Athanas alpheusophilus, Marin, 2017
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4324.1.3 |
|
publication LSID |
lsid:zoobank.org:pub:77728C1D-430E-4B84-98Be-462E2A1F3Aaf |
|
DOI |
https://doi.org/10.5281/zenodo.6046834 |
|
persistent identifier |
https://treatment.plazi.org/id/6C7F0C4E-FFC2-5C6E-38DE-461CC4E9FE51 |
|
treatment provided by |
Plazi |
|
scientific name |
Athanas alpheusophilus |
| status |
sp. nov. |
Athanas alpheusophilus View in CoL sp. nov.
( Figs. 1–6 View FIGURE 1 View FIGURE 2 View FIGURE 3 View FIGURE 4 View FIGURE 5 View FIGURE 6 )
Athanas japonicus Kubo, 1936 .—Marin, 2013: 61, Fig. 24–3a, b.
Material examined. Holotype: male (cl 5.6 mm, tl 11.0 mm), ZMMU Ma 5839, Russia, Sea of Japan, Posjeta Bay, Vitjaz, 42°36′7″N 131°10′43″E, depth 1–2 m, Zostera beds, yabby pump, from burrows of Alpheus breviscristatus , coll. I. Marin, 14 June 2015. Paratypes: 12 ovigerous females and 7 males), LEMMI, same collection data as for holotype.
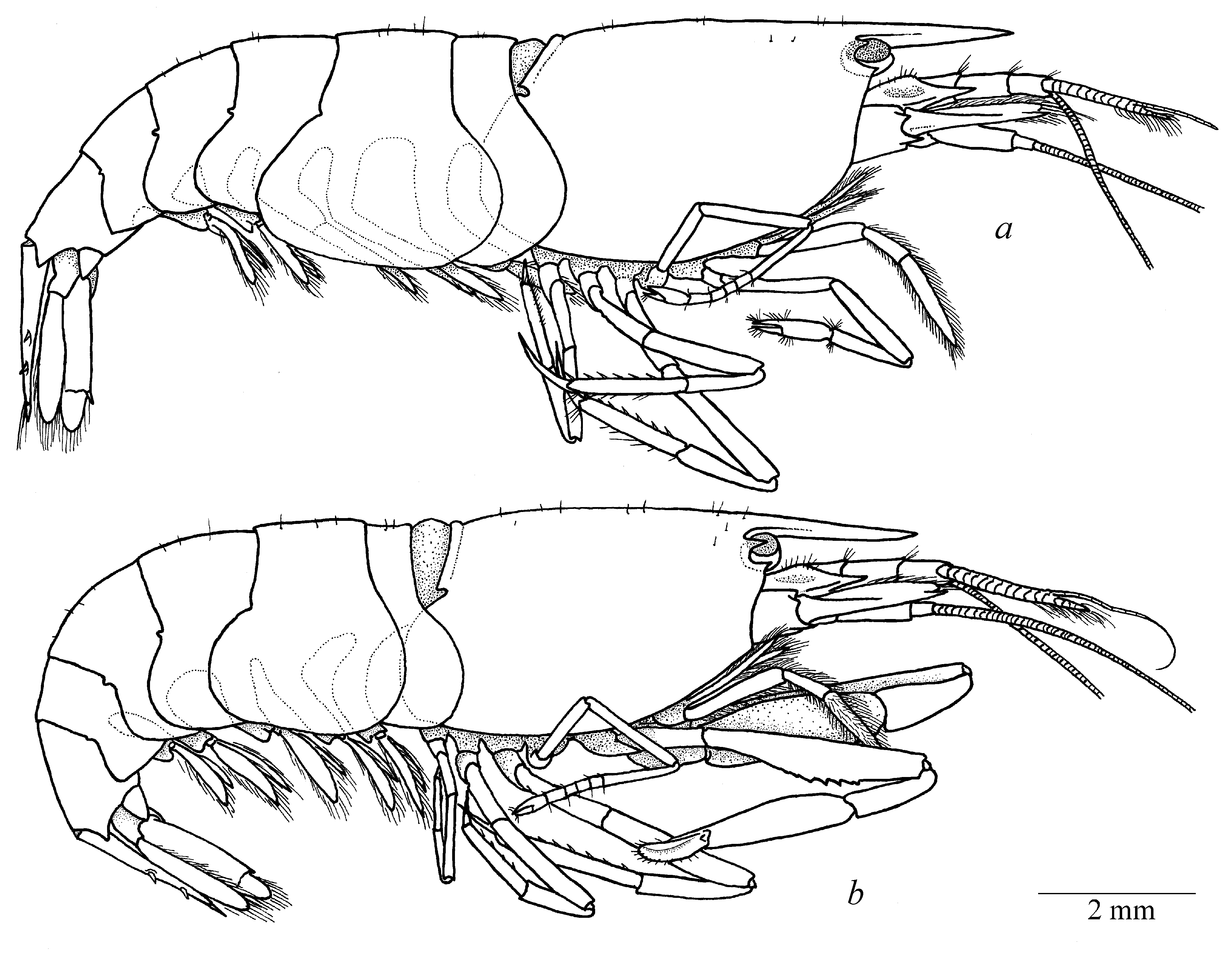
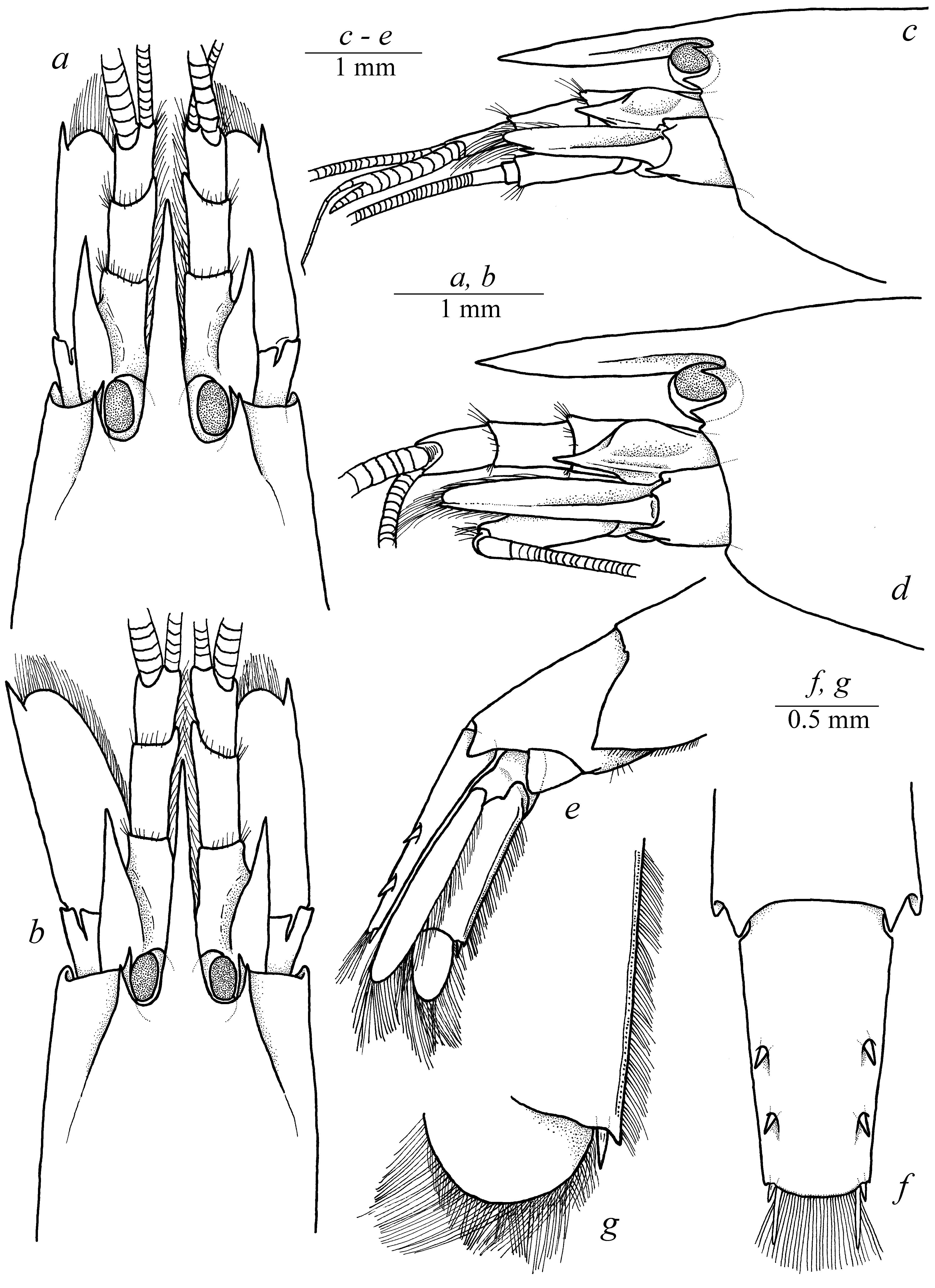
Description. Small-sized shrimp with moderately slender, subcylindrical body ( Fig. 1 View FIGURE 1 ). Carapace smooth, sparsely covered with simple erect setae ( Fig. 1 View FIGURE 1 ). Rostrum long and slender, almost reaching distal margin of second antennular article ( Fig. 2 a, b View FIGURE 2 ), distally acute, without dorsal or ventral ridges, with well-marked lateral ridges ( Fig. 2 c, d View FIGURE 2 ). Extra-corneal and infra-corneal teeth well developed, sharp, extra-corneal slightly smaller than infra-corneal ( Fig. 2 c, d View FIGURE 2 ). Pterygostomial angle feebly marked, sometimes bluntly protruding ( Fig. 2 c, d View FIGURE 2 ). Cardiac notch well developed ( Fig. 1 View FIGURE 1 ).
Pleomeres I–IV with ventrally rounded pleura, pleura of pleonite V with blunt posteroventral angle ( Fig. 1 View FIGURE 1 ); pleonite VI with large subtriangular articulated plate and bluntly produced posterolateral angle ( Fig. 2 e View FIGURE 2 ). Telson ( Fig. 2 f View FIGURE 2 ) subrectangular, slightly tapering distally; dorsal surface with two pairs of stout spines, situated at about 1/2 and 1/4 of telson length, respectively; posterior margin rounded, posterolateral angle with 2 pairs of submarginal spines, the lateral spine is about 3 times shorter ( Fig. 2 f View FIGURE 2 ).
Eyes only partly concealed by carapace, most-posterior portion invisible in dorsal and lateral views; cornea well developed and pigmented, occupying most of eye peduncle ( Fig. 2a–d View FIGURE 2 ).
Antennula ( Figs. 2 a, b View FIGURE 2 , 3 a, b View FIGURE 3 ) with antennular peduncle relatively slender; basal segment about twice longer than wide, with strong tooth on mesioventral margin, stylocerite relatively slender, acute distally, overreaching distal margin of basal antennular segment, but not reaching mid-length of second segment of antennular peduncle; latter about 0.8 times as long as basal segment, slightly longer than distal segment, about 1.5 times as long as wide in dorsal view; distal segment about 1.5 times longer than wide in dorsal view.
Antenna ( Fig. 2 a, b View FIGURE 2 ) with basicerite fairly stout, bearing strong, sharp ventrolateral tooth overreaching distal margin of the segment ( Fig. 2 c, d View FIGURE 2 ); scaphocerite subrectangular, reaching distal margin of antennular segment and distal margin of carpocerite; distolateral tooth strong, acute; distolateral tooth, convex, sloping posteriorly overreaching distal margin of scaphocerite blade ( Fig. 2 c, d View FIGURE 2 ).
Mouthparts typical for the genus. Maxilliped III slender, pediform; coxa with ear-shaped, distally subacute lateral plate; arthrobranch absent; antepenultimate segment about 6 times as long as wide, with a long slender subdistal spinule (or spine-like seta) on distoventral margin; penultimate segment about twice longer than wide; ultimate segment about 4.5 times as long as wide, tip furnished with long strong setae, without marked spines.
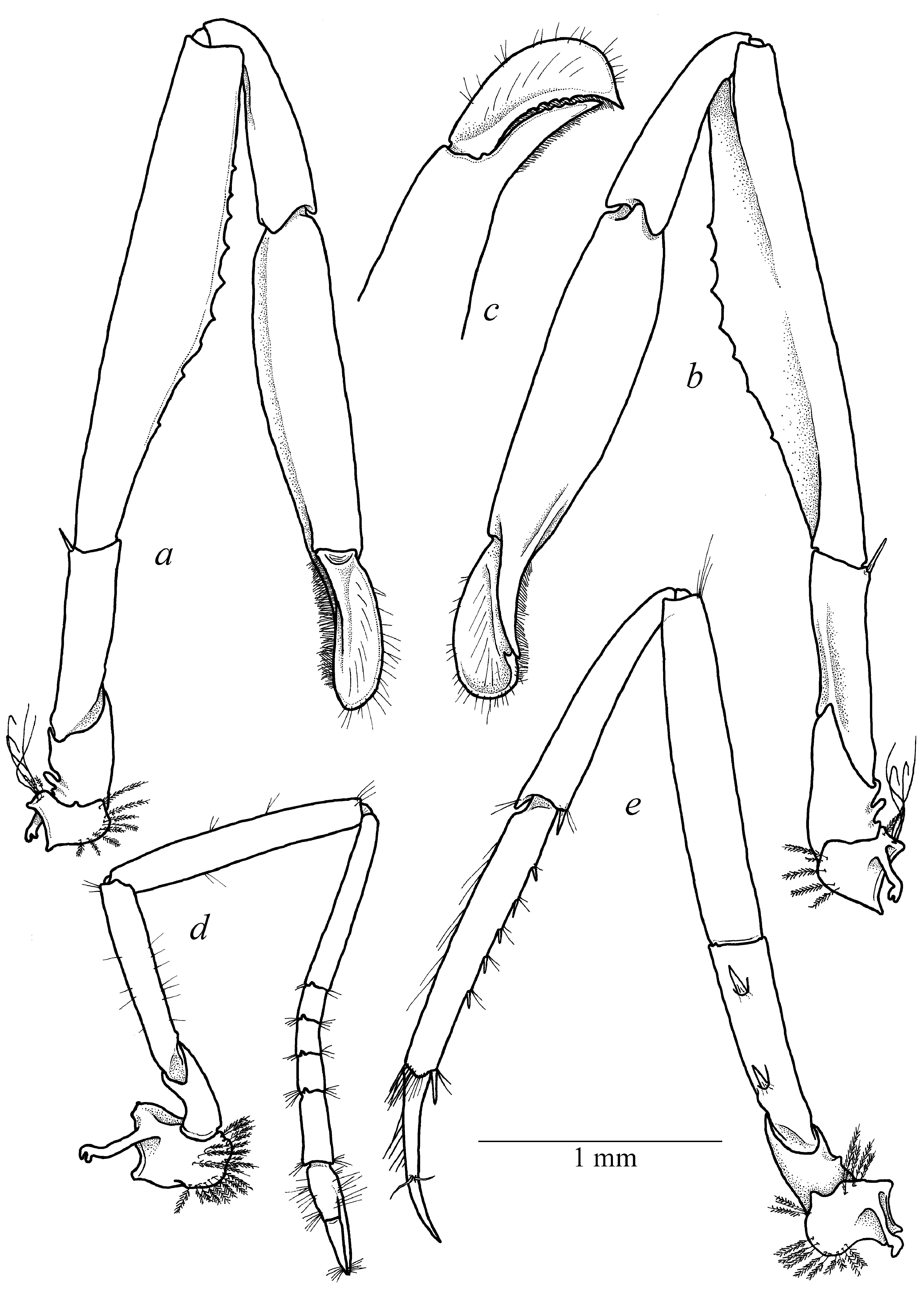
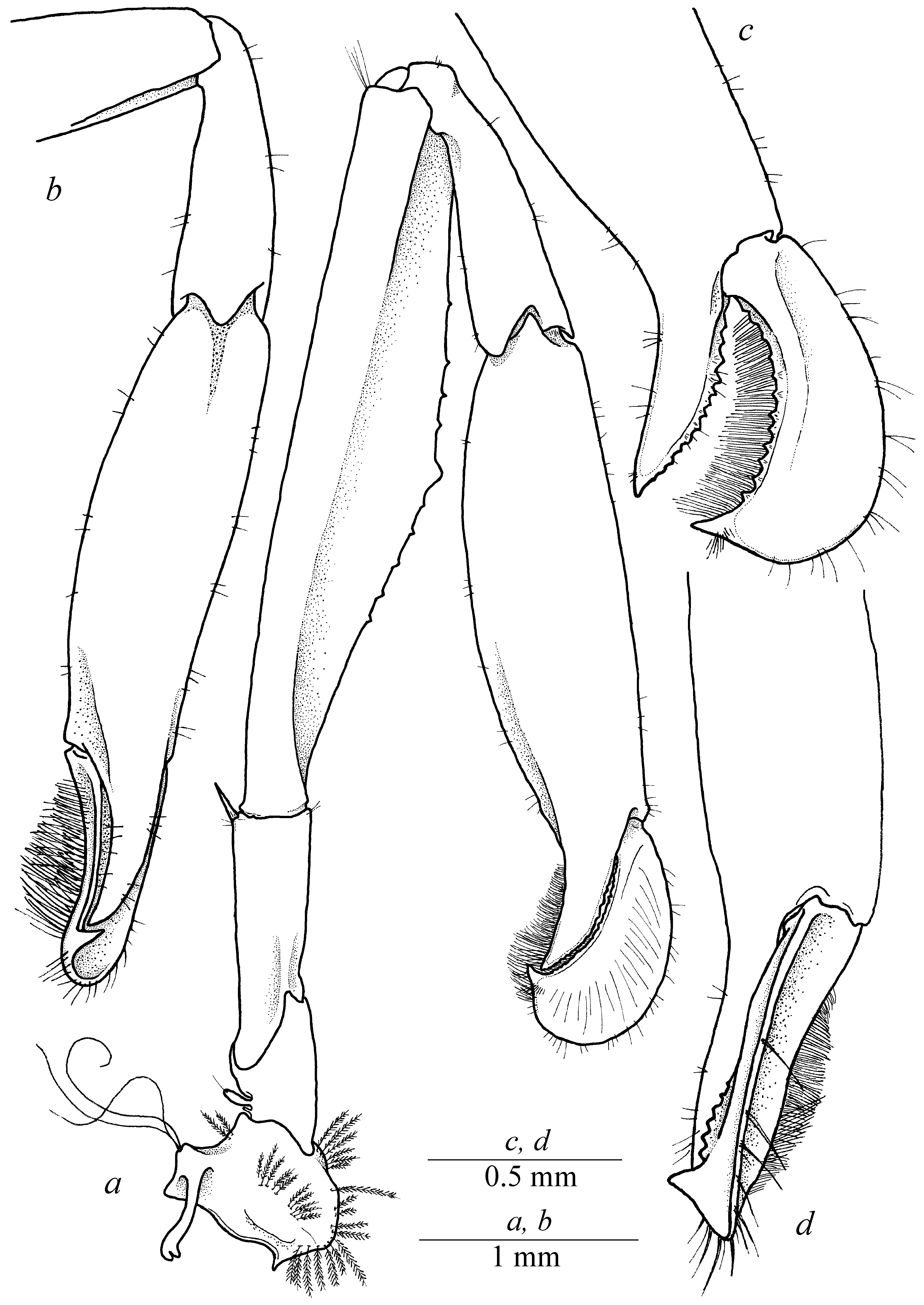
Pereiopods I (chelipeds) differ in males and females. Chelipeds relatively small, subequal and symmetrical ( Figs. 1 a View FIGURE 1 , 3 c View FIGURE 3 ) in females, with coxa and basis unarmed, coxa with epipod; basis with minuscule rudimentary exopod; ischium about 4 times as long as wide, with unarmed margin; merus relatively slender, with straight margin, about 7 times as long as wide, slightly longer than ischium and carpus; carpus relatively short, slender, distally widening, with rows of long simple setae along distodorsal margin; palm ( Fig. 3 d View FIGURE 3 ) smooth, subcylindrical, with straight lateral margins, about twice longer than wide; fingers slender, subcylindrical, straight, about 4 times longer than wide, with straight cutting margins. Chelipeds greatly enlarged, subequal in size, but slightly asymmetrical in shape ( Fig. 3 e View FIGURE 3 ) in males, carried folded with ventral portion of palm fitting in ventrally deeply excavated merus in life ( Fig. 1 b View FIGURE 1 ). Major cheliped ( Fig. 5 View FIGURE 5 ) with stout coxa and short basis, both unarmed; coxa with epipod; basis with minuscule rudimentary exopod; ischium about 2.5 times longer than wide, with single long simple spine in distodorsal angle; merus significantly broadened medially, ventral surface excavated, excavated lobe armed with several blunt small teeth along distal margin; carpus relatively slender, vase-shaped, distally widening, about 3 times longer than maximum width; palm smooth, subcylindrical, medially inflated distinctly flattened, with moderately convex dorsal and strongly convex ventral margins, without lateral teeth; polex (fixed finger) ( Fig. 5 b, c View FIGURE 5 ) subcylindrical, tapering and curved in distal part, with sharp tip, cutting margin with numerous small teeth, unarmed with dense rows of setae; dactylus ( Fig. 5 c, d View FIGURE 5 ) equal in size to polex, tapering and curved in distal part, with sharp tip, cutting margin with numerous small teeth, with well-marked large dorsal crest, outer lateral part of finger furnished with dense row of long simple setae, forming dense lateroventrally directed setal brush. Minor cheliped ( Figs. 3 e View FIGURE 3 , 4 a–c View FIGURE 4 ) morphologically similar and only slightly smaller than major cheliped.
Pereiopod II ( Fig. 4 d View FIGURE 4 ) relatively slender; coxa and basis short, coxal with small epipod; basis without exopod; ischium unarmed, about 6 times as long as wide, about 1.5 times shorter than merus; merus unarmed, with straight margins, about 6 times as long as wide; carpus with 5 segments with ratio approximately equal to (proximal to distal) 4:1:1:1:2; chela simple, slender, slightly shorter that fifth carpal segment; fingers simple, as long as palm.
Ambulatory pereiopods (III–V) moderately slender. Pereiopod III ( Fig. 4 e View FIGURE 4 ) with short coxal segment and small epipod; basis short, as long as wide; ischium about 3 times as long as wide, with 2 small ventrolateral spines; merus unarmed, about 5.5 times as long as wide, unarmed; carpus about 4 times as long as maximal width, widening distally, unarmed; propodus about 6 times as long as wide, with 5 minute ventral spines and one pair of distoventral spinules; dactylus relatively slender, simple, curved distally, about half of propodus length. Pereiopods IV and V similar to pereiopod III, but more slender; propodus of pereiopod V armed with 2 minute spines in proximal third of length and several rows of short setae distally (propodal cleaning brush); dactylus simple, slender, slightly curved.
Uropod with exopod armed with fairly stout distolateral spine ( Fig. 2 g View FIGURE 2 ); diaeresis almost straight, without specific features.
Gill/exopod formula: 5 pleurobranchs (PI–V); 0 arthrobranchs; 0 podobranchs; 4 mastigobranchs (MxpIII, PI– III); 5 setobranchs (PI–IV); 3 well-developed exopods (MxpI–III) + 1 rudimentary exopod (PI).
Colour. Body and appendages deep red; white dorsal line stretching from rostral tip through mid-dorsal area of carapace to distal margin of pleomere VI; distal parts of uropodal exopod white; some small isolated white spots present on lateral surface of carapace and pleurae ( Fig. 7 View FIGURE 7 ).
Size. Athanas alpheusophilus sp. nov. is a relatively large species within the genus. Carapace length reaching 6.0 mm and total length about 12 mm in males, while females are slightly larger with carapace length reaching 7.0 mm and total length about 14 mm.
Ecology. All described specimens were collected from burrows of large snapping shrimp Alpheus brevicristatus built at depths of 1–2 m into muddy sand among Zostera beds. The species appears to be associated with A. brevicristatus as it has never been found outside of the host burrows at the type locality or any other investigated areas along Russian coast of the Sea of Japan. Within the burrows of A. brevicristatus , Athanas alpheusophilus sp. nov. lives in small groups composed of several ovigerous and non-ovigerous females and one or two mature males.
Etymology. The specific name refers to the association with larger host alpheid shrimp of the genus Alpheus Fabricius, 1798 , which burrows are inhabited by the new species; from Alpheus + philus (Greek for loving).
Distribution. Presently known only from the type locality in Vityaz Bay (42°36′7″N 131°10′43″E), Russian coast of the Sea of Japan. The species probably belongs to cold current living fauna of the Sea of Japan inhabiting northern mainland coast influenced by Liman cold current from the Sea of Okhotsk ( Fig. 7 View FIGURE 7 ).
Taxonomic remarks. Athanas alpheusophilus sp. nov. belongs to increasingly heterogeneous and possibly non-monophyletic “ Athanas dimorphus ” species group (cf. Anker & Jeng 2007) characterized mainly by the general shape of enlarged chelipeds (pereiopods I), especially in males, including their ability to be carried flexed, with chela fitting in merus specially excavated for this purpose. The new species shows more close similarities with the representatives of “ Athanas japonicus ” species group described and presently known only from Japan, including Athanas ohsimai Yokoya, 1936 , Athanas japonicus Kubo, 1940 , Athanas lamellifer Kubo, 1940 (considered as junior synonym of A. japonicus (after Miya & Miyake 1968)).
At the same time, the new species can be clearly separated from A. ohsimai by longer rostrum reaching the distal antennular segment (vs. reaching the midlength of second antennular segment in A. ohsimai (Yokoya 1936)) , different proportions in pereiopod I with longer carpus both in males and females (carpus of pereiopod I about half of propodal length in males ( Figs. 3 e View FIGURE 3 , 4 a, b View FIGURE 4 , 5 a, b View FIGURE 5 ) and equal to chela length in females ( Fig. 3 c View FIGURE 3 )) (vs. carpus about 1/3 of propodal length in males and markedly shorter than chela in females of A. ohsimai (Yokoya 1936: Fig. 1B, L View FIGURE 1 ) and different form of dactylus with marked dorsal crest ( Figs. 3 e View FIGURE 3 , 4 a–c View FIGURE 4 , 5 a–d View FIGURE 5 ) in the new species.
From A. lamellifer View in CoL the new species can be clearly separated by shorter antennal stylocerite not reaching midlength of second antennular segment (vs. overreaching distal margin of second antennular segment in A. lamellifer View in CoL (Kubo 1940: Fig. 4B View FIGURE 4 )), equal in size and similar in shape pereiopods I in males (vs. unequal in size and dissimilar pereiopod I in males of A. lamellifer View in CoL (Kubo 1940: Fig. 4D View FIGURE 4 , E’)), different proportions in pereiopod I with longer carpus both in males and females ( Figs. 3 e View FIGURE 3 , 4 a, b View FIGURE 4 , 5 a, b View FIGURE 5 ) (vs. carpus of pereiopod I about 1/3 of propodal length in males of A. lamellifer View in CoL (Kubo 1940: Fig. 4D, E View FIGURE 4 )) and different form of dactylus with marked dorsal crest ( Figs. 3 e View FIGURE 3 , 4 a–c View FIGURE 4 , 5 a–d View FIGURE 5 ) (vs. dactylus of major pereiopod I without distal crest in A. lamellifer View in CoL (Kubo 1940: Fig. 4 View FIGURE 4 D’)).
From A. japonicus Kubo, 1936 View in CoL the new species can be clearly separated by different form and proportions of pereiopods I both in males and females as well as different body coloration. The new species possess large slightly unequal in size but similar in shape pereiopods I in males ( Fig. 1 b View FIGURE 1 ) and small similar pereiopods I in females ( Fig. 1 a View FIGURE 1 ) while it was indicated in the original description of A. japonicus (Kubo 1936) View in CoL that “ first pair of pereiopods robust as compared with the other legs, asymmetrical in size, usually larger in the right side than the left in both sexes, in male they are stouter than in female and slightly outreach the antennal scale ”. Such unequal and dissimilar pereiopods I in females were also described in Mya and Miyake (1968) as “Pattern II A–1” and “Pattern B” (see Mya & Miyake 1968: Fig. 5B, C View FIGURE 5 ), while pereiopods I characteristic for females of Athanas alpheusophilus View in CoL sp. nov. were probably indicated as “Pattern A” ( Mya & Miyake 1968: Fig. 5A View FIGURE 5 ); dactylus of pereiopods I in males is less than half of propodus while in Kubo’s description (1936: Pl. XIII: C, I) it significantly larger than half of propodal length. Such large dactylus is also characteristic for Athanas cf. japonicus View in CoL described by Anker (2003: Figs. 14–17) and Mya and Miyake (1968: Fig. 5F View FIGURE 5 ). Dactylus of both male pereiopods I in males of Athanas alpheusophilus View in CoL sp. nov. is armed with marked dorsal crest ( Fig. 3 e View FIGURE 3 , 4 a–c View FIGURE 4 , 5 a–d View FIGURE 5 ) also referred by Mya and Miyake (1968) as “Pattern IV B” ( Mya & Miyake 1968: Fig. 5G View FIGURE 5 ). At the same time, such significant feature is missing both in the description and figures given by Kubo (1936: pl. XIII: C, H, I) for A. japonicus View in CoL . Pattern of pereiopods I similar to the original description of A. japonicus View in CoL (Kubo, 1936: Pl. XIII C, H, I) is probably presented by Mya and Miyake (1968: Fig. F) as “Pattern IV A”. Thus, it is possible to conclude that combination of small equal-andsimilar pereiopods I in females and large unequal-and-similar pereiopods I armed with large dorsal dactylar crest in males characteristic for Athanas alpheusophilus View in CoL sp. nov. show a unique pattern within “ Athanas japonicus View in CoL ” species complex. One more feature allowing separating the new species and A. japonicus View in CoL is the body coloration. Kubo (1936) mentioned that body coloration of A. japonicus View in CoL is “ deep blue ” (Kubo 1936: 46) that is characteristic for many Athanas View in CoL species (e.g. Anker 2003), while body coloration of Athanas alpheusophilus View in CoL sp. nov. is generally deeply red ( Fig. 6 View FIGURE 6 ).
It is also worth noting that proportions of pereiopods I in males of Athanas alpheusophilus View in CoL sp. nov. are mostly similar to A. japonicus View in CoL reported by Kubo (1936) and clearly differs from A. ohsimai View in CoL described by Yokoya (1936: Fig. 1B, L View FIGURE 1 ), A. lamellifer View in CoL (after Kubo 1940: Fig. 4D, E View FIGURE 4 ) and Athanas cf. japonicus Kubo View in CoL described by Anker (2003: Figs. 14–17). Both A. ohsimai View in CoL and A. lamellifer View in CoL can be easily separated from Athanas alpheusophilus View in CoL sp. nov. (as well as from A. japonicus View in CoL too) by significantly shorter carpus of pereiopods I in males: carpus in A. japonicus View in CoL (after Kubo 1936) and Athanas alpheusophilus View in CoL sp. nov. ( Fig. 3 e View FIGURE 3 ) accounting for at least half of propodal length while carpus is much shorter, not exceeding 1/3 of propodal length in Athanas cf. japonicus View in CoL (after Anker 2003), A. lamellifer View in CoL (after Kubo 1940) and A. ohsimai View in CoL (after Yokoya 1936). Male pereiopods I with long carpus were also described by Mya and Miyake (1968) as “Pattern III” ( Mya & Miyake 1968: Fig. E). Thus, Mya and Miyake (1968) synonymized A. lamellifer View in CoL and A. japonicus View in CoL using this feature incorrectly and Athanas lamellifer Kubo, 1940 View in CoL could be considered as valid species until more careful and complete revision will be accomplished. Besides, some previous records of Athanas japonicus View in CoL from Indo-West Pacific (e.g. Banner & Banner 1973; Anker 2003) need to be revised.
The only Athanas species recorded north from the latitude of the description of the new species is Athanas squillophilus Hayashi, 2002 , which can be clearly separated from the new species by longer rostrum reaching distal antennular segment (vs. reaching midlength of second antennular segment in A. squillophilus (Hayashi 2002: Fig. 2A View FIGURE 2 )) as well as shorter stylocerite, different position of dorsal spines on telson and morphology of chelipeds (pereiopods I) both in males and females (see Hayashi 2002: Fig. 3 View FIGURE 3 ). Moreover, carpus of pereiopod II in A. squillophilus consist of 6 segments ( Hayashi 2002: Fig. 3D View FIGURE 3 ) while carpus of pereiopod II consists of 5 segments ( Fig. 4 d View FIGURE 4 ) in the new species. By these features, A. squillophilus seems not to be closely relative to the new species.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Athanas alpheusophilus
| Marin, Ivan 2017 |
Athanas alpheusophilus
| Marin 2017 |
Athanas alpheusophilus
| Marin 2017 |
Athanas alpheusophilus
| Marin 2017 |
Athanas alpheusophilus
| Marin 2017 |
Athanas alpheusophilus
| Marin 2017 |
Athanas alpheusophilus
| Marin 2017 |
Athanas alpheusophilus
| Marin 2017 |
A. lamellifer
| Kubo 1940 |
A. lamellifer
| Kubo 1940 |
A. lamellifer
| Kubo 1940 |
A. lamellifer
| Kubo 1940 |
A. lamellifer
| Kubo 1940 |
A. lamellifer
| Kubo 1940 |
A. lamellifer
| Kubo 1940 |
A. lamellifer
| Kubo 1940 |
A. lamellifer
| Kubo 1940 |
Athanas lamellifer
| Kubo 1940 |
A. japonicus
| Kubo 1936 |
A. japonicus
| Kubo 1936 |
A. ohsimai
| Yokoya 1936 |
Athanas cf. japonicus
| Kubo 1936 |
A. ohsimai
| Yokoya 1936 |
A. japonicus
| Kubo 1936 |
A. japonicus
| Kubo 1936 |
Athanas cf. japonicus
| Kubo 1936 |
A. ohsimai
| Yokoya 1936 |
A. japonicus
| Kubo 1936 |
Athanas japonicus
| Kubo 1936 |
Athanas
| Leach 1814 |
Athanas
| Leach 1814 |