Tendra zostericola, NORDMANN, 1839
|
publication ID |
https://doi.org/10.1111/j.1096-3642.2005.00179.x |
|
DOI |
https://doi.org/10.5281/zenodo.10545379 |
|
persistent identifier |
https://treatment.plazi.org/id/6F0C243F-FF80-256F-FCD8-667F0336FEFE |
|
treatment provided by |
Diego |
|
scientific name |
Tendra zostericola |
| status |
|
TENDRA ZOSTERICOLA NORDMANN, 1839 View in CoL
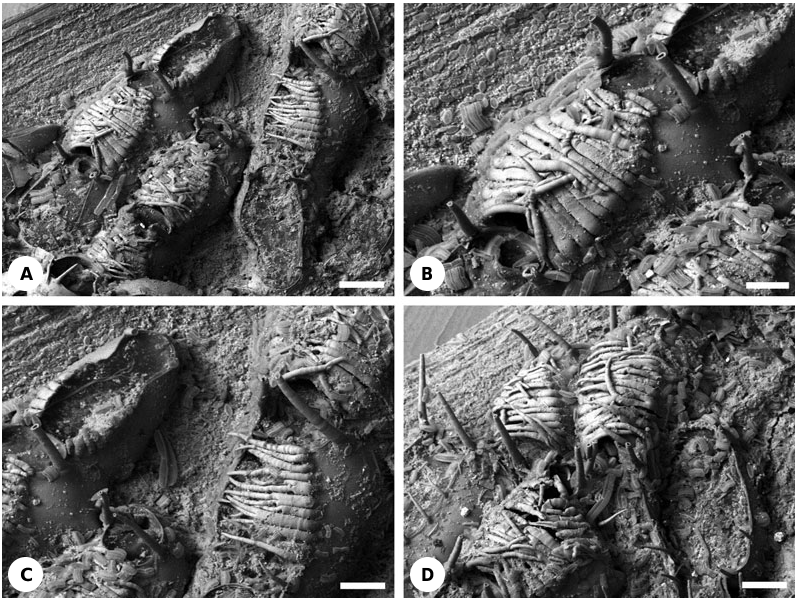
( FIGS 1A- D View Figure 1 , 21A View Figure 21 )
Material: NHM 11.10.1.489, Recent, Sebastopol Bay, Black Sea, collected by A. Ostroumoff.
Description: Non-brooding autozooids possess two oral and sometimes one or two, occasionally more, proximal mural spines that are more slender and shorter than the oral spines ( Fig. 1A, D View Figure 1 ). Oral spines are truncated, their tips probably having a terminal membranous window (cf. Ostrovsky, 1998). Mural spines are acute.
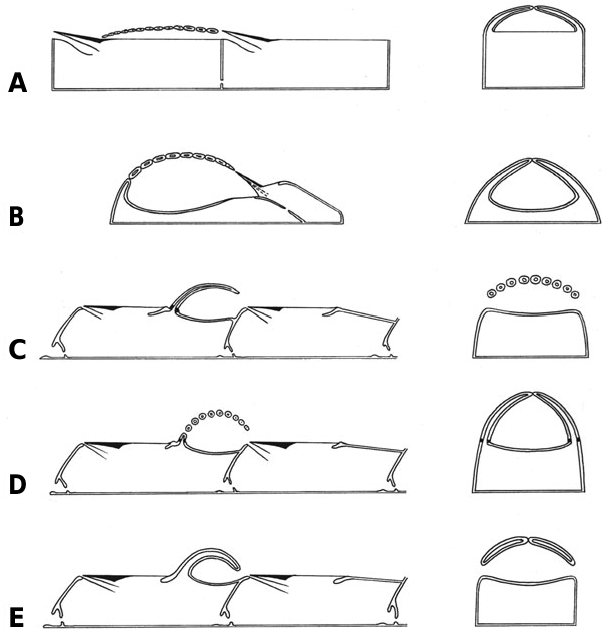
In addition to the oral spines, the left and right sides of the mural rim in brooding autozooids have a row of horizontally inclined, basally flattened spines ( Fig. 1A, B, D View Figure 1 ). These are long and acute, overarching the frontal membrane to give a brooding cavity between this membrane and the undersides of the spines ( Fig. 21A View Figure 21 ). Each row consists of 10–15 spines (up to 17, according to Repiachoff, 1875 and Levinsen, 1909; 13–18, according to Occhipinti Ambrogi & d’Hondt, 1981), growing towards the opposite row. The proximal part of the opesia is devoid of spines, leaving a gap for oviposition of the eggs and for release of the larvae. This entrance to the brooding cavity may be open but in most instances it is plugged by the operculum of the maternal zooid ( Fig. 1A View Figure 1 ), similarly to the so-called cleithral ovicells found in neocheilostomes. The brood chamber has two openings, proximal and distal.
During development of the brood chamber, the proximal spines evidently commence growth before the distal spines ( Fig. 1A, C View Figure 1 ). They meet the spines growing towards them from the opposite side along the midline of the zooidal frontal surface. In some instances spine growth immediately ceases at this point, but in other cases the spines overgrow one another, sometimes even reaching the opposite side of the mural rim ( Fig. 1B View Figure 1 ). Spine arrangement varies, from rather regular to chaotic, and from very tight (though not fused laterally) to loose with slits between adjacent spines ( Fig. 1D View Figure 1 ). Within the same brood chamber, spines can also be very variable in shape: wide or narrow, flat or more cylindrical, unbranched or bifid ( Fig. 1C View Figure 1 ), straight or curved, and growing along or above the spine lattice. In rare examples only one spine row is developed, either on the left or right side of the zooid ( Fig. 1C View Figure 1 ). In addition, some zooids have up to nine thin mural spines of different length which are not in contact with the spines from either the same or the opposite side of the zooid ( Fig. 1D View Figure 1 ) (see also Levinsen, 1909). It is difficult to envisage such a loose construction serving as an effective brood chamber.
All of the oral and mural spines are articulated, as indicated by the presence of a ring furrow at the base of each spine. In contrast, brood-chamber spines appear not to be articulated; unlike oral and mural spines, none are broken off basally. However, sections of decalcified material are required to confirm this supposition. The cavities of all spine types are apparently confluent with the visceral coelom of the zooid distal of the maternal zooid.
Brood chambers are formed near the distal margins of colonies, often being located at the growing edge. In many instances several brooding zooids are found in the same longitudinal row, one following the other ( Fig. 1D View Figure 1 ).
Remarks: The term ‘acanthostegal’ or ‘acanthostegous ooecia’ was introduced by Levinsen (1902, 1909) for the unusual brood chambers constructed of spines in Tendra zostericola and Heteroecium amplectens ( Hincks, 1881). The first superficial description of the ‘cellule treillissées’ and developing embryos inside these brood chambers was given by Nordmann (1839: 191) for Tendra . Repiachoff (1875: 132) remarked that these zooids ‘play a role of the ovicells’ known in a majority of the Cheilostomata . Following Repiachoff, Reingard (1875) thought that embryos developed inside the body cavity of specialized zooids in this species. However, he believed that they could not be compared with ovicells since they possess a polypide and an ovary.
Ostroumoff (1886) was the first to recognize the actual position of the developing embryos in the space between the frontal membrane and the overarching spines. Paltschikowa-Ostroumowa (1926) and Braiko (1967) further studied different aspects of reproduction in Tendra zostericola (see also Occhipinti Ambrogi & d’Hondt, 1981, and references therein), which is known from the Mediterranean as well as the Black Sea.
Where several brooding zooids are present successively within the same longitudinal row, all of these zooids (possibly excepting the most distal one) will have produced their own eggs as well as brooding the embryos of the proximal neighbouring zooid. As in cheilostomes with ovicells, formation of the brood chamber by any particular zooid is presumably triggered by the development of an ovary in its proximal neighbour.
GENUS HETEROECIUM HINCKS, 1892 HETEROECIUM AMPLECTENS ( HINCKS, 1881)
( FIGS 2A- D View Figure 2 , 21B View Figure 21 )
Material: NHM 99.5.1.702, Recent, Western Australia, T. Hincks Collection.
Description: Non-brooding autozooids possess seven mural spines surrounding the frontal membrane. Six of these spines are short, with blunt roundish tips. The seventh spine is several times longer than the others, though approximately equal in width, and is placed on the proximal gymnocyst just behind the frontal membrane. In contrast with the other mural spines, it is basally articulated.
So-called brooding zooids are actually complexes of two zooids, a proximal (maternal) autozooid (probably an autozooidal polymorph) and a distal kenozooid ( Fig. 21B View Figure 21 ). Laterally juxtaposed and flattened spines overarch the frontal wall of the kenozooid to form the brooding cavity ( Figs 2A, B View Figure 2 , 21B View Figure 21 ). The nonarticulated spines, numbering 15–17, start their growth on the mural rim of the kenozooid and meet along the midline above the calcified frontal wall, forming a sort of medial keel. Cavities of the spines are confluent with the visceral coelom of the kenozooid ( Fig. 2C View Figure 2 ). The brood chamber has the shape of an elongated hemisphere, opening proximally where it is plugged by the operculum of the maternal zooid. Brood chambers are always formed in the axils of branch bifurcations, and there is never a zooid distal of the brood chamber. Communication between the maternal autozooidal polymorph and the distal kenozooid is via simple pores ( Figs 2D View Figure 2 , 21B View Figure 21 ). The floor of the brood chamber is calcified, except for a peculiar area in its proximal part which is membranous with two lateral appendages ( Figs 2C, D View Figure 2 , 21B View Figure 21 ).
Remarks: The first description of this species was given by Hincks (1881) who correctly recognized the spinose structures as brood chambers. However, he believed them to be single zooids with ‘the upper portion of the zooecial aperture... much extended’ and ‘roofed in by a number of (soldered) marginal spines’. Hincks also wrote that ‘the zooecium... is divided into two chambers – one for the polypide, the other for the embryo’ (1881: 130). In a later work, Hincks (1892) introduced the genus Heteroecium and repeated the description mentioned above. Levinsen (1909: 148) described a second species, H. brevispina (as ‘Var. brevispina n.’), with some clear differences (larger size, more mural spines, shorter proximal spine, etc.) but a brood chamber having the same structure. Not surprisingly, Levinsen (1909: 148) did ‘not understand the significance of the situation of the aperture behind the area formed by the spines’, since he thought that the brood chamber consisted of a single zooid. Nevertheless, he correctly understood the structure of the brooding cavity, and, following Ostroumoff (1886), wrote that ‘the acanthostegal ooecia... are cavities internally limited by the covering membrane of the zooecium and externally by a cover made up of two rows of hollow spines’ ( Levinsen, 1902: 17). Images of H. brevispina recently placed by Dr P. E. Bock on the Bryozoa Home Page website (http://www. civgeo.rmit.edu.au/bryozoa/cheilostomata/tendridae/ heteamp.html) show the brood chamber to be constructed of 12–13 flattened, nonarticulated spines. Closure of the brood-chamber opening by the zooidal operculum is also clearly seen.
The unusual membranous area in the floor of the brood chamber is interpreted as a rudiment of the frontal membrane to which are attached the parietal muscles involved in tentacle crown eversion in the autozooids. Sections of decalcified material would help to resolve this issue.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.