Colobaea pectoralis (Zetterstedt), 1847
|
publication ID |
https://doi.org/10.11646/zootaxa.4840.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:56993BCA-1A3E-415E-A765-0D55AB3E7A97 |
|
DOI |
https://doi.org/10.5281/zenodo.4405594 |
|
persistent identifier |
https://treatment.plazi.org/id/7170D74F-6A18-FFCB-FF4F-FEC58FACDCCB |
|
treatment provided by |
Plazi |
|
scientific name |
Colobaea pectoralis (Zetterstedt), 1847 |
| status |
|
Colobaea pectoralis (Zetterstedt), 1847 View in CoL
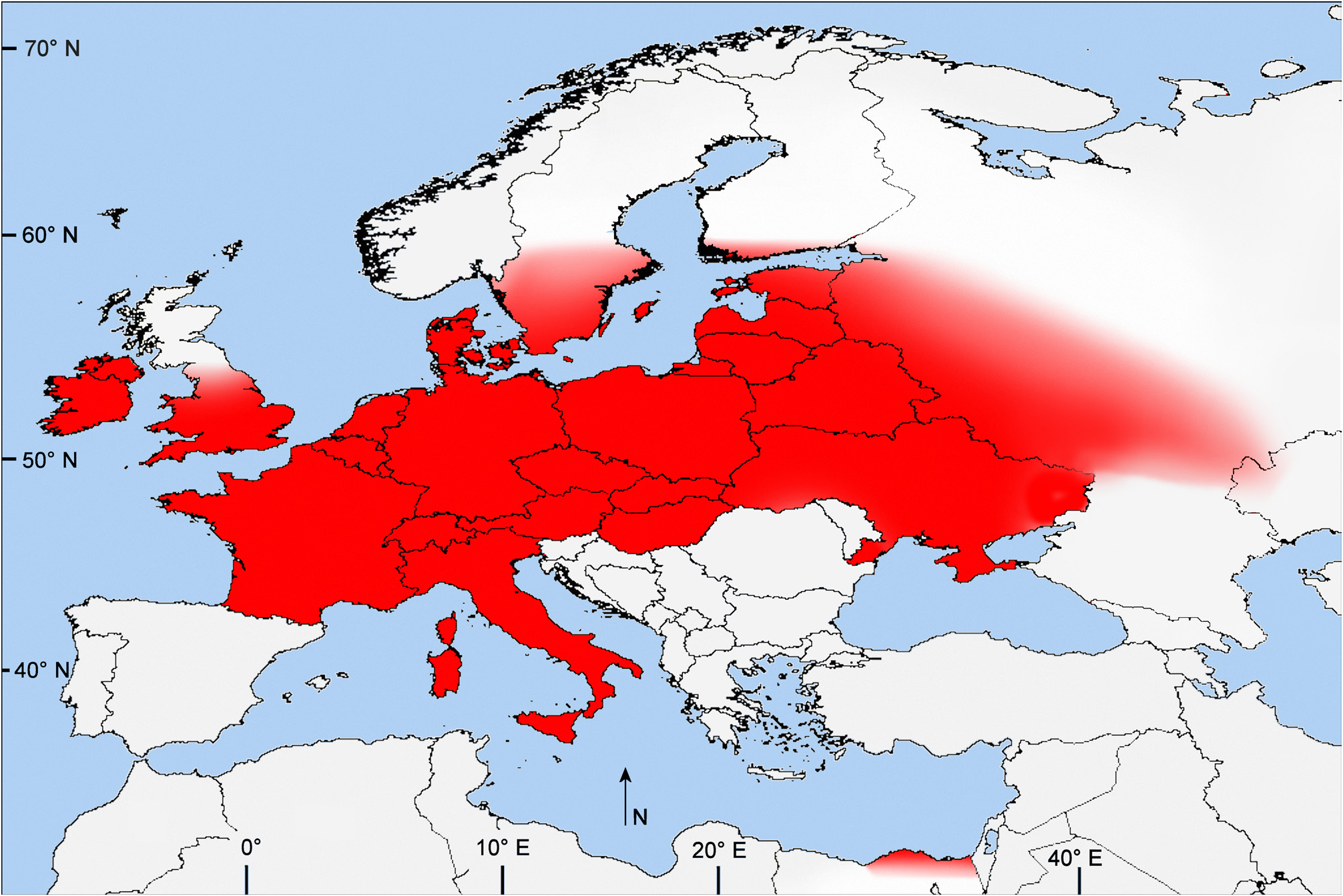
Map 14 View MAP 14
(BNN 6035; FT 6012–15, 6447)
Colobaea pectoralis and all other species of Colobaea except C. bifasciella have color patterns similar to those of many species of Pteromicra . Colobaea pectoralis is distinguished from other species of Colobaea by the yellow anepisternum and anepimeron, elongate black stripe along the upper margin of the anepisternum, and slightly infumated crossveins.
Type locality: Kallunge (Gotland, Sweden), lectotype in the MZLU. Colobaea pectoralis is known from northern and central Europe, extending from Ireland ( County Clare) and Finland south throughout southern England, France, and Belgium to the Czech Republic ( Pieštany ) and east to the St. Petersburg area, the Russian Upland Area ( Black Soil Region south of Moscow ), and northern Kazakhstan.
Becker’s (1903) record from Cairo, Egypt, may pertain to C. punctata , which was not recognized as a distinct species until Lundbeck (1923), or to a related species.
Dates of capture records of adult flies range from 19 April (Jakobstad, Finland) to 17 September (Copenhagen, Denmark).
Laboratory rearings and observations are based on puparia and adult flies, all collected in Zealand , Denmark: at Snøgedam, Hillerød ( FT 6012–15 ) ( 5 July–1 September 1960) and at Vollerup Mose, near Sorø ( FT 6447 ) ( 18 May 1964) .
Adult flies were swept from low, emergent vegetation around a small, densely shaded pond (Snøgedam). Newly formed puparia were found in shells of Anisus vortex lying on a mat of Lemna and at the bases of Sparganium simplex . A few puparia were found in shells of A. vortex attached to cases of Limnephilus Leach sp. ( Trichoptera ) at Snøgedam. Shells of Bathyomphalus contortus (L.) containing puparia were found among emergent vegetation in an exposed marsh (Vollerup Mose). Lundbeck (1923), Rozkošný (1967), Nienhuis (1970), and Przhiboro (2001) reared adult flies from puparia found in shells of A. vortex in Denmark, Czech Republic, the Netherlands, and northeastern Russia, respectively.
Adult flies collected during the summer mated between 5 July and 28 August. Four pairs that emerged between 11 and 18 January 1961 from puparia formed in the laboratory during the previous autumn were placed in separate breeding vials. All four pairs copulated within 48 hours after emergence, and one pair mated within 24 hours; Nienhuis (1970) also noted that adult flies mated on the day of emergence. Each mating lasted 10–60 minutes, with pairs often copulating several times daily. A distinct difference was noted in the copulatory posture of C. pectoralis from that of all other species of Sciomyzidae in which the copulatory posture is known except Ectinocera borealis Zetterstedt (Tetanocerini) . Males of most other species place their foretarsi on the head of the female, along the inner margins of her compound eyes. Males of C. pectoralis , however, almost always place their foretarsi on the anterolateral angles of the female’s thorax. The midlegs of the male hold the basicostal margins of her wings, while his hind tarsi grasp the sides of her postabdomen.
Females collected during early July oviposited between 7 July and 2 September. Those that emerged in the laboratory during January laid viable eggs four days after emergence. Although sprigs of fresh moss, strips of fresh Typha leaves, and living A. vortex snails were present, females oviposited mainly on wet peat moss at the bottom of the breeding jars. Females frequently were observed to wriggle down through a tangle of dry moss to oviposit onto the wet moss. Females always laid eggs singly and distributed them at random.After incubation periods of 2–5 days at 24°C, the eggs hatched between 12 July and 3 September and during January.
Lundbeck (1923) evidently thought that C. pectoralis was host specific. After stating that he had reared adult flies only from puparia in shells of A. vortex (as “ Planorbis vortex ”), he added that C. bifasciella and C. pectoralis “are each found only in 1 species of snail though in the places where I collected them also other species of small snails were present.” ADB and LVK documented larvae of C. pectoralis successfully attacking two additional species of snails: 1) puparia were found in nature in shells of B. contortus that the larvae had evidently killed and consumed, and 2) larvae killed and consumed small individuals of Planorbis planorbis during laboratory rearings in addition to A. vortex . The larvae never attacked larger individuals of P. planorbis , nor did they consume freshly crushed aquatic snails.
Most laboratory rearings were conducted with A. vortex . Newly hatched C. pectoralis larvae readily attacked living A. vortex . The larvae penetrated between the mantle and shell, adjacent to the upper side of the aperture, until only their posterior spiracles remained exposed. When reared individually with one snail per larva, the snail usually did not die until after the first larval molt; the larva then continued to feed in the decaying snail tissues. Larvae that attacked full-grown snails completed their development in those individuals, whereas larvae that attacked smaller snails left the emptied shell of the first host and attacked a second and sometimes even a third snail before pupariating. Under crowded rearing conditions, several first-instar larvae would attack and feed within a single snail; such snails usually died within 24 hours. After consuming the tissues of the first snail, these larvae then attacked another snail, either individually or together. Second-instar larvae that were removed from the liquefied tissues of a dead A. vortex and then placed with a living snail attacked it immediately. Upon contact, these larger larvae would slash at the exposed foot or mantle of the snail, causing the snail to retract into its shell. The larvae would continue to slash and hook at the exposed tissues. With each contact, the snail would retract farther. The larvae fed voraciously as they penetrated into the shell. By the time the larvae were in the third stadium, the snail tissues had become a black, putrid, viscous mass. Each larva remained immersed in the liquefied tissue up to its posterior spiracles and consumed most of this material before pupariating. Total duration through all three larval stadia required 8–10 days.
Larvae of C. pectoralis moved about less actively than did larvae of other sciomyzid species studied by the authors. They were never seen crawling more than a few millimeters up the dry sides of the rearing containers, even when they had not eaten for a day or more. Larvae readily attacked snails lying on wet moss, but larvae apparently did not encounter snails held only a few millimeters above the surface of the wet moss by sprigs of drier moss. When dead snails containing actively feeding larvae were submerged, the larvae quickly wriggled out of the shells but did not float to the surface, remaining instead at the bottom, defecating and extending/retracting their posterior spiracles.
Puparia were formed in the laboratory between 26 July and 10 August. Most larvae pupariated in snail shells, with one puparium being formed in each shell. When as much as half of the snail shell had been chipped away to allow observation of a larva throughout its development, the larva eventually left the shell and pupariated on wet filter paper on the bottom of the rearing container or on the dry sides of the container. Some puparia that were formed outside of the shell retained the typical curved shape, but others, especially those formed in a corner of a rearing box or under wet paper, were distorted. Puparia were formed within shells at various distances from the aperture. For example, the anterior end of one puparium was 1½ whorls in from the aperture, while the anterior third of another puparium extended beyond the aperture. Usually the anterior end of the puparium was situated at a distance of about one whorl inside the edge of the aperture. Pupariating larvae produced no septum material such as that excreted and formed into an operculum-like structure by C. americana . Pupae became visible through the translucent integument 2–3 days after the puparia were formed.
Dates of emergence of adult flies indicate that C. pectoralis overwinters within the puparium and that a facultative diapause or period of quiescence may develop in pupae formed during late summer and autumn. Lundbeck (1923) reared adult flies from puparia collected during spring. ADB and LVK examined Lundbeck’s specimens in the ZMUC, where 21 adult flies are pinned with their puparia and with the A. vortex shells in which those puparia had been formed. The shells are labeled as having been collected 12 April 1906 at Utterslev Mose (a bog about 6 km northwest of Copenhagen), but no dates of emergence of the adult flies are noted on the labels. Rozkošný (1967) obtained three adult flies ( 1♀, 2♂) on 9 and 13 April from puparia collected 25 March 1965 at Musov, Moravia. Nienhuis (1970) obtained seven adult flies between 10 and 27 April from puparia collected 4 April 1969 in Herweg, The Netherlands. Przhiboro (2001) obtained 1♂ on 20 May 1998 from a puparium in a shell of A. vortex collected 7 May 1998 on the shore of Anninskoe Lake near St. Petersburg, Russia. ADB and LVK found puparia in shells of B. contortus on 18 May 1964; adult flies ( 5♀, 3♂) emerged between 23 and 30 May. Puparia found in shells of A. vortex between 22 June and 8 August 1960 and held at room temperature produced adult flies ( 4♀, 5♂) 1–11 days after they had been collected. Other puparia found in shells of A. vortex at the same place and at about the same time ( 19 July–8 August 1960) and exposed to 4°C and 76% relative humidity from 23 November to 31 December 1960 produced adult flies ( 2♀, 3♂) between 12 and 18 January 1961. These puparial periods ranged from 159 to 183 days. Similarly, puparia formed in the laboratory between 26 July and 10 August 1960 and exposed to 4°C and 76% relative humidity from 23 November to 31 December 1960 produced adult flies ( 2♀, 5♂) between 11 and 18 January 1961. The longest puparial period, 263 days, was that of a puparium formed in the laboratory on 28 July 1960; it produced 1♀ on 17 April 1961. Duration of the nonquiescent puparial period of summer generations of C. pectoralis was about 11 days.
Among field-collected adult flies held in the laboratory, 1♂ lived 68 days and 1♀ lived 70 days, but the others lived only 6–28 days.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
SuperOrder |
Heterobranchia |
|
Order |
|
|
SuperFamily |
Succineoidea |
|
Family |
|
|
Genus |