Midbody paranota ca 1.5 × as wide as prozonite, slightly inflated, marginal groove distinct, anterior corner smoothly convex, posterior corner smoothly convex without projecting posteriorly on any rings, but with 2–3 very small tooth-like irregularities, each bearing small seta; lateral margin very slightly convex, in lateral view slightly oblique (anterior lower) at ca 2/3 ring height. Midbody metatergites ca 2.2 × as wide as long. Pore formula 5, 7, 9, 10, 12, 13, 15–18; ozopore small, round, opening dorsolaterally close to margin near posterior corner of paranotum. Spiracle small, round, opening on short, crater-like elevation; on diplosegments anterior spiracle just above and anterior to first leg, posterior spiracle about midway between leg bases ( Fig. 2E View Figure 2 ). Sternites moderately setose, as wide as long, transverse impression deep, longitudinal impression indistinct. Anterior legs with prefemur greatly swollen dorsally, femur less so ( Figs 6A, 6B View Figure 6 ); swellings begin leg 3, gradually decrease to leg 15; tarsus long, slightly curved, ca 1.6 × as long as femur on anterior legs, but proportionally shorter posteriorly; relative podomere lengths tarsus>(prefemur, femur)>(postfemur, tibia). Sphaerotrichomes on 3–4 most distal podomeres, shafts tapered ( Fig. 2C View Figure 2 ). Brush setae on distal end of coxa/trochanter, prefemur, base of femur; brush setae unbranched, tapering ( Fig. 2D View Figure 2 ). Gonopore on distomedial bulge of leg 2 coxa, pro- tected by thin cowl ( Figs 2A, 2B View Figure 2 ). Short brushes of fine setae on sternite between legpairs 3, 4 and 5. Leg 6 and 7 bases ( Fig. 5A View Figure 5 ) well- and equally separated; no leg 6 sternal tab; leg 7 tab short, thin, with brush of fine setae; anterior edge of sternite medially with paired, conjoined brushes of fine setae on low protuberance. Pre-anal ring moderately setose; hypoproct subtrapezoidal; epiproct from above tapering smoothly to rounded tip, extending slightly past anal valves. Spinnerets ( Fig. 2F View Figure 2 ) in square array; setae with tightly-fitting basal sheaths; dorsal setae unprotected, ventral setae in shallow depression.
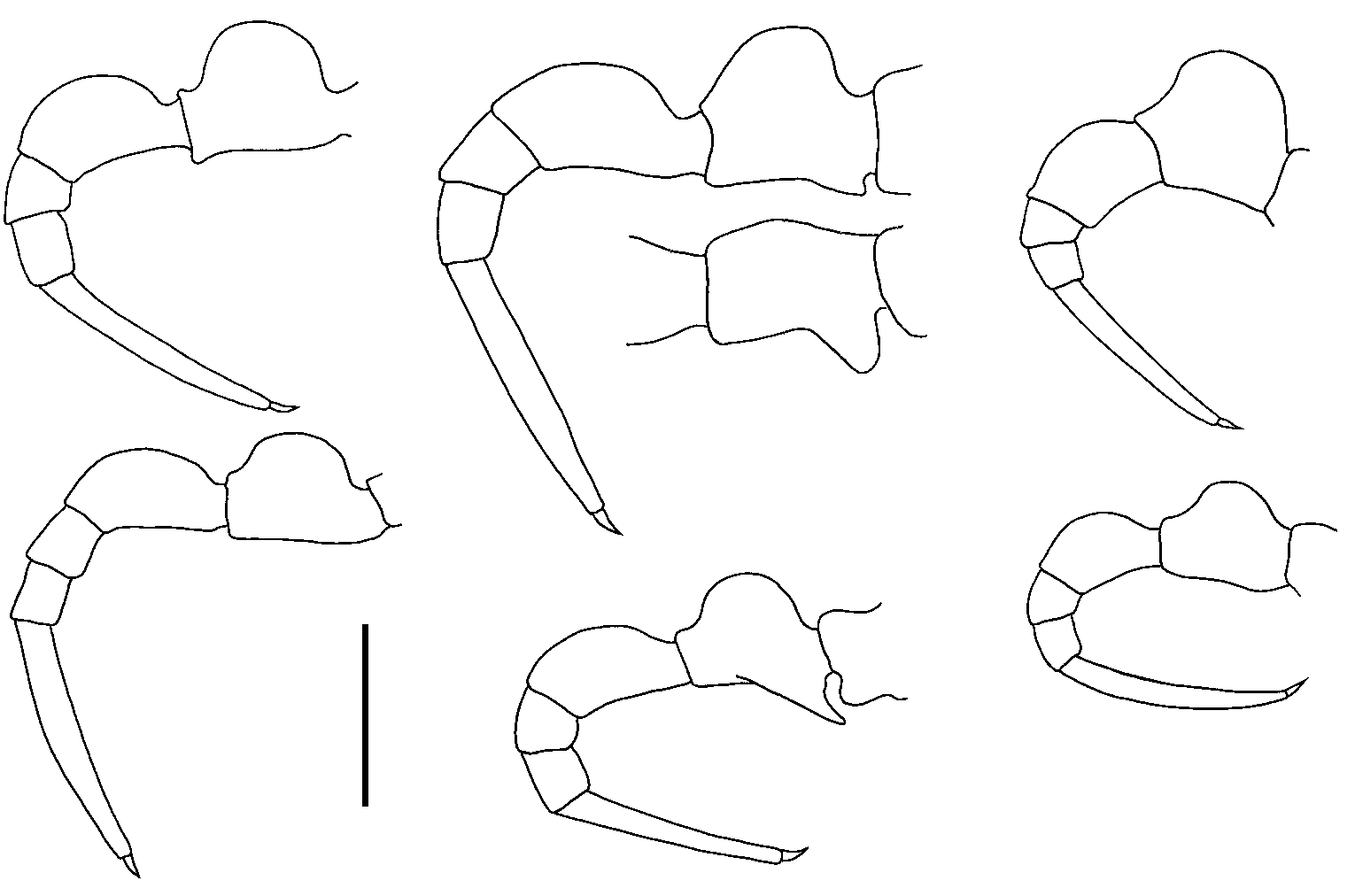
Gonopod aperture ovoid, ca 1/2 as wide as ring 7 prozonite, posterolateral margins raised. Telopodites straight, parallel but slightly divergent apically; extending nearly to leg 5 bases when retracted. Gonocoxa short (ca 1/3 length of telopodite), more or less truncated-conical with anterior side longer than posterior; lateral surface projected distally as rounded lobe pressed to telopodite base, medial surface slightly projected
distally; with sparse long setae distally on posterolateral surface. Gonocoxae weakly
joined distomedially. Cannula prominent, inserting basomedially in depression on te-
lopodite. Telopodite ( Fig. 7A View Figure 7 ) a distally tapering cylinder with setose base protruding
posteriorly as rounded lobe; with small, discrete, setose bump on anteromedial side of
base; and with buttress-like ridge on anterolateral surface from articulation with gono-
coxa to ca 1/3 telopodite height. Numerous minute setae near cannula insertion; fine,
tapering setae on telopodite base, on posterolateral surface and in longitudinal row of
three long setae on lateral surface at about half telopodite height. Telopodite also with
narrow, discrete zone of 30–40 stout, rod-like setae on posteromedial surface from
ca 1/3 to 2/3 telopodite height; these setae often broken or broken away, but sockets
always visible. Apex of telopodite with cluster of six major processes, labelled here and
in Fig. 7A View Figure 7 from lateral to medial:
(1) broad Y-shaped process flattened anteroposteriorly, divided at 1/3 to 1/2 the process height, the two tips acute [‘ta1’ of Verhoeff (1936)];
(2) similar, slightly smaller, less deeply divided process [‘ta2’];
(L) laminate process resembling bird’s head in profile, flattened mediolaterally, broadly curved distally with posterior, hook-like extension [‘ti’];
(S) short, thorn-like solenomere curving posteromedially [‘sl’];
(3) short process possibly continuous with solenomere, divided into posterolaterally directed tab with rounded tip, and posteromedially directed rod with several apical teeth [‘n’];
(4) thin, rod-like process arising posteromedially below the telopodite apex and bending very slightly posteriorly, with a flattened tip [‘psl’].
In addition, very short finger-like process arising just lateral to process 3 and directed posterolaterally. Prostatic groove curving first anteriorly, then posteriorly from base and running more or less directly to solenomere, passing posterior to base of process 4.
Female as large as male or slightly smaller, legs more slender and prefemora and femora not swollen, sternites ca 1.2 × as wide as long. Epigynum ca 1/3 width of ring 2, posterior margin produced medially as small, rounded triangle ( Fig. 3C View Figure 3 ). Cyphopods not examined.
Distribution. Common and sometimes locally abundant in eucalypt forest over ca 25 000 km 2 in central and eastern Tasmania from sea level to at least 960 m elevation, including Schouten and Maria Islands and Forestier and Tasman Peninsulas, but absent from much of the inland northeast ( Fig. 23 View Figure 23 ).
T. armatum is parapatric with T. hickmanorum sp. n. in the west of its range, notably along the Tasmanian biogeographical divide known as the Mersey Break ( Mesibov 1999), and is sympatric or parapatric with T. clarksonorum sp. n. and T. gerdiorivum sp. n. along the East Tamar Break ( Mesibov 1994, 1997). It may be parapatric with T. orientale sp. n. in the Eastern Tiers.
T. armatum may have been introduced to Tasmanian localities outside its natural range. Adults are often found under loose eucalypt bark, and might be carried from place to place in shipments of logs and firewood. For example, the specimen in Fig. 1A was collected in 2007 at Tahune Forest Reserve in southern Tasmania, well within the range of T. warra sp. n. and well outside the main range of T. armatum . Another specimen was found near Tahune Bridge in the Reserve in 1994. Tahune Forest Reserve is a much-visited tourist attraction and the road through the Reserve has been used by log-carting vehicles for many years.
Remarks. Verhoeff (1936, p. 14) thanks “Prof. Nicholls an der University of Western Australia, Perth-Crawlay [Crawley]” for material from Lake Leake. George Edward Nicholls was Professor of Biology at the University of Western Australia from 1921 to 1947. He is known to have visited Tasmania in 1928, 1929 and 1939 ( Nicholls 1943, p. 142) and it is likely that T. armatum was collected during one of the first two visits.
The type material was located by Dr Jörg Spelda and imaged by him at my request. It consists of three registered museum lots. A20033578 is a slide mount with disarticulated legs, antennae, metatergites and right and left gonopods. From images of this mount it is clear that Verhoeff used this slide to draw his Figs 3 View Figure 3 and 4 View Figure 4 (leg 3) and Figs 7 View Figure 7 and 8 View Figure 8 (medial views of left gonopod). A20033579 is a slide mount with a leg, a ring 7 metatergite and a joined pair of gonopods. Th e latter were used by Verhoeff for his Figs 5 View Figure 5 and 6 View Figure 6 (posterior view of left gonopod). Verhoeff’s description is thus based on two males. A 20052437 consists of two male trunk pieces in alcohol (dried out at some time in the past). Th e original label has been lost but the pieces are likely to be from the two males dissected by Verhoeff for the slide mounts. Only one of the slide mount labels, for A20033578, specifies Lake Leake, Tasmania, but since Verhoeff used both slides for his description and does not mention another locality, it is also likely that both males illustrated were from Lake Leake.
The gonopod drawings ( Figs 5–8 View Figure 5 View Figure 6 View Figure 7 View Figure 8 ) in Verhoeff (1936) are clear and accurate, but Verhoeff offers no evidence for the homologies he proposes for the apical processes on the telopodite, and I am reluctant to use his names for these processes.
T. armatum varies very little over its large range in colour and morphological details. Specimens from lower elevations tend to be slightly larger, to ca 15 mm in length.
| VI |
Mykotektet, National Veterinary Institute |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Tasmaniosoma armatum Verhoeff, 1936
| Mesibov, Robert 2010 |
Tasmaniosoma armatum
| Verhoeff 1936: 12 |