Cyclodomorphus TODO Bibron, TODO
|
publication ID |
https://doi.org/ 10.3853/j.0067-1975.47.1995.1 |
|
DOI |
https://doi.org/10.5281/zenodo.4662071 |
|
persistent identifier |
https://treatment.plazi.org/id/6E1687E8-E121-FFDD-2A66-11A36889F8D4 |
|
treatment provided by |
Felipe |
|
scientific name |
Cyclodomorphus TODO Bibron, TODO |
| status |
|
Cyclodomorphus TODO Bibron, TODO
Cyclodus Casuarinae Dumeril & Bibron, 1839: 749 . Lectotype: MNHP 7131, Bruny Island , Tasmania (Peron & Lesueur).
Cyclodus nigricans Peters, 1875: 621 . Holotype: 2MB 8193, Australia (Flower).
Hemisphaeriodon tasmanicum Frost & Lucas, 1894: 227 . Lectotype: MV D2087, Tasmania (c. Frost).
Diagnosis. A moderately large Cyclodomorphus (maximum SVL 174 mm), differing from all other species in the genus in the combination of prefrontals usually contacting, postnarial groove absent, postmental usually contacting two infralabials on each side, subcaudal scales 68-84, and dorsal colour pattern often present and complex in adults (dark edges to scales and dark streaks basally and centrally on scales).
Description. Nasals usually broadly to narrowly separated (55.6%, n = 117), less commonly in point to narrow contact (21.4%) or moderate to broad contact (20.5%), rarely a median internasal present (2.6%); prefrontals usually in moderate to broad contact (55.2%, n = 116), less commonly in narrow contact (31.0%), rarely in point contact (1.7%), narrowly to broadly separated (10.3%), or separated by a small median scale (1.7%); transversely enlarged nuchals 0-6 on each side (x = 2.4, sd = 1.08, n = 225), usually three (48.0%) or two (26.2%); loreals two bilaterally; supraoculars usually three bilaterally, rostral two in contact with frontal, second largest (98.3%, n = 116), rarely two unilaterally (1.7%), reduction due to fusion of first and second supraoculars (n = 1) or second and third supraoculars (n = 1); supraciliaries 5-7 (x = 6.0, sd = 0.29, n = 232), usually six (91.8%); presuboculars 1- 3, usually two (82.2%, n = 231), rarely one (0.4%); postsuboculars 2-5 (x = 3.6, sd = 0.56, n = 231), usually four (55.4%); upper palpebrals 7-12 (x = 8.2, sd = 1.00, n = 69); lower palpebrals 7-12 (x = 9.3, sd = 0.95, n = 68); secondary temporals usually in a-configuration bilaterally, rarely in {3-configuration unilaterally (n = 2) or bilaterally (n = 1); supralabials 6-8 (x = 7.0, sd = 0.30, n = 232), usually seven (90.9%), third-last below centre of eye, separating pre- and postsuboculars; infralabials 6-9 (x = 7.4, sd = 0.56, n = 227), usually seven (56.4%) or eight (40.1%); usually first two infralabials contacting postmental (n = 110), rarely one only unilaterally (n = 4) or bilaterally (n = 1); ear small, usually with a single small lobule along rostral margin (81.2%, n = 218), rarely two (6.4%) or lobules absent (12.4%).
Body scales in 22-26 (x = 23.3, sd = 1.00, n = 108) longitudinal rows at midbody; scales in paravertebral rows not or only slightly broader than adjacent scales, 61-73 (x = 66.7, sd = 2.87, n = 107); subcaudal scales 68-84 (x = 76.9, sd = 3.89, n = 69); lamellae below fourth toe 9-14 (x = 11.5, sd = 1.03, n = 201).
SVL 41.5-174 mm (n = 114); AGL/SVL 51.8-69.1% (x = 62.0%, n = 112); TL/SVL 63.6-133.6% (x = 103.9%, n = 70); FLL/SVL 12.7-21.4% (x = 15.8%, n = 114); HLL/SVL 17.5-26.2% (x = 21.5%, n = 114); FLL/HLL 64.2-85.7% (x = 73.6%, n = 115); HL/SVL 13.6-24.6% (x = 17.2%, n = 113); HW/HL 57.9-75.2% (x = 67.9%, n = 113); HD/HL 40.4--61.1% (x = 50.8%, n = 112).
Coloration (in preservative). Adult coloration variable.
Dorsal ground colour olive-grey to green, rarely reddish. Rarely immaculate, usually with narrow to broad dark brown or black lateral margins to most dorsal body and tail scales, giving solid to broken narrow dark stripes on body and at least tail base. Many individuals also with multiple fine mid brown-grey or russet streaks basally and centrally on most scales, which may in extreme development obscure the dark stripes and ground colour. Some individuals with a few dorsal body and tail scales also dark brown to black edged apically, in rare extreme development leading to irregularly defined narrow dark bands across back and tail.
Head dorsum usually immaculate, but in some strongly patterned individuals with dark flecks or spots along margins of some head shields.
Laterally, body and tail with dorsal ground colour and predominantly striped pattern grading evenly into ventral colour and predominantly banded pattern.
Face olive-grey or green, sutures black edged, especially subocular supralabials and less commonly other circumocular scalation, giving a dark mask about eyes.
Venter olive-green to blue-grey, rarely immaculate, usually with scattered scales black, especially along apical and lateral margins, sometimes with cream bases, aligned to give a series of narrow dark bands, usually in the form of irregular vermiculations on the body, more regular and on alternate scale rows on tail. Throat variably patterned, from immaculate through a few scattered dark flecks or spots, to three or four solid narrow dark bands cranial to level of forelimbs.
Limbs above with dorsal ground, ventrally with ventral ground, with varying development of dark markings corresponding to dorsal and ventral patterns.
Soles and palms yellow, occasionally with light brown calli or low tubercles.
Rare individuals entirely melanistic, or in one case (MV D11218) with dorsal pattern and ground colour largely obscured by broad, black, nearly confluent bands.
Juveniles with red to olive-green dorsal ground, body dorsum with dark scales and/or scale margins tending to align transversely to give narrow, closely spaced dark bands on body and at least tail base. Nape with two similar but broader and more pronounced dark bands, the more cranial extending rostroventrolaterally towards ears, continuing below ears as one or two narrower bars. Head dorsum of neonates often with a dark brown wash, especially over parietal shields. Subocular supralabial and usually some adjacent circumocular shields solid black, giving a black mask over eyes. Coloration otherwise as adults. Dark nape bands are the most longlasting element of juvenile coloration, but rarely persist as solid elements beyond SVL 50 mm.
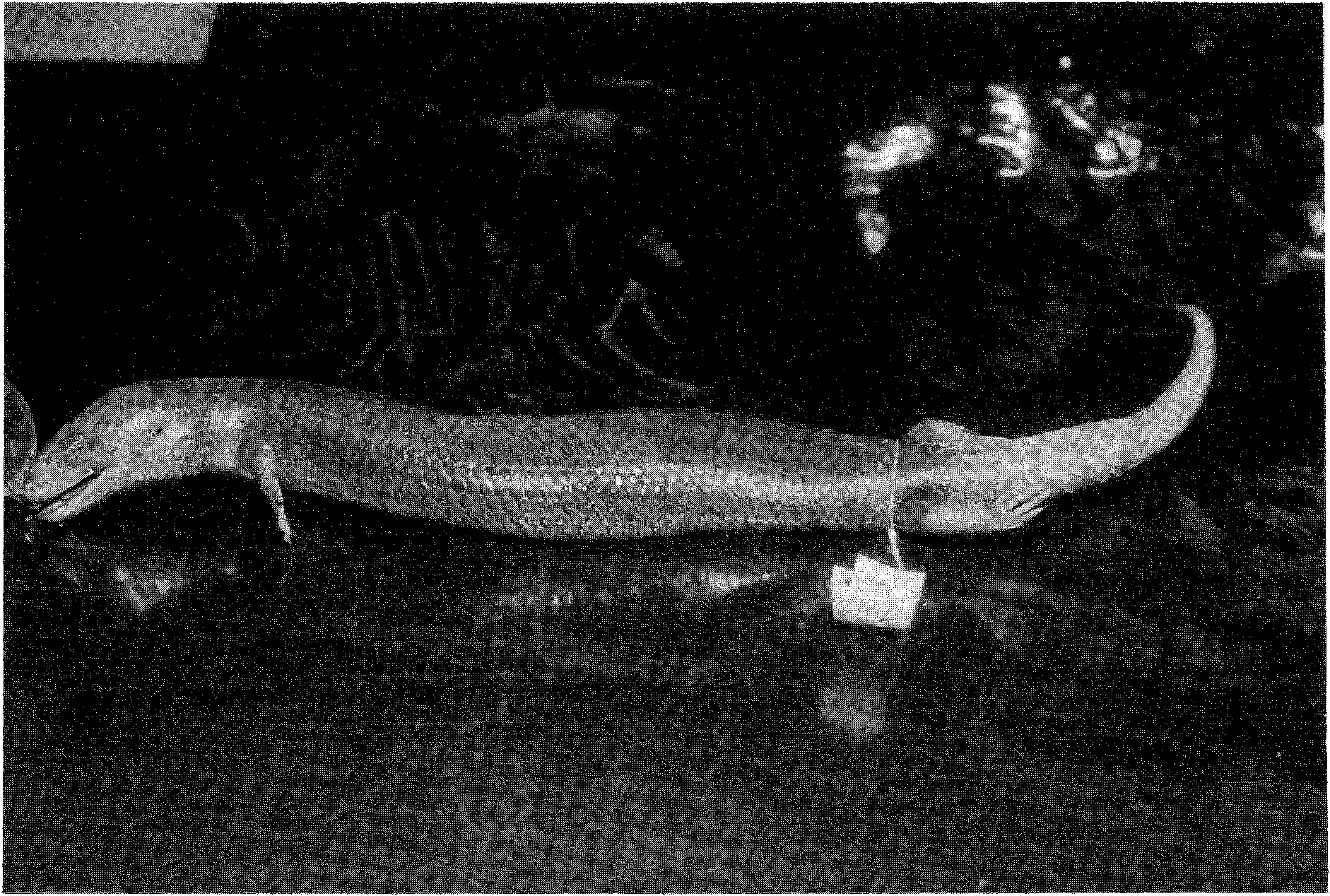
Coloration (in life). Three lizards (AM R65206-08) had a faint lavender tint to the sides of the body, the smallest individual having the throat and body and tail venter yellowish-brown (A. Greer field notes). An individual from Mount Wellington ( Fig. 4 View Fig ) had the pale parts of the body venter and flanks yellow, the iris mid-brown and the tongue dark blue-black.
Allometry ( Table 3 View Table 3 ). With respect to SVL, AGL and TL show positive allometry, while HL and limb lengths show negative allometry. With respect to HLL, FLL shows negative allometry, while HD shows negative allometry with respect to HL.
Sexual dimorphism. No significant differences were detected in the degree of separation/contact of nasals or prefrontals, or in mean number of nuchals, presuboculars, postsuboculars, supraciliaries, lower palpebrals, supralabials, infralabials, midbody scales, subcaudal scales or subdigital lamellae (t-tests). Significant differences were detected between males and females in mean number of upper palpebrals (males: x = 7.9, sd = 0.60, n = 25; females: x = 8.4, sd = 1.10, n = 42; t65 = 2.06') and paravertebral scales (males: x = 65.4, sd = 2.51, n = 33; females: x = 67.5, sd = 2.90, n = 57; t88 = 3.44'*').
Mature females (SVL 103-174 mm; x = 127.6 mm, sd ::: 16.97, n ::: 48) were much larger than mature males (85-126 mm, x ::: 107.8 mm, sd ::: 12.48, n = 21; MannWhitney U test, Z = 4.416***).
Females have significantly longer bodies, shorter tails and legs and shorter but broader heads than males, although the differences in proportions are slight in most characters ( Table 4 View Table 4 ).
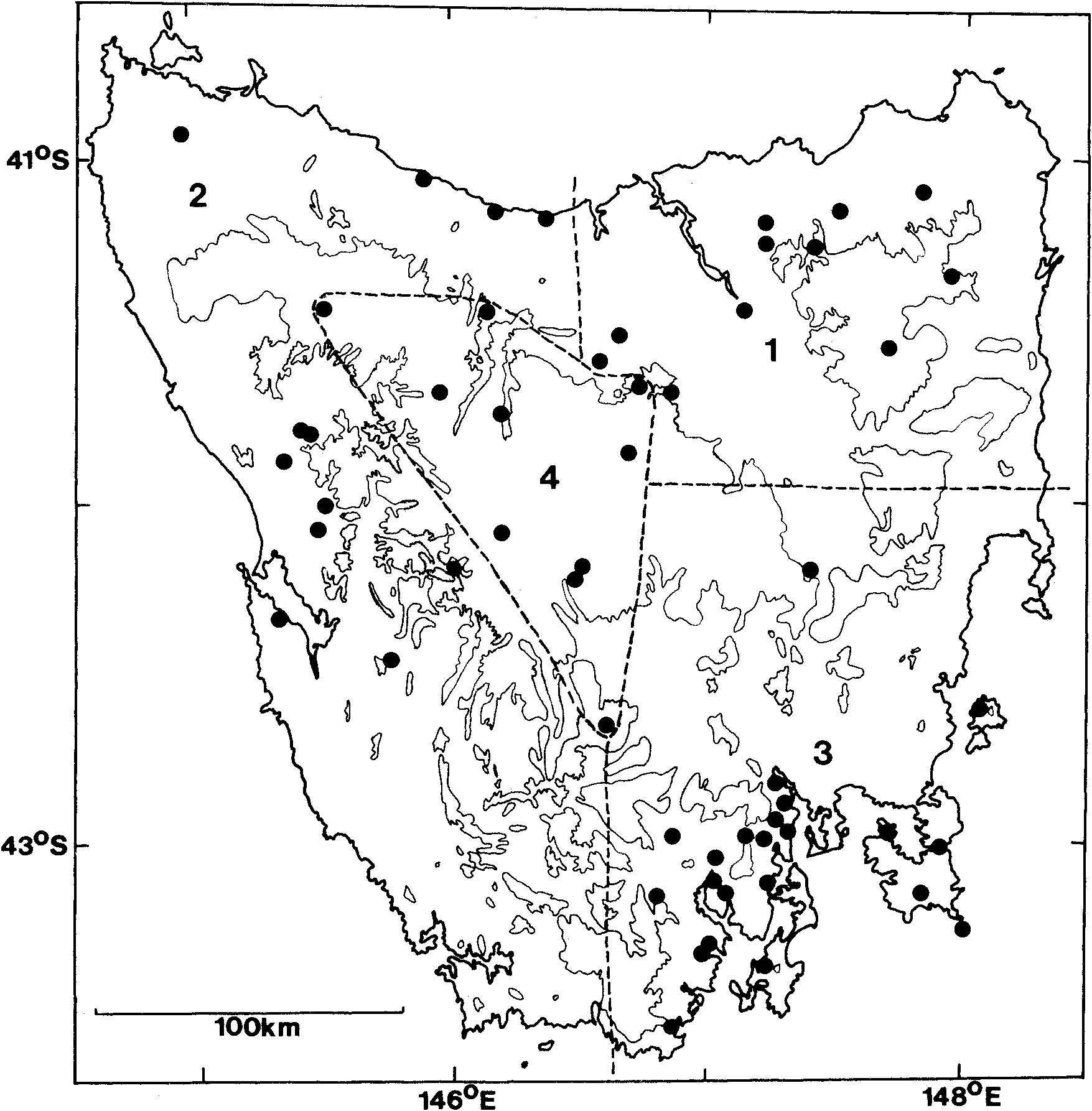
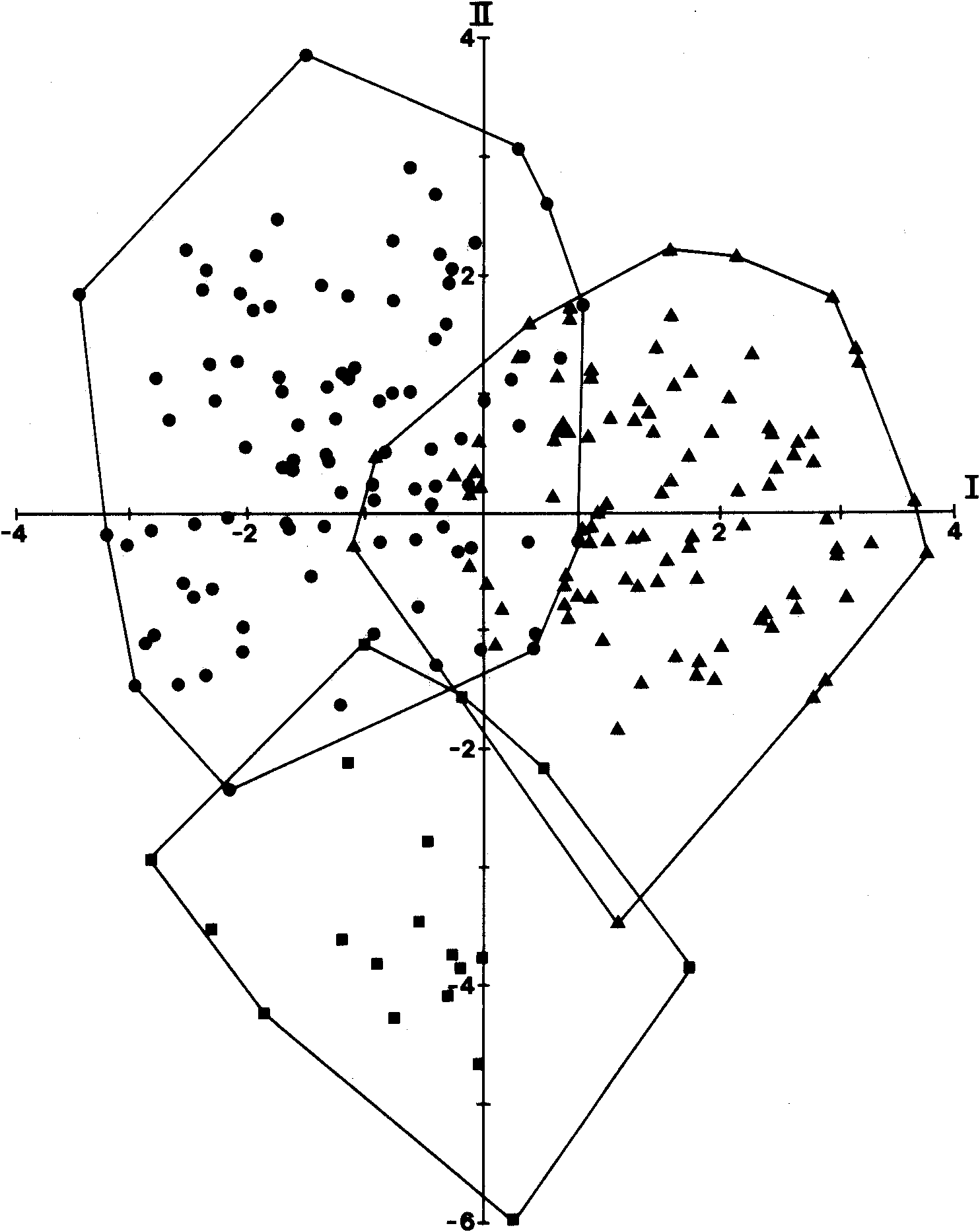
Distribution. Northern, central and eastern Tasmania, from sea level to the central plateau ( Fig. 2 View Fig ). Also Betsy ( Green & Rainbird, 1993), Brony, Maria and Tasman Islands on the east coast. Although there are no specimen-based records from the south-west of Tasmania, sight records exist for Mount Anne and Mount Melaleuca (A. Dudley, M. Hutchinson, pers. comm.).
Type material. Cyclodus casuarinae was described by Dumeril & Bibron (1839) mostly from a single MNHP specimen from NouveIle Hollande. A second specimen, in the collection of the Zoological Society of London, is mentioned in their description of coloration. Dumeril and Bibron did not specifically designate a holotype. However, the Paris specimen (MNHP 7131) has consistently been considered the holotype ( Dumeril & Dumeril, 1851; Guibe, 1954; Brygoo, 1985; Cogger et al., 1983), and must therefore be considered to be lectotype, nominated by assumption of holotype status (Article 74b of the Code of Zoological Nomenclature). The lectotype has been subsequently identified as collected by Peron and Lesueur from Bruny Island during the Baudin Expedition of 1801-04 ( Dumeril & Dumeril, 1851; Guibe, 1954; Brygoo, 1985). This specimen is presumably one of the "quelques beaux Iezards analogues aux Scinques, differant toutefois essentiellement des animaux de celle famille par l'elegance des formes et le rapport des proportions" observed by Peron (1807) on Bruny Island between mid January and early February 1802 (see also MacFarlane & Triebel, 1937, for a reprint of Peron's account). The whereabouts of the Zoological Society of London paralectotype are unknown. That collection was largely dispersed to other national and provincial collections in 1852, and the remaining specimens disposed of in 1856 ( Sclater, 1901).
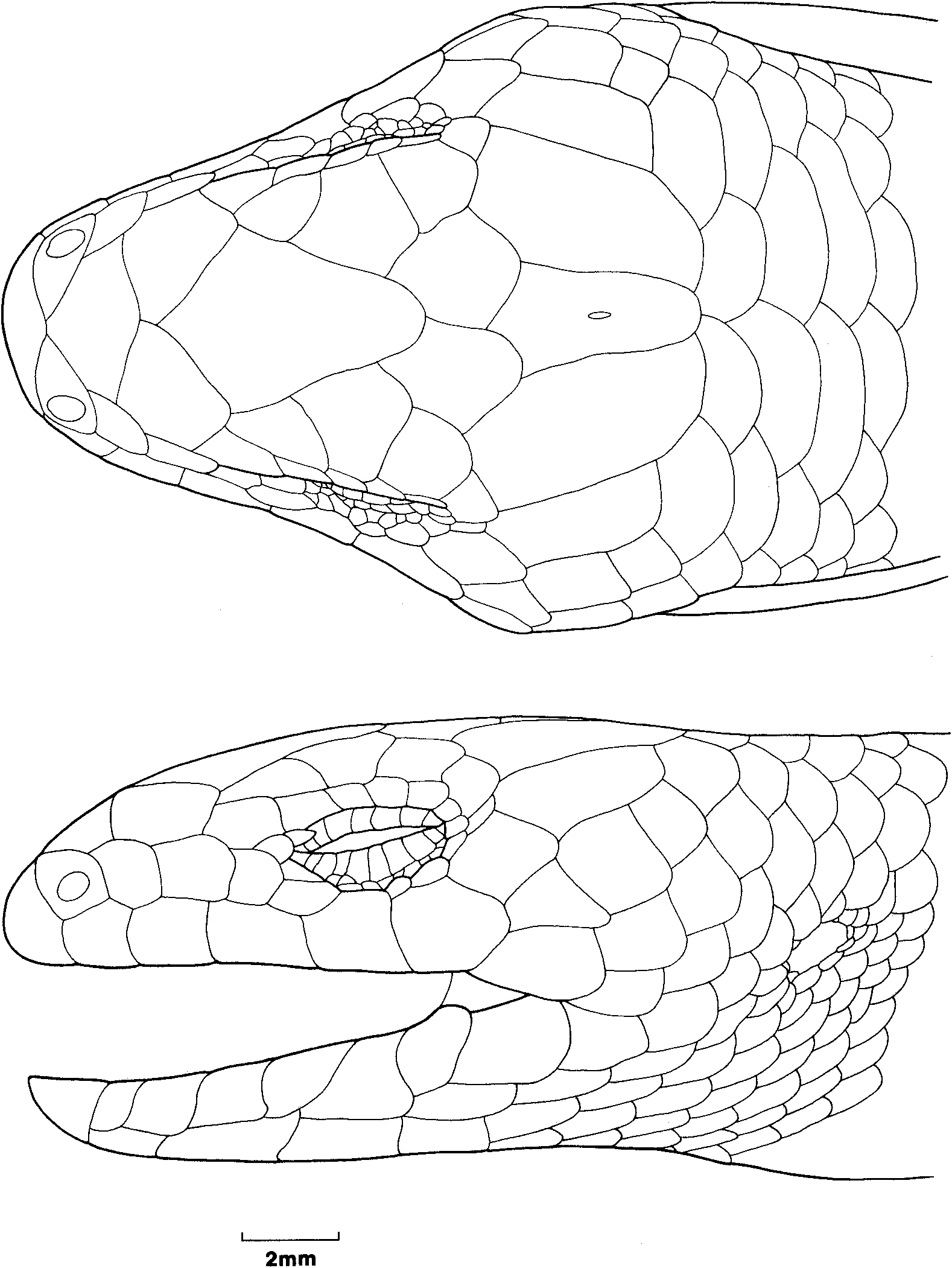
The lectotype of C. casuarinae ( Fig. 5 View Fig ) has the following combination of character states: nasals narrowly separated; prefrontals in narrow contact; supraoculars three; presuboculars two; postsuboculars three; supraciIiaries six; supralabials seven; infralabials 7/8, first two contacting postmental; nuchals three; temporals in a-configuration; midbody scales 24; paravertebral scales 68; tail regenerated from 12th subcaudal; subdigital lamellae 14/13; SVL 145 mm; AGL 95.5 mm; TL 25.5 mm (original part) + 28 mm (regenerate); FLL 20 mm; HLL 26 mm; HL 21.2 mm; HW 15.2 mm; HD 11.1 mm. Although the specimen has been eviscerated, the large size suggests that it was female. Most of the measurements and scalational characters are in close agreement with those given in the type description, only the tail length (62 mm vs 53.5 mm) being noticeably different .
While the type description does not give a precise locality for the lectotype and the regenerated tail precludes use of subcaudal counts to accurately assign it to a population, the more precise locality given by Dumeril & Dumeril (1851) and subsequent authors, based on MNHP catalogue data, is sufficient to assign the name to the Tasmanian taxon.
The description of Cyclodus (Homolepida) nigricans by Peters (1875) is brief, mentioning only the dark coloration, 7/6 supralabials, two loreals longer than high, 25 midbody scales, and 70 scales along the body between lower jaw and vent. Peters considered that the combination of these features and a long snout differentiated his species from C. casuarinae , although he gave no comparative data for the latter species. Cyclodus nigricans was placed in the synonymy of C. casuarinae by Boulenger (1887).
Peters did not give any locality for his species, nor did he explicitly indicate the extent of his type series or its provenance or repository. Although he states at the end of the description that he found additional specimens of the species in collections sent to him by Prof. Flower of the Royal College of Surgeons in London, it is clear from the single set of scale counts and the asymmetry reported in supralabial scales (seven left, six right) that he described his species from only a single specimen. A single specimen (ZMB 8193; Australia; pre: Flower) is identified as the type in Berlin, and in coloration and the direction of asymmetry of the supralabial shields agrees with Peters' description, although Peters counts one fewer supralabial and 25 midbody scales rather than the 24 that I count. The holotype of C. nigricans ( Figs 6 View Fig , 7 View Fig ) has the following combination of character states: nasals in moderate contact; prefrontals in moderate contact; supraoculars three; presuboculars two; postsuboculars 3/4; supraciliaries 617; supralabials 817; infralabials 8/9, first two contacting postmental; rostral ear lobules one; nuchals three; temporals in a-configuration; upper and lower palpebrals nine; midbody scales 24; paravertebral scales 67; tail regenerated from 34th subcaudal; subdigital lamellae 13; SVL 148 mm; AGL 97 mm; FLL 21 mm; HLL 30 mm; HL 22.9 mm; HW 16.3 mm; HD 11.7 mm. The coloration is uniformly dark browniblack dorsally and dark but with evidence of darker macules centrally on scales ventrally. Although the gonads have not been examined to confirm the sex, the large size of the specimen suggests that it is female. As with C. casuarinae , the regenerated tail of this specimen precludes use of the sole completely diagnostic character to assign the name to this species. However, the canonical variates analysis ( Fig. 3 View Fig ) unequivocally (98.9% probability) identifies the type as Tasmanian (67.6% probability of being from south-east Tasmania). Further, I am aware of two other individuals with uniformly dark coloration, both from Tasmania (A. Dudley, pers. comm.).
Hemisphaeriodon tasmanicum was described by Frost & Lucas (1894) from material collected by Baldwin Spencer from Lake St Clair. They initially only compared their species with Cyclodomorphus gerrardii (then in the monotypic genus Hemisphaeriodon ), but later (Lucas & Frost, 1896), after examining additional material, recognised its affinities with C. casuarinae and placed their species in its synonymy.
Although Frost and Lucas did not indicate the number of specimens on which their description was based, it is clear from the variation expressed ("one to four pairs of nuchals", ventral surface "greyish or brownish") that more than one individual was involved. This is further borne out by their later (Lucas & Frost, 1896) mention of "specimens" from Lake St Clair. No indication was given of the repository of their material, and no types were located by Cogger et al. (1983). Amongst the material examined for this paper are five MV specimens from Frost's collection (D2087-90, D2092, Tasmania, received 12 October, 1915, but not registered until 23 June, 1943; A.J. Coventry, pers. comm.) and a single AM specimen (R4142) from Lake St Clair, collected by Spencer and donated by Lucas. The AM animal forms part of a collection from Lucas that includes a syntype of Ablepharus rhodonoides , described by Lucas & Frost (1896) in the same paper that synonymised H. tasmanicum . Frost & Lucas (1894) placed tasmanicum in Hemisphaeriodon on the basis of enlarged maxillary teeth, while Lucas & Frost (1896) synonymised it partly on palatal osteology. The mouths of both R4142 and D2087 have been opened subsequent to preservation, in the former case by fracturing the mandibles, in the latter by transecting the adductor musculature, and the palatal mucosa has been stripped back, allowing access to the palatal elements. Both specimens are similar in coloration and in position and condition of preservation. The measurements and scalation of D2087 are very close to those presented by Frost & Lucas (1894), only the midbody count (24) being outside the variation given in the description (26). However, counts of 26 midbody scales are very rare in C. casuarinae (only seen in two of 108 specimens examined) and it is possible that the count provided by Frost and Lucas is erroneous. On the basis of collection data and the dissection of the oral cavity, I believe that AM R4142 is certainly a syntype, and on the basis of the similarity between this specimen and D2087, and between the latter and the type description, that MV D2087 is also a syntype. Of the other four MY specimens from Frost's collection, three (D2088-89, D2092) are strongly patterned, and cannot be syntypes (Frost and Lucas emphasised the unpatterned dorsum of their species). The other specimen, D2090, while unpatterned, has been more neatly set, is in a much better state of preservation, and has clearly been treated very differently to the two identifiable syntypes.
Because of its closer correspondence to the measurements provided by Frost & Lucas (1894), I nominate MV D2087 as lectotype, leaving AM R4142 the only identifiable paralectotype.
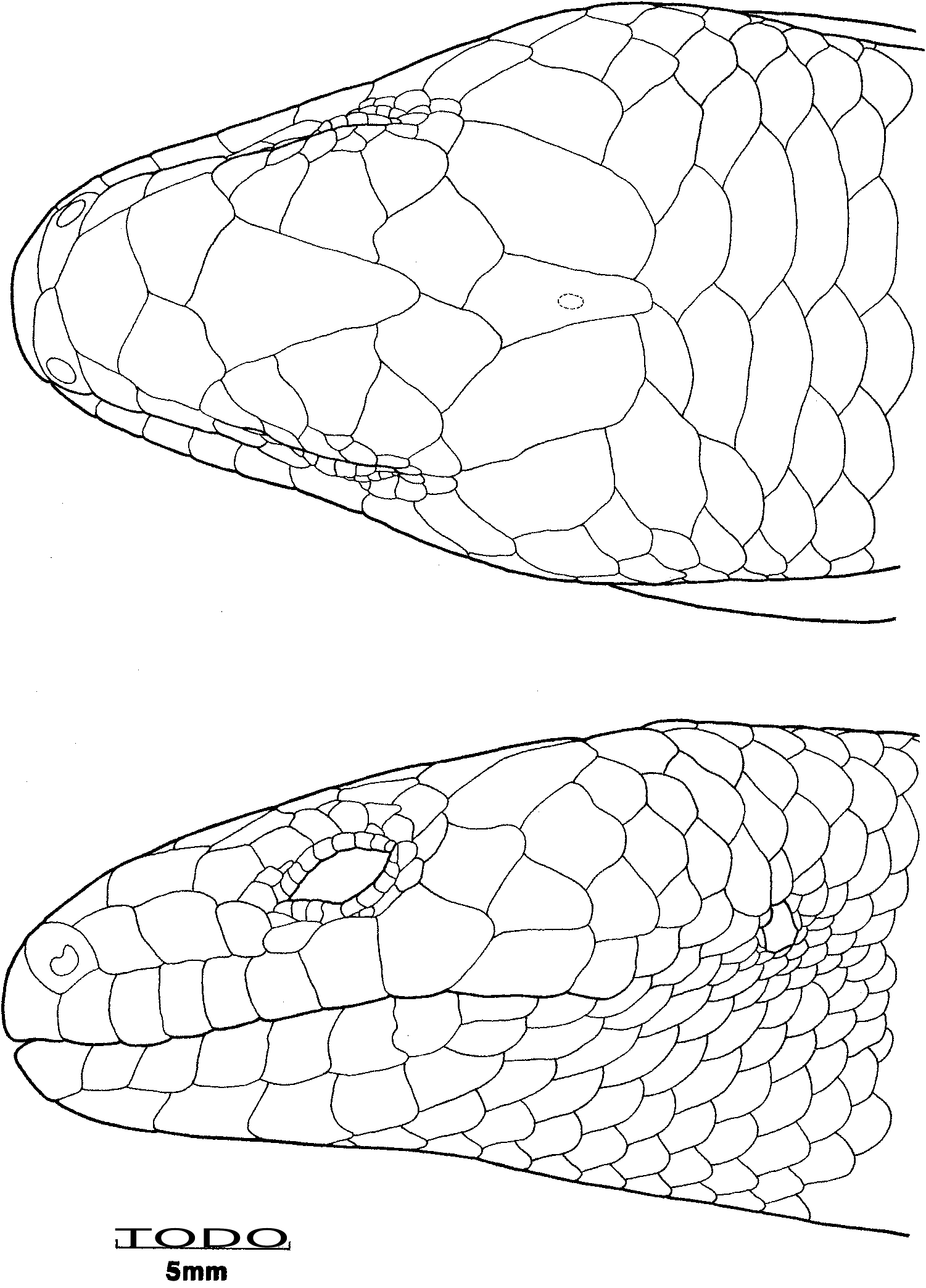
The lectotype of H. tasmanicum ( Figs 8 View Fig , 9 View Fig ) has the following combination of character states: nasals separated; prefrontals in narrow contact; supraoculars three; presuboculars two; postsuboculars four; supraciliaries six; supralabials six; infralabials seven, first two contacting postmental; nuchals three; upper palpebrals eight; lower palpebrals nine; temporals in a-configuration; midbody scales 24; paravertebral scales 62; subcaudal scales 71; subdigital lamellae 11/10; SVL 101 mm; AGL 63 mm; TL 101 mm; FLL 17 mm; HLL 22.5 mm; HL 15.9 mm; HW 11.9 mm; HD 7.6 mm. Both the type locality (Lake St Clair) and the morphology of the two type specimens clearly identify the name as belonging to the Tasmanian taxon.
Habitat and habits. Rawlinson (1974) describes C. casuarinae as terrestrial, commonest in clearings bordered or surrounded by dense arboreal vegetation, and using exposed patches of low vegetation or litter for basking sites and microenvironments under fallen logs and in deep litter for periods of inactivity. In his tabulation of habitat preferences ( Rawlinson, 1974, table 11.5) he records the species from savanna woodland, dry sclerophyll forest and wet sclerophyll forest.
Three more general publications give probably composite accounts of the ecology of members of the C. casuarinae complex, although the authors of all three have had some experience with the Tasmanian species. Wilson & Knowles (1988) describe the species as crepuscular to nocturnal, sheltering in grass tussocks and beneath leaf-litter, logs and surface debris in dry sclerophyll forest, woodlands, heathlands and swamplands, particularly where ground cover is dominated by tussock grasses. Ehmann (1992) records the species from "coastal plains, dunes, river flats, valleys and ranges. Vegetation of forests, woodlands, heathlands and tussock grasslands. In relatively closed vegetation structures, the species inhabits clearings, edges and other natural canopy openings that are invaded by dense low ground cover, especially spreading tussock grass." The species "shelters under embedded fallen logs, deep litter, stones and the procumbent spreading apron around the base of large tussock grasses and low bushes. It forages during the day and on warm nights, stalking and ambushing insects, snails, grubs and small lizards under partial cover." Cogger (1986) reports the species from a wide variety of habitats, from "coastal heaths and sand dunes to the dry sclerophyll forests of the ranges. Common in coastal grazing lands. Normally crepuscular to nocturnal, it is usually found during the day in leaf-litter or under fallen timber."
Specific data are available for few specimens. Green (1977) reported the species to be uncommon at his Maggs Mountain study site, with only three records. One adult was collected by a roadside in wet sclerophyll forest. Among the material examined for this study, habitat and microhabitat data are available for AM R65206-08 (under pieces of thin wood/timber which were very dry underneath, on a north-east facing grassy slope with lots of timber and large dead trees scattered about the paddocks; A. Greer field notes), AM R65209-1O (under dry rubbish in a cleared south-facing sloping paddock with rock outcrops, boards, rubbish and tree pieces scattered throughout, below a eucalypt forest; A. Greer field notes) and AM R70069-72 (under sheet iron in grassland bordered by dry sclerophyll forest; G. Stephenson field notes).
For a series of six lizards, Rawlinson (1974) reported a voluntary thermal minimum of 27.1°C, a voluntary thermal maximum of 39°C and a mean active temperature of 32.6°C.
Fleay (1952) reports predation by the Tasmanian Devil, Sarcophilus harrisii .
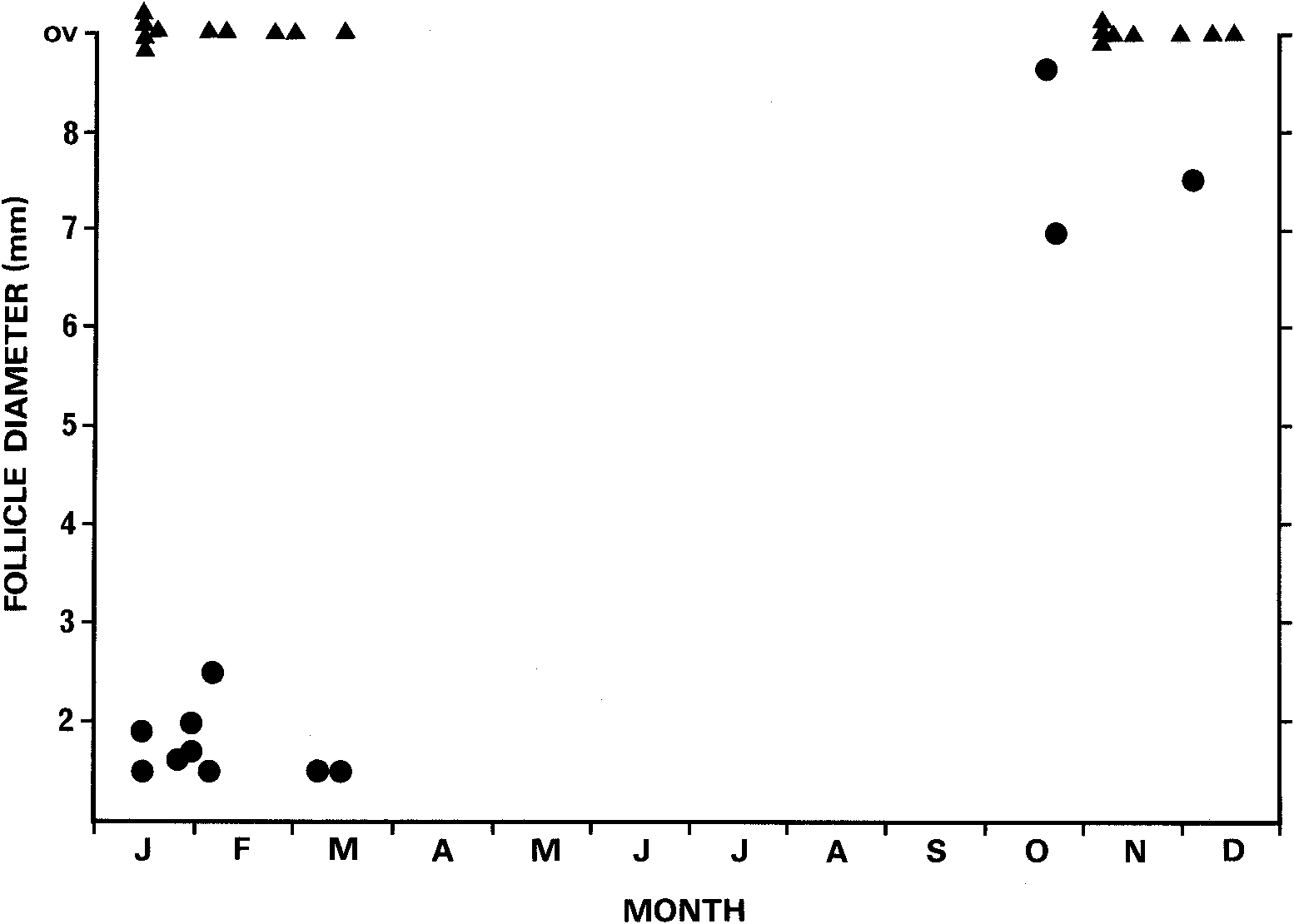
Reproduction. Adult females are available only from between October and March ( Fig. 10 View Fig ). In this period, females with grossly enlarged ovarian follicles were only present between late October and early December , while oviducal yolks and embryos were present between early November and March . Between January and March , non-gravid females with small ovarian follicles were also collected. The data, being pooled over many years, are insufficient to determine whether the occurrence of both gravid and non-gravid material in summer reflects non-annual reproduction or variation in the timing of parturition. However, I suspect from the occurrence of enlarged ovarian follicles over almost a two month period, the occurrence of fully scaled and pigmented embryos in females collected as early as 19 January ( TM C127 ) and as late as March ( OVM 1972.3.17 b), and the existence of neonates born January and March ( MV D39151 View Materials -56 , SVL 41.5- 44.5 mm, born to D39139 View Materials , collected 31 January, 1967; OVM 1984.3.13 b-g, SVL 42.5-45 mm, born after 4 weeks captivity to 1984.3.13 a, collected 9 February, 1984; SAM R8784-8785, SVL 42 mm; collected January, 1967) that the latter is the case. Rawlinson (1974) reported parturition in mid to late February .
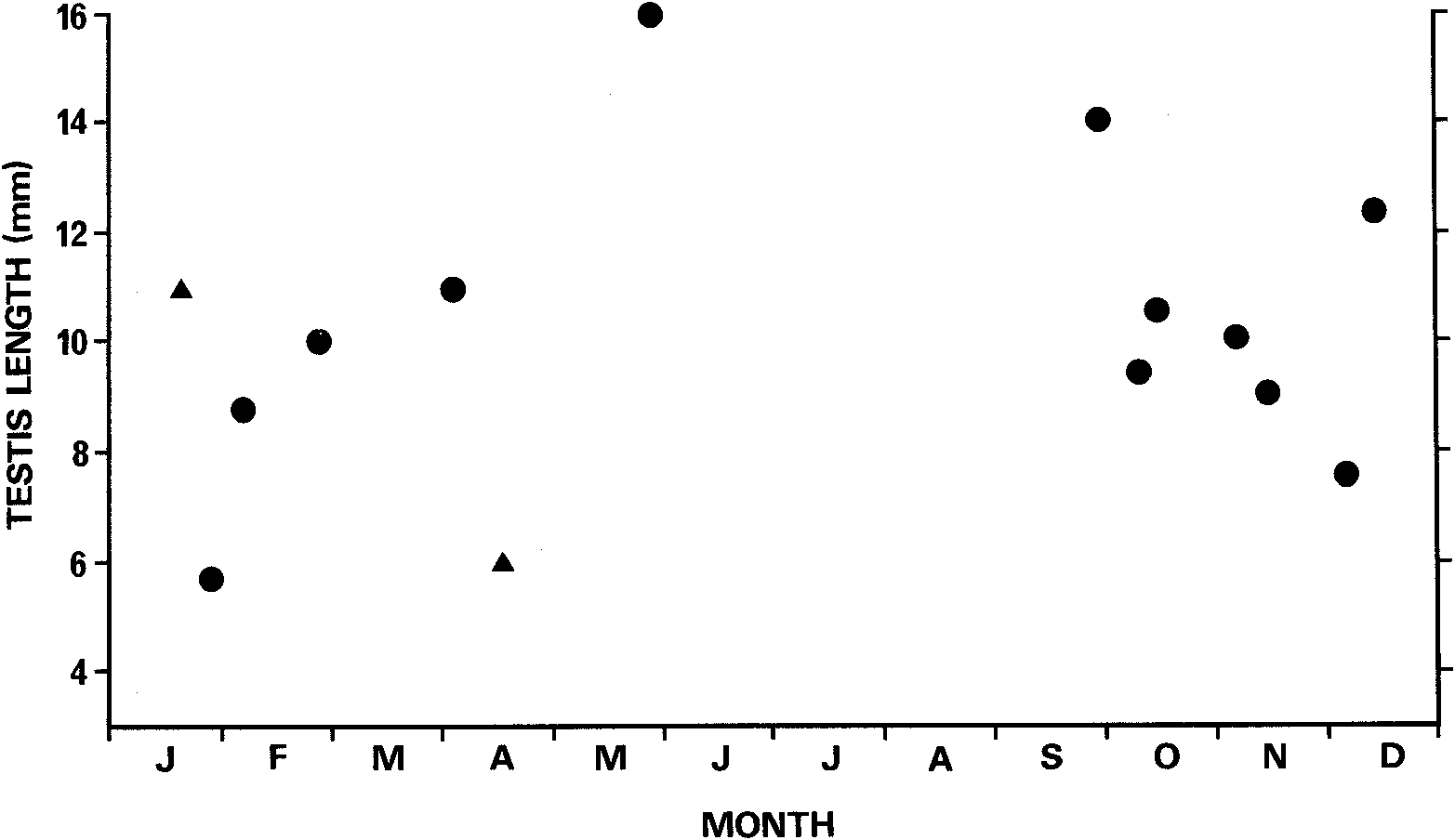
Mature males have turgid testes throughout the year ( Fig. 11 View Fig ). The largest testes were seen in males collected 27 May, 30 September and December. The latter two dates correspond to the inferred timing of ovulation, but the May date is well before this period. Rawlinson (1974) stated that copulation occurs in Spring.
Females (SVL 103-174 mm, x = 127.6, sd = 16.90, n = 25) carried from 4-14 (x = 7.6, sd = 3.06, n = 26) enlarged yolking ovarian follicles or oviducal yolks or embryos. There was a significant positive correlation between litter size and maternal SVL (litter = 0.117SVL - 7.219; r = 0.655*'*).
There are several literature reports of litter size for C. casuarinae , although in the absence of associated locality data most could refer to any of the three species here recognised. Worrell (1963) reported "about six young", Frauca (1966) a litter of six, Peters (1967) 6-8 young, Bustard (1970) a litter of 19, Swanson (1976) around five young, Wilson & Knowles (1988) 2-17, usually about four, and Ehmann (1992) up to 19, usually about seven. More precise figures are given by Rawlinson (1974), Greer (1989) and Shine & Greer (1991), who give 2-7 (x = 4.1, n = 15), 4-14 (x = 6.8, n = 8) and 4-14 (x = 6.87, n = 8) respectively.
Sex ratio. Overall, the ratio of mature males:females was 21:49, significantly different to 1:1 (XI = 10.41*"), although animals were only available between September and May. Seasonally, there was a significant difference in sex ratio between the gestation period and other months (male:female; November-March, 9:29; April-October, 6:3; Xl = 6.05*).
Specimens examined. 1. NORTH-EAST TASMANIA: MV D1051, Scottsdale; D39139 View Materials , D39151 View Materials -56, 16 km SSW Scottsdale; QVM 1006, Launceston; 1940.302, Montana; 1942.220, Deloraine; 1943.142, Hill Street, Launceston; 1944.76, Lebrina; 1962.3.28 a-b, 1963.3.12 -13, Winnaleah; 1969.3.8.a-b, Sideling, Launceston-Scottsdale road; 1972.3.188, south-east slope Ben Lomond; 1981.3.96, North Lilydale; 1984.3.13 ag, Liffey; 1987.3.74, Dairy Plains; TM C48, St Columba Falls, Pyengana. 2. NORTH-WEST TASMANIA: AM R37702-03, Zeehan; R37704-06, 4 miles west Queenstown; MV D915, Emu Bay; D39138 View Materials , 24 km east Marrawah; D39140 View Materials , 25 km south-east Zeehan; D39142 View Materials , 17 km south-west Smithton; D39143 View Materials , 6.4 km east Queenstown; D39144 View Materials , Collingwood River, 44 km east Queenstown; QM J41561 View Materials , Franklin River, below Goodwins Peak; J41562 View Materials , south side Macquarie Harbour; QVM 1958.3.7, Burnie; 1969.3.13, near Devonport; 1972.3.19, Ulverstone; 1972.3.121, Renison Bell. 3. CENTRAL TASMANIA: AM R4142 (paralectotype of H. tasmanicum ), Lake St Clair; R65206-08, 17.4 km north Breona via Highway 5; R65209-1O, north-east side Bronte Lagoon, just north Lyell Highway; MV D56347- 49, Mount Field; D56658, 2.6 km south Bronte Lagoon; NTM R9292, Cradle Mountain; QVM 1962.3.40, Erriba; 1964.3.3, Waratah Hellyer Spur road; 1972.3.17 a-b, Great Lake; 1976.3.22, Maggs Mountain; 1979.3.37, 1979.3.39, Maggs Mountain Hut; SAM R8784-85, south extreme, Lake Sinclair [St Clair]; R8798, Lake Sinclair [St Clair]. 4. SOUTH-EAST TASMANIA: AM 4785, Ouse River; R10053, Catamaran; R68001, Russell Falls National Park; R70069-72, Russell River, 5 km north Judbury in Huon Valley; R107594, Eaglehawk Neck; ANWC R3071, Huon River, 14 km east Judbury; MNHP 7131, Bruny Island (type of C. casuarinae ); MV D29, Port Arthur; D2593, Port Esperance; D5733-34, Tasman Island; D7919-20, Kingston; D7991, Ridgeway; D1l218, Huon Valley; D39128 View Materials , 9.6 km west Geavestown; D39133 View Materials , Coal Mines; D39141 View Materials , 11.3 km south Huonville; D39145 View Materials , 5.6 km east-south-east Longley; QVM 1972.3.16, Antill Ponds; TM C39, Kettering; C1l4, Lunawanna, South Bruny Island; C126-27, Lower Longley; C258, Dover; C267ab, Cygnet; C273, Maria Island; C305, West Moonah; C318, Counsel Creek, Maria Island; C997, Wellesley Street, South Hobart. UNLOCALISED: AM R2917, no data; R14402, north Tasmania; MV D161l, D2087 (lectotype of H. tasmanicum ), D2088-90, D2092, D4919, QM 113774, QVM 1950.3.1, SAM R2231, R6131, Tasmania; SAM R59, " South Australia "; 2MB 8193, Australia (type of C. nigricans ).
| MNHP |
Princeton University |
| TM |
Teylers Museum, Paleontologische |
| MV |
University of Montana Museum |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Cyclodomorphus TODO Bibron, TODO
| Shea, Glenn M. 1995 |
Hemisphaeriodon tasmanicum
| Frost, C. & AH & S. Lucas 1894: 227 |
Cyclodus nigricans
| Peters, W. 1875: 621 |
Cyclodus Casuarinae Dumeril & Bibron, 1839: 749
| Dumeril, AM & G. Bibron 1839: 749 |