Anopheles lepidotus Zavortink, 1973
|
publication ID |
https://doi.org/ 10.5281/zenodo.211343 |
|
DOI |
https://doi.org/10.5281/zenodo.6178204 |
|
persistent identifier |
https://treatment.plazi.org/id/6D0FD20B-FB19-FFB9-B5C1-B7C0145AF903 |
|
treatment provided by |
Plazi |
|
scientific name |
Anopheles lepidotus Zavortink, 1973 |
| status |
|
Anopheles lepidotus Zavortink, 1973 View in CoL
Anopheles (Kerteszia) boliviensis Theobald, 1905 View in CoL , of Komp & Osorno-Mesa, 1936: 415 (3*, L*); Komp, 1937: 500 (3*, L); Lane, 1953: 279 (3*, L*); Komp, 1956: 40 (3, L, biology and distribution, in part); Stone, Knight & Starcke, 1959: 35 (3, L, taxonomy, distribution, in part); Forattini, 1962: 448 (3*, L); Aragão, 1964: 76 (biology and distribution, in part). Anopheles (Kerteszia) lepidotus Zavortink 1973: 17 View in CoL (3*, Ƥ* [misidentification], L, key to females incorrect, biology and distribution, in part); Knight & Stone, 1977: 58 (only 3, L, distribution, in part).
Overview. Because Komp & Orsono-Mesa (1936) assigned two males with larval skins to An. boliviensis View in CoL , numerous publications before Zavortink (1973) were unknowingly addressing An. lepidotus View in CoL instead of An. boliviensis View in CoL . Numerous articles following Zavortink (1973) that addressed An. lepidotus View in CoL were correct, in part, as the male, larva and genitalia are An. lepidotus View in CoL . However, post-1973 articles addressing An. lepidotus View in CoL females and their structures were incorrect and should now be interpreted as An. pholidotus View in CoL . Also, post-1973 published discussions of the biology, distribution, medical significance, and the phylogenetics of An. lepidotus View in CoL are only partially correct. A good example of the above can be found in Gonzalez & Carrejo (2009) where, as in Zavortink (1973), the larval and male genitalia keys and descriptions correctly separate the two species, but the adult female key and associated characters in the text do not. As in other publications, the distribution records in this report need to be re-assessed. Country records are revised here, but within country provincial and local records for the two species need to be reevaluated.
Male and female diagnostic characters. ( Figs. 3 View FIGURE 3 , 4 View FIGURE 4 A; Table 1). Maxillary palpus with apical pale scales on all 5 palpomeres; palpomere 1 with apical white scales; pedicel with dorsal and ventrolateral patches of small white spatulate scales; mid-portion of proboscis with large area of variable pale scaling on dorsum, laterally, and/or on venter; postspiracular area with small ventral patch of white scales (may be present or absent on males); wing normally with 7 pale fringe spots; M1+2 fork with white scales (may be 1–3 scales or obscure on males); hindtarsomeres 1 and 2 without apical pale scales on dorsum; terga I, III–VIII normally with white scales; female cerci with erect white scales; male gonocoxite with erect white scales to apex; accessory setae on gonocoxite of unequal length; internal seta of gonocoxite with long gradually attenuated tip.
FEMALE ( Fig. 3 View FIGURE 3 , Table 1). Head. Pedicel with dorsal and ventrolateral patches of small white spatulate scales; palpomeres 1–5 with white scales on distal half or apex, palpomere 1 with several apical white scales, palpomere 2 with apical 0.6 pale-scaled, distal half of palpomere 3 with long white area ventrolaterally and long dark area dorsomesally before white apex, palpomeres 4,5 with very small white-scaled area at apex; palpomeres 1,2 with erect black scales; proboscis dark-scaled on basal 0.1, with white or translucent scales dorsally, laterally and/ or ventrally (variable) on median 0.5, dark-scaled on apical 0.3. Thorax ( Fig. 3 View FIGURE 3 ). Scutal integument with 2 submedium and 2 lateral longitudinal dark lines; scutum with pale scales on acrostichal and dorsocentral rows on anterior 0.4; long erect setae on scutum pale except for stout dark setae at anterior ends of acrostichal and dorsocentral rows (rarely extending posteriorly) and dark setae over wing root and infrequently in prescutellar area; scutellum may have pale and dark setae, with several black scales on mid-region and infrequently laterally; antepronotum with long white scales, occasionally with small patch of black scales at mesal end; postpronotum with pale pruinose area posteroventrally; proepimeron and subspiracular areas with pale pruinose areas in line with that on postpronotum; postspiracular area with small ventral patch of white scales in line with anterior pruinose areas and white scales on upper mesokatepisternum; pruinose areas and connecting white scales form upper lateral white line on side of thorax from postpronotum to mesokatepisternum; lower lateral pale line on side of thorax incorporates pruinose areas on the metameron, lower mesepimeron, middle of mesokatepisternum, and propleuron; propleuron without scales, usually with 1 long seta; prespiracular area with setae and several white scales; mesepimeron with 1 long vertical “C-shaped” row of long white scales that extends downward from upper mesepimeral setae to middle of mesepimeron. Wing. ( Fig. 3 View FIGURE 3 ). Wing with 5 pale spots on costa—a small humeral spot that starts at or just distal to humeral crossvein and 4 large sector, accessory sector, subcostal, and preapical pale spots, preapical pale spot extends to apex of R1; pale subcostal spot extends to R2+3; pale fringe spots at wing apex usually consist of two small spots located at tips of R2 and R3,occasionally merged into one larger spot (combined R2 and R3 fringe spots); posterior fringe spots occur at tips of M1, M2, M3+4, CuA, and 1A, usually increasing in size from M1 to 1A; vein R4+5 with small pale spot at base, apex with dark fringe scales; base of vein M dark-scaled, pale-scaled at M1+2 fork; base of vein CuA usually dark-scaled before pale-scaled mcu crossvein; 1A dark-scaled to distal end, or rarely with 1–3 pale scales at tip. Legs. ( Fig. 3 View FIGURE 3 ). Fore- and midcoxae with white scales; foretarsomeres 4,5 normally dark-scaled, 4 occasionally with small dorsal pale patch; hindtarsomere 1 with small basal pale spot, small postbasal dark band, dark-scaled dorsally to apex, but pale-scaled ventrally from postbasal dark band to apical dark band; hindtarsomere 2 dark dorsally from base to apex, with long pale area on venter from near base to near apex; hindtarsomere 3 dark dorsally except for small apical pale band, pale ventrally from near base to apex; hindtarsomere 4 dark-scaled on basal 0.3–0.4 and pale-scaled to apex; hindtarsomere 5 dark-scaled; hindtarsomeres 1–3 dark from dorsal view except for small basal pale spot on Ta-III1 and small apical pale spot on Ta-III3; Ta-III4 dark basally and pale apically, and Ta-III5 dark; from ventral view Ta-III1 is nearly all pale from near base to small dark tip, Ta-III2 pale except for small dark areas at base and apex, Ta-III3 pale from near base to apex; Ta-III4 and Ta-III5 as described for dorsal view. Abdomen ( Fig. 3 View FIGURE 3 ). Terga and sterna with long, erect, spatulate, white and brown scales; tergum I with several long erect white scales on apicomesal area, sternum I without scales, tergum II with apicomesal patch of brown partially erect scales, sternum II with curved mesal row of long erect white scales, terga III–VII with long erect white scales either laterally (III–V) or lateral and basal (VI–VII), terga III–V with mesal patch of brown erect scales beyond base (III) or extending from base to apex (IV,V), those on V forming basal and apical transverse rows of long erect scales, sterna III–V with postbasal row of erect white scales, tergum VI with small patch of brown basomesal erect scales and distinct apical row of brown erect scales, sternum VI with basal row of erect white scales and apical row of erect brown scales, tergum VII with basal row of erect white scales and apical row of mixed erect white and brown scales, sternum VII nearly covered with erect long brown scales and few white lateral scales, tergum and sternum of VIII covered with long erect white scales.
MALE. Males possess the same diagnostic characters ( Table 1) as females and generally have a habitus matching the females. Occasionally characters on males may be less evident or absent, these include the posterior extension of white scales in the acrostichal row on the scutum, absence of a small ventral patch of scales on the postspiracular area, fewer (1–3) white scales on wing fork M1+2, scales not so dense on wing veins, and very faint posterior fringe spots on the wing. Certain characters like the costal pale spots on the wing may appear larger on males. Genitalia. ( Fig. 4 View FIGURE 4 A). Tergum VIII with median broad erect spatulate white scales; gonocoxite with erect white scales to apex; 2 accessory setae on gonocoxite of unequal length, longest seta flattened and broadened apically with a pointed tip, shortest seta with broad rounded tip; internal seta of gonocoxite with long gradually attenuated tip; aedeagus long slender without leaflets; ventral lobe of claspette with dense long spicules mesally near rounded bare apex, with shorter more scattered spicules basally and laterally, lateral margin with broad narrowing and bluntly rounded lobe joining basal stem at emarginate angle; dorsal lobe composed of 2 stems, each with 3–4 sinuous flattened setae.
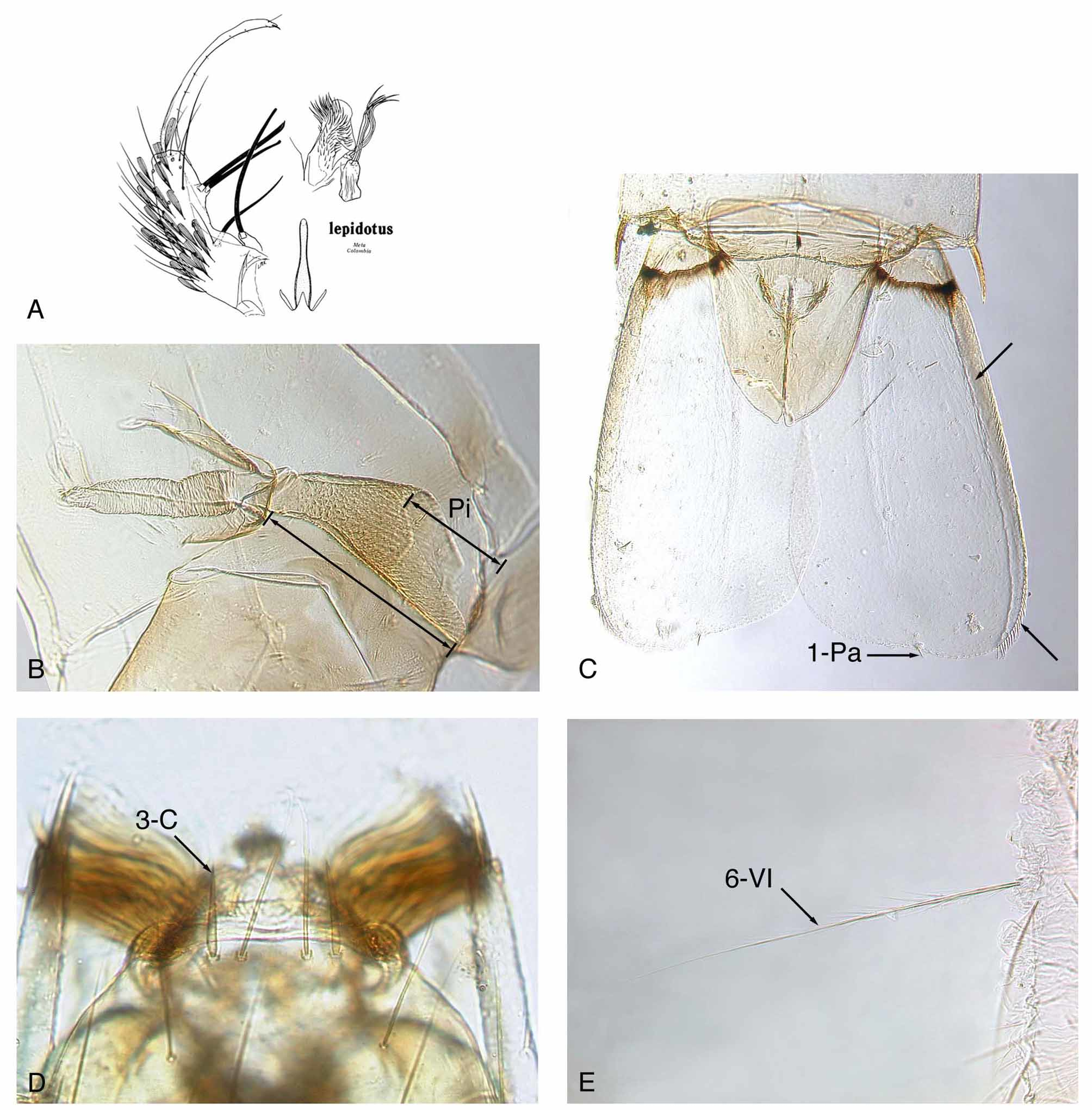
Pupal diagnostic characters ( Fig. 4 View FIGURE 4 B,C). Pinna exceptionally long, 0.41–0.55 (mean 0.46) trumpet length; seta 1-IX short, very thick, acutely pointed; lateral margin of paddle ( Fig. 4 View FIGURE 4 C) exceptionally thick, usually sclerotized and pigmented on basal portion; paddle with midrib absent or weakly developed; paddle asymmetrical, with relatively straight apical margin at seta 1-Pa, and without long filamentous spicules on distal 0.33 of lateral margin.
Pupa. Integument light to medium brown, most pigmentation on trumpet and segments I–IV; setae very thin and weak, single or with few branches. Cephalothorax. Trumpet medium brown, angusticorn, without meatal cleft, with wide opening, pinna exceptionally long, 0.41–0.55 (mean 0.46) trumpet length (n = 12); seta 13-CT or alveolus present. Abdomen. Setae 0-VII,VIII long, frail, nearly as long as seta 1-VII; 1-II–VII short, frail, single or with few distal branches; 1-IX very short, thick, sharply pointed; 9-I very short, 9-II,III weakly developed, 9-III slightly longer than 9-II, 9-IV,V length ratio 0.19–0.50 (mean 0.33), 9-V,VI length ratio 0.38–0.61 (mean 0.52), 9- V–VIII well developed, spine-like, darkly pigmented, with long sharp dorsolateral aciculae; 10-VI present; 14-III absent. Paddle. Base with distinct darkly pigmented transverse line; paddle asymmetrical, lateral half considerably longer than mesal half, paddle index (L/W) 1.72–2.06 (mean 1.92), apical margin straight near seta 1-Pa on both lateral and mesal sides; lateral edge exceptionally thick, sclerotized and pigmented, extending to near tip; midrib absent or very weakly developed; spicules on lateral margin small, wide, and acute, beginning on basal 0.33, with larger and longer stout spicules on distal 0.33 approximately equal length of 1-Pa; lateral spicules in 2 rows, one directed dorsally and the other ventrally, except in most distal row of longer stout spicules; distal 0.33 of lateral margin of paddle without long sinuous or straight spicules that are much longer than 1-Pa; seta 1-Pa short, stout, sharp pointed, not filamentous, inserted mesal to tip of paddle; 2-Pa short, filamentous, usually inserted cephalad and some distance from 1-Pa.
Larval diagnostic characters ( Fig. 4 View FIGURE 4 D,E). Seta 3-C very thick, short, usually sharply pointed; 11-P very short; 1-I small with 4 or 5 simple branches; palmate setae (1-II–VI) only on 5 segments, moderately open, not brush-like; 2-IV exceptionally long, equal in thickness to 6-IV, usually simple, rarely with aciculae; 3-VIII with few branches near base; 6-VI stout, long, with median length aciculae on basal 0.33 and shorter aciculae more distal, without strong basal branches; 9-IV–VI nearly equal length of 7-IV–VI, with 2–5 branches; pecten spines from side view with single mesal row of stout aciculae extending out to tip.
Larva. Integument light brown on head, abdominal plates, and sclerotized structures on segments VIII and X. Head. Antennal seta 3-A as long as or only slightly longer than 2-A; 3-C very thick, short, usually sharply pointed, infrequently tip split or with small aciculae; distance between both 2-C, measured between the outer adjacent margins of the two alveoli, narrow, not more than 2.5 times distance between outer adjacent alveoli margins of 2-C and 3-C on one side; 5-C simple; 11-C usually with 2-6 small distal branches. Thorax. Seta 11-P very short. Abdomen. Seta 1-I with 4–5 simple branches; palmate setae on five segments (II–VI); palmate setae moderately open and well formed, not brush-like; 1-VII short with 2 or 3 simple branches; 2-IV exceptionally long, stout, equal thickness to 6-IV, usually simple, rarely with aciculae; 3-VIII with 2 or 3 large branches on basal 0.33; 5-II–V plumose, with branches along main stem; 6-VI as stout and long as 6-V, with frail median length aciculae on basal 0.33, and short aciculae more distally, without strong branches near base; 9-IV–VI nearly equal length of 7-IV–VI, with 2–5 branches; 8-S present; pecten spines (18–23) of nearly equal length, from side view with single row of long stout aciculae; seta 1-X very long, attenuated, approximately 2 times saddle length; most anterior ventral brush seta (4a- X) short, usually <0.33 length of seta 4b-X; ventral brush (4-X) with 9 pairs of setae.
Egg. Unknown.
Specimens Examined ( Anopheles lepidotus ). Seventy specimens were examined (19Ƥ, 133, 14Le, 17Pe, 7G) from three countries as follows. COLOMBIA: Meta Department, Restrepo, holotype (specimen B, pinned), 3, with slides of associated larval exuviae and genitalia, from leaf axil of bromeliad, XII-1935, Orsono-Mesa, in NMNH; paratype (specimen A mounted on slide), 13, with slides of associated larval exuviae and genitalia, from same locality and collection as holotype. ECUADOR: Napo Province, Yasuni National Park, Tiputini Biodiversity Station, EC104, -2, -3, 1Ƥ, 2LePe, from bromeliad, 29-X-1998, R. Wilkerson; EC126(1), 1Ƥ, biting human, 1-XI- 1998, R. Wilkerson; EC151, -1 through -4, 43, 4LePe, 2G, from bromeliad, 4-XI-1998, R. Wilkerson; EC166, - 101, -102, -2 through -4, -6, 3Ƥ, 33, 3Le, 4Pe, 3G (2M in ETOH), from bromeliad, 7-XI-1998, R. Wilkerson; EC168, 5Ƥ (3 in ETOH), biting human, 7-XI-1998, R. Wilkerson; EC256, -100, -103, -105 through -109, -1 through -3, 6Ƥ, 43, 3Le, 7Pe, from bromeliad, 26-V-1999, R. Wilkerson. PERU: Junín Province, Mission Cutivireni, PE349, 1Ƥ, biting human in hut, 20-III-1985, Falcone and others; PE359, 1Ƥ, biting human in canopy, 22- III-1985, Hayes, Harrison & Savage; Junín Province, Puerto Ocopa, PE346, 1Ƥ, biting human in hut, 26-II-1986, Calderón & Hayes.
Bionomics. Anopheles lepidotus is a true “bromelicolus” species that occurs at relatively low elevations in remote or semi-remote tropical forests on the Amazonian slopes of the Andes in South America. It has been collected at elevations between 234 and 483 m, but not at elevations of 1,700 m like An. auyantepuiensis ( Harbach & Navarro, 1996) , or above 2,000 m like An. boliviensis , An. gonzalezrinconesi , An. pholidotus , and An. rollai ( Navarro et al., 2010) . Specimens were collected as larvae in arboreal bromeliads in Colombia and Ecuador and reared to adults with associated exuviae of the immature stages, and in human landing collections (HLC) in Ecuador and Peru at ground and canopy levels. To date, nine host-seeking An. lepidotus females were captured (out of 19 known females) in human landing collections. Seven of those females were captured in the canopy on open platforms at 15 m height ( Peru) and at 34 m height ( Ecuador), whereas two females were captured in Peru at ground level inside unscreened houses. One of the last two specimens was collected engorged while feeding on a human. The three females collected (1 canopy, 2 at ground level) in Peru in 1985–86 came to humans in February and March shortly after dark (1800–1900 hr), although HLC continued at ground level throughout the night. The remaining six females captured by HLC were taken in November 1998 at 34 m in the canopy in Ecuador between 1800–1900 hr. These collections during the dry summer months differ from 1942–43 collections of An. boliviensis in Villavicencio, Colombia, which were most common during the wet season between April and October ( Bates, 1945). However, Bates qualified those data by stating, “The capture data must be viewed with some suspicion because the species has predominantly late diurnal and crepuscular habits, and the captures were made at midday.” Larval collections from bromeliad axils (species unknown) at varying heights up to 34 m resulted in 11 males and 16 females that were reared in Ecuador during October and November 1998, plus the two males (holotype and paratype) collected during December 1935 from bromeliad axils at an unspecified height in Colombia. Larvae collected during the Ecuador trip survived well after collection, but development of the successive instars and stages was lengthy.
Species associated with An. lepidotus and captured by HLC between 1600–2000 hr during a canopy (15 m) collection (PE359) in Mission Cutivireni, Peru (12o S, 74o W) in 1985 were: Aedes sp.; Ochlerotatus (Chrysoconops) fulvus (Wiedemann) (as Aedes (Och.) fulvus (Wiedemann) ; see Reinert et al. (2008)); Anopheles (Anopheles) fluminensis Root ; An. (Nyssorhynchus) oswaldoi (Peryassú) ; An. (Nys.) rangeli Gabaldón, Cova Garcia & Lopez; Chagasia ablusa Harbach ; Ch. bonneae Root ; Haemagogus (Conopostegus) sp.; Hg. ( Haemagogus ) sp.; Psorophora (Janthinosoma) ferox (von Humboldt); Ps. (Grabhamia) dimidiata Cerqueira ; and Sabethes (Sabethoides) chloropterus (von Humboldt). Species associated with An. lepidotus , captured at ground level by HLC between 1800–0600 hr inside a house (PE349) in Mission Cutivireni, Peru in 1985 were: Ochlerotatus fulvus (as Ae. fulvus ); An. fluminensis ; An. (Ano.) intermedius (Peryassú); An. (Nys.) nuneztovari Gabaldón; An. (Nys.) oswaldoi ; An. (Nys.) rangeli; An. (Nys.) trinkae Faran ; An. (Nys.) sp.; Ch. bonneae Root ; Ps. (Gra.) cingulata (Fabricius); Ps. (Jan.)? horrida (Dyar & Knab); and Ps. (Psorophora) lineata (von Humboldt). Taxa associated with An. lepidotus in larval collections from bromeliad axils at 34 m height in Napo, Ecuador (0o 38’17”S, 76o 08’ 42” W) in 1998 were: EC104, Wyeomyia sp.; EC151and EC166, Culex spp.
Distribution. Anopheles lepidotus is only known from four localities in three countries on the Amazonian slopes of the Andes. Those sites are Colombia (Meta, Restrepo, and Buena Vista), Ecuador (Napo, Yasuní National Park, Tiputini Biodiversity Station), and Peru (Junín, Mission Cutivireni, and Puerto Ocopa). The Ecuador and Peru collections represent the first confirmed records of this species in those two countries. The female previously identified as An. lepidotus from Cochabamba, Bolivia ( Zavortink, 1973) is actually An. pholidotus .
Medical significance. Prior to this study, An. lepidotus was considered a fairly abundant species in areas of Colombia where malaria transmission occurred. Quiñones et al. (1984) proposed that An. lepidotus females were the primary Kerteszia species biting humans and the probable vector in an area of high malaria endemicity in Colombia. However, subsequent to that study specimens were sent to us for examination (courtesy of Dr. Suarez, SNEM, Colombia) and they were not An. lepidotus , but An. pholidotus . Reared specimens from the above 1984 study area and examinations of dissected male genitalia also confirmed that the correct species was An. pholidotus , not An. lepidotus ( Escobar et al., 2010) . Since the holotype and paratype male of An. lepidotus were reared from larvae in 1935 in Meta, Colombia, there have been no other confirmed specimens of this species collected and preserved from the type locality or Colombia. During the last 28 years only an additional 30 specimens of An. lepidotus have been collected in three sites in Peru and Ecuador. Zavortink (1973) thought that An. lepidotus was the dominant and most important species in the Meta Department of Colombia, but we found that only two specimens (types) of An. lepidotus are known from Meta (and Colombia). Thus, the biological information and medical importance for the specimens previously identified as An. boliviensis and subsequently assigned to An. lepidotus by Zavortink actually apply to An. pholidotus or An. boliviensis (to a lesser extent). For all of the above reasons, we consider An. lepidotus an uncommon (or inaccessible in the forest canopy) or rare species, and unlikely to be involved in the transmission of human malaria parasites on a large scale. However, An. lepidotus was the only Kerteszia species collected biting humans in Ecuador and Peru, and it obviously has an affinity for human blood in the canopy and at ground level. This suggests it may become infected with malaria by feeding on primates in the canopy and then transmit the parasites to humans at a later time. Deane (1967, 1988) determined that in the State of Amazonas, Brazil, adjacent to Peru, Plasmodium malariae was the major simian malaria parasite, which occurred in 25 monkey species in Brazil. Sulzer et al. (1975) and Sulzer et al. (1978) conducted three malaria surveys in Mission Cutivireni, our 1985–86 collection site, and declared the locality a hyperendemic area for Plasmodium vivax and P. m a l a r i a e, with 97.2% of the Ashaninka Amerindians infected in the initial studies. Hayes et al. (1987) conducted vector studies at Mission Cutivireni and determined that An. trinkae , a ground feeding species, was the primary malaria vector at the Mission and in Puerto Ocopa, but the Plasmodium species were not identified and no Kerteszia species dissected. Forattini (2002) and Collucci & Sallum (2003) discussed the role of several arboreal Kerteszia species in the transmission of malaria parasites, particularly P. m a l a r i a e (= P. brasilianum ) in primates and humans in Brazil and other areas of South America Thus, humans living in or moving into areas like Mission Cutivireni on the eastern slopes of the Andes might be exposed to P. malariae in a simian/arboreal Anopheles / human cycle like those described above by Forattini (2002) in Brazil, or similar to the macaque/ Anopheles /human P. knowlesi cycles now known in peninsular Malaysia, Malaysian Borneo, and other parts of southeast Asia (Vythilingam et al., 2008; Tan et al., 2008).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Anopheles lepidotus Zavortink, 1973
| Harrison, Bruce A., Ruiz-Lopez, Freddy, Falero, Guillermo Calderon, Savage, Harry M., Pecor, James E. & Wilkerson, Richard C. 2012 |
Anopheles (Kerteszia) boliviensis
| Knight 1977: 58 |
| Zavortink 1973: 17 |
| Aragao 1964: 76 |
| Forattini 1962: 448 |
| Stone 1959: 35 |
| Komp 1956: 40 |
| Lane 1953: 279 |
| Komp 1937: 500 |
| Komp 1936: 415 |