Scinax tropicalia, Novaes-E-Fagundes & Araujo-Vieira & Entiauspe-Neto & Roberto & Orrico & Solé & Haddad & Loebmann, 2021
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4903.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:5ABDA0B2-BC31-47E1-A988-CC3307D5FD71 |
|
DOI |
https://doi.org/10.5281/zenodo.4562840 |
|
persistent identifier |
https://treatment.plazi.org/id/777CE11D-4B64-FF89-FDE0-9FB5874AFBF3 |
|
treatment provided by |
Plazi |
|
scientific name |
Scinax tropicalia |
| status |
sp. nov. |
Scinax tropicalia sp. nov.
Scinax x-signatus View in CoL — Dias et al. (2014a: Table 1 View TABLE 1 ), Mira-Mendes et al. (2018: Table 1 View TABLE 1 , Fig. 4 View FIGURE 4 ).
Scinax cf. x-signatus View in CoL — Dias et al. (2014b: Table 2 View TABLE 2 ), Rojas-Padilla et al. (in press: Table 1 View TABLE 1 , Fig. 4G View FIGURE 4 , Appendix 1).
Scinax sp. aff. hayii View in CoL — Roberto & Loebmann (2016: 136, 146, Tables 1 View TABLE 1 and 3 View TABLE 3 , Supplementary Table 1 View TABLE 1 and Fig. 11 View FIGURE 11 ), Araujo-Vieira et al. (2020: 18).
Generic and phylogenetic placements. The new species is assigned to Scinax View in CoL based on the three synapomorphies of the genus: webbing between toes I and II not extending beyond the subarticular tubercle of Toe I; origin of the m. pectoralis abdominalis through well-defined tendons; and m. pectoralis abdominalis overlapping the m. obliquus externus ( da Silva 1998; Faivovich 2002; Faivovich et al. 2005). The new species is a member of the S. ruber View in CoL clade ( Faivovich 2002; Faivovich et al. 2005) by having a combination of pectoral fold present, external vocal sac, and medial slip of the m. extensor digitorum comunis longus of the hand with a single insertion on the dorsum of metacarpal IV, which differentiate adults of species of the S. ruber View in CoL clade from all those of the S. catharinae View in CoL clade.
Holotype. MZUESC 20440 View Materials , adult male, campus of the Universidade Estadual de Santa Cruz ( UESC), municipality of Ilhéus, state of Bahia, Brazil [datum WGS84; -14.795694°, -39.172645°, about 30 m above sea level (a.s.l.)], collected 18 July 2018 by G. Novaes-e-Fagundes.
Paratypes. A total of 65 adults (47 males and 18 females). Nineteen specimens from the type locality. MZU- ESC 20420, 20436, 20438 (females), 20324, 20413–20419, 20421–20425, 20437, 20439, CFBH 44691 View Materials (males), collected on different dates between 25 March and 17 July 2018 by G. Novaes-e-Fagundes. Two specimens from the state of Ceará, Brazil. CFBH 25423 View Materials , 25425 View Materials (males) from Parque das Trilhas (-4.265833°, -38.935278°), Serra de Baturité, municipality of Guaramiranga, collected 23 January 2009 by D. Loebmann .
Forty-four specimens from other localities in the state of Bahia, Brazil. MHNJCH 1258 , 1260 , 1373 , 1522 (females), 1257, 1418 (males), Morro do Mara (-13.899639°, -39.952515°), between municipalities of Jequié and Jitaúna , collected on different dates in August , October , and December 2018 and April 2019 by Deivson F. O. Bastos. MZUESC 9176 View Materials (male), RPPN Fazenda Capit„o (-14.323356°, -39.076132°), municipality of Itacaré, collected 29 November 2010 by Tadeu Medeiros. MZUESC 10214 View Materials (male), Fazenda Santo Antônio, municipality of Ibicaraí (-14.857932°, -39.59182°), collected 4 February 2012, unknown collector. MZUESC 10330 View Materials (male), Reserva Serra Bonita (-15.383°, -39.550°), municipality of Camacan, collected 1 December 2010 by Iuri R. Dias. MZUESC 17933 View Materials (male), Parque Estadual Serra do Conduru (-14.493444°, -39.135833°), municipality of Uruçuca, collected 16 November 2016 by Débora Cruz. MZUESC 20449 View Materials , 20453 View Materials (females), 20450 (male), Fazenda Bonfim (-14.610685°, -39.356982°), municipality of Uruçuca, collected 29 October 2018 by V. G. D. Orrico, Omar Rojas-Padilla, and Vinícius Q. Menezes. MZUESC 20193–20195 View Materials , 20197–20201 View Materials , (males), 20427 (female), Ilha Pequena (-13.939611°, -39.015778°), MZUESC 20208 View Materials , 20209 View Materials , 20228 View Materials (males), 20243 (female), CFBH 44689 View Materials , 44690 View Materials (males), Ilha Grande (-13.912077°, -39.002759°), municipality of Camamu, collected on different dates in November 2016, 2017, March and May 2017, and July 2018 by G. Novaes-e-Fagundes, Leildo Carilo-Filho, and César Alexandre. MZUESC 20311 View Materials (male), Piracanga (-14.215941°, -38.99201°), municipality of Maraú, collected 20 April 2018 by G. Novaese-Fagundes. MZUESC 20408 View Materials , 20409 View Materials (females), 20410 (male), CFBH 44693 View Materials (male), Parque Nacional Serra das Lontras (-15.17961°, -39.34554°), municipality of Arataca, collected 19 and 21 February 2018 and 11 and 12 April 2018 by Omar Rojas-Padilla. MZUESC 20411 View Materials (female), 20412 (male), CFBH 44692 View Materials (female), Estaç„o Ecológica Wenceslau Guimar„es (-13.595717°, -39.719767°), municipality of Wenceslau Guimar„es, collected 29 and 30 April 2018 by Marcos Vila-Nova. MZUESC 20433 View Materials , 20435 View Materials (males), Fazenda Bom Pastor (-14.683556°, -39.1714°), municipality of Ilhéus, collected 8 July 2018 by Renan N. Costa. MZUESC 16647 View Materials , 20443 View Materials , 20444 View Materials (females), 20445, 20446 (males), Fazenda Provis„o (-14.650479°, -39.21483°), municipality of Ilhéus, collected 7 August 2016 by un-known collector or 18 July 2018 by Iuri R. Dias and Caio V. Mira-Mendes, MZUESC 21756 View Materials (male), Serra da Jibóia (-12.871967°, -39.48164°), municipality of Elísio Medrado, collected between 23 February and 3 March 2015 by Iuri R. Dias .
Referred specimens. Three adult specimens. CFBH 27973 View Materials , route to Piraí do Norte , municipality of Gandu , state of Bahia, Brazil, collected 25 January 2011 by Tuliana Brunes , Michele Gonçalves , and Nelson Rodrigues. CFBH 25880 View Materials , Parque das Trilhas, Serra de Baturité , municipality of Guaramiranga , state of Ceará, Brazil, collected 21 March 2010 by I. J. Roberto and Paulo T. Brito. MNRJ 55443 View Materials , Sítio Horizonte Belo, Serra de Baturité, municipality of Pacoti, state of Ceará collected 31 January 2007 by I. J. Roberto .
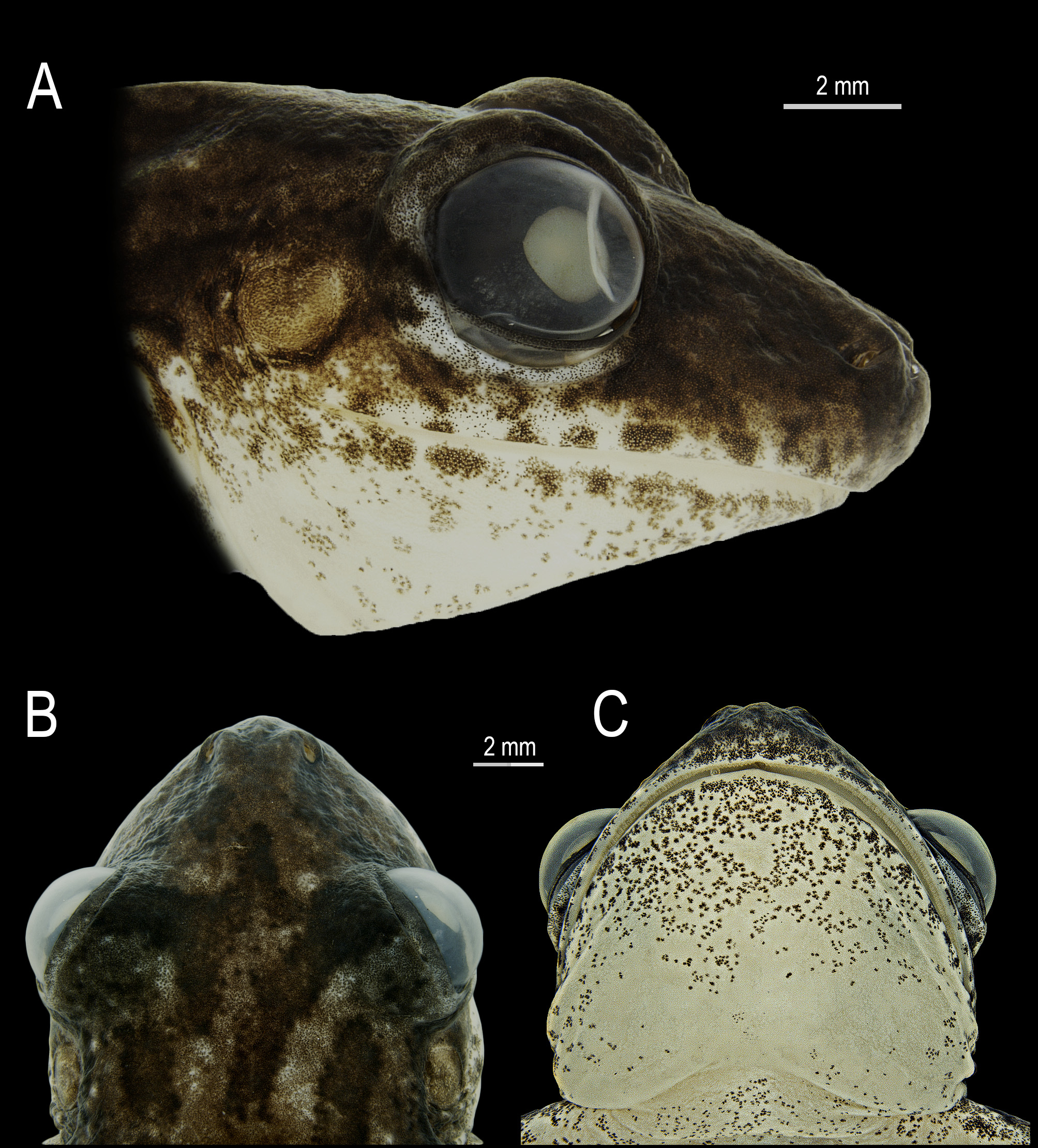
Description of the holotype. Adult male (SVL = 34.8 mm) in a good state of preservation, with a muscle sample tissue (TUESC 1411) removed from the right shank and preserved in 96% ethanol and frozen prior to fixing the voucher specimen in formalin ( Fig. 1 View FIGURE 1 ). Head as wide as long, HL 32.8%, and HW 33.1% of SVL. Snout slightly protruding in profile ( Fig. 2A View FIGURE 2 ), nearly rounded in dorsal view ( Fig. 2B View FIGURE 2 ). Nostrils dorsolateral, elliptical, protruded; IND 22.9% of HW. Canthus rostralis marked and convex. Loreal region concave. Eyes large and protuberant, ED 36.3% of HW, and 12.0% of SVL. Pupil horizontal and subelliptical. Palpebral membrane translucent, not reticulat-ed, its margin with a thin dark rim. Tympanum distinct, round, small, TD 40.4% of ED. Tympanic annulus rounded, with upper portion hidden by the supratympanic fold. Supratympanic fold barely evident, from the upper portion of the tympanum to the insertion of the arm. Tongue ovoid, free laterally and posteriorly, shallowly notched posteriorly. Vomerine teeth in two barely separated transverse series, each bearing five (right) and six (left) teeth, between choanae. Choanae oval. Vocal slits present, nearly parallel to mandible. Vocal sac subgular, median, ventrally not reaching pectoral region and not occupying space between head and body, with a slight medial constriction in the posterior portion, which gives it a bilobate shape when inflated and deflated ( Figs. 1B View FIGURE 1 and 2C View FIGURE 2 ). Pectoral fold present, with pre- and postaxillar elements. Axillary membrane absent.
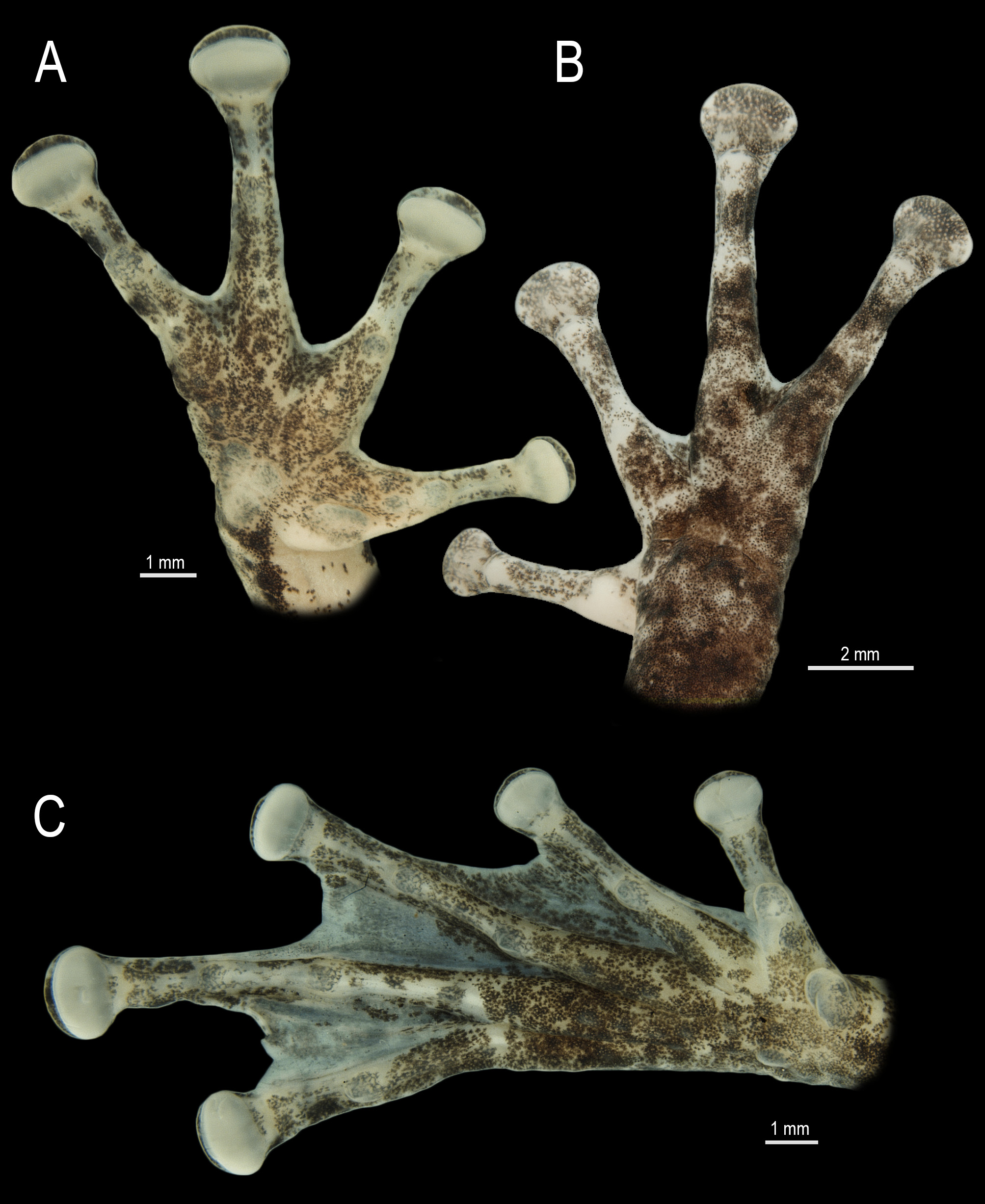
Forelimbs not hypertrophied, upper arm thinner than forearm, hands proportionally large, HAL 30.8% of SVL. Small, round, ulnar tubercles on ventrolateral margin of forearms. Fingers not swelled ( Fig. 3A, B View FIGURE 3 ). Relative finger length II<III=V<IV. Discs elliptical, large (3FD 111.8% of TD), wider than long; disc of Finger II smaller than others. Subarticular tubercles single, conical; relative evidence IV<V<III<II. Supernumerary tubercles absent. Accessory palmar tubercles few, small, single, round. Thenar tubercle single, elliptical; palmar tubercle flat, nearly triangular, bilobate. Webbing basal between fingers. Slightly thickened, light-colored nuptial pad covering the Metacarpal II dorsomedially, extending from the thenar tubercle base, only obscuring its outer margin, to subarticular tubercle ventrally ( Fig. 4 View FIGURE 4 ). Spicule-shaped papillary epidermal projections absent on nuptial pads. Hindlimbs non-hypertrophied, more robust (transversally wider) than forelimbs, TL 52.7% of SVL, FL 44.4% of SVL. Toes not swelled, fringed ( Fig. 3C View FIGURE 3 ). Relative toe length I<II<V<III<IV. Discs elliptical, wider than long, slightly smaller than discs of fingers, 4TD 90.5% of 3FD. Subarticular tubercles single, conical, and rounded. Supernumerary tubercles small, single, and rounded. Inner metatarsal tubercle single, elliptical; outer metatarsal tubercle single, slightly marked, smaller than inner tubercle, approximately one-third of its size. Webbing formula I 2 + –2 + II 1–2 III 1 + –2 ½ IV 2 + –1 V (right foot). Fringe on lateral margin of Toe V extends along the margin of the sole by a poorly developed ridge that reaches the outer metatarsal tubercle. Small, round tubercles on the ventrolateral margin of tarsus.
Cloacal opening directed posteriorly at upper level of thighs. Skin texture areolate on abdomen, flanks, and posterior and ventral surface of thighs; granular to tubercular on region between tympanum and arm insertion, lateral margin of forearms and tarsi, subcloacal region, and heels; shagreened to granular on dorsum of head, trunk, and limbs; smooth on throat, groin, and hidden parts of limbs. There is no evidence of any thickened or glandular area in pectoral region and forelimbs.
Measurements (mm). SVL 34.8; HL 11.4; HW 11.5; SL 6.1; IND 2.6; IOD 7.6; ED 4.2; EN 4.0; TD 1.7; HAL 10.7; FL 15.5; TL 18.3; 3FD 1.9; 4TD 1.7.
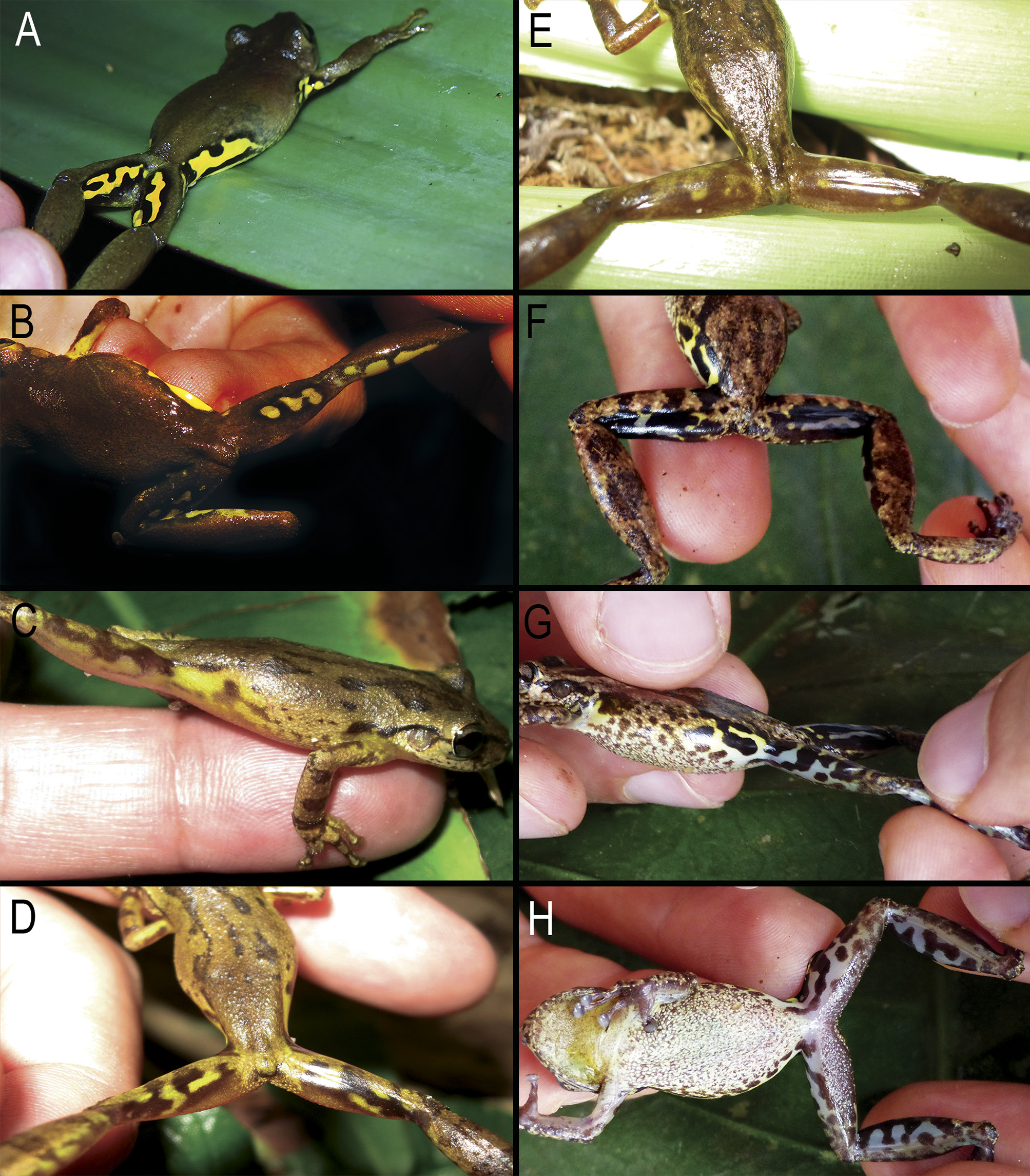
Coloration in life. Dorsal color pattern brown with small, irregular, dark brown blotches and two larger longitudinal ones, extending from the posterior corner of the eyes to the sacral region ( Fig. 5 View FIGURE 5 ). Several scattered light brown or yellow small spots present on dorsum. On dorsum of head, at the level of eyes, a dark brown fragmented marking resembling the outline of a duck´s foot ( Fig. 5A, B View FIGURE 5 ). Dorsolateral, longitudinal, dark brown blotches from the posterior corner of the eyes, through upper margin of tympanum, to the anterior portion of the flanks, near the axillae. Dark brown blotches on upper lip, near the infra-orbital margin of the eye, and on snout covering the nostrils. Canthus rostralis covered by dark brown irregular blotches. Iris bronze with black thin reticulations, golden thin halo bordering the pupil, short vertical black marking below the posterior fold of the pupil, and horizontal black band in its central portion. Dark brown transverse bars on dorsal surfaces of fingers, toes, forelimbs, and thighs, and large blotches on shanks and tarsus. Nuptial pads light-colored. Discs brown dorsally and translucent bluish gray ventrally. Toe webbing covered by brown melanophores. Ulnar and tarsal tubercles light brown or gray. White bones. When calling at night, the holotype presented a general brighter and yellow coloration than that on the inactivity call period described above (compare Fig. 5A, B View FIGURE 5 ).
The following description is based on the freshly euthanized individual ( Fig. 5 View FIGURE 5 C–E). Chest and belly yellow with scattered small brown spots. Throat orange yellow, also finely pigmented with brown spots on anterior portion. Thighs light brown pigmented with darker brown spots. Axillae, inguinal region, and hidden surfaces of thighs, shanks, and tarsi light yellowish green, with medium to large size, rounded and irregular, black blotches. Flanks light yellow. Palms and soles brown.
Coloration in preservative. Coloration pattern is similar but paler than that of the living specimen. Light yellow or green coloration on flanks, throat, chest, belly, axillae, inguinal region, and hidden surfaces of thighs, shanks, and tarsi faded and became light beige or cream white after two years in ethanol 70%. Iris coloration became pale blue.
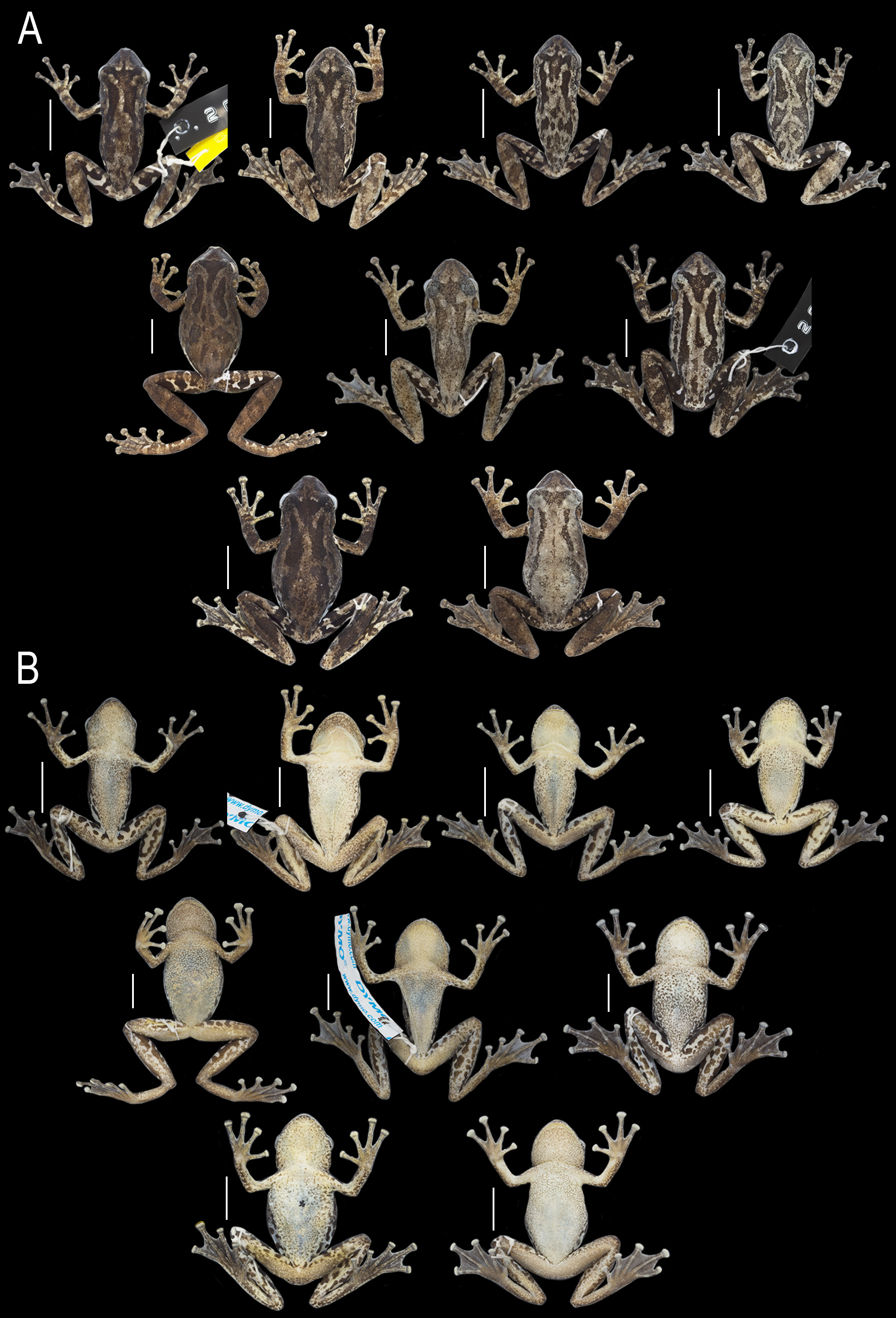
Variation. Morphometric variation of specimens of the type series is in Table 1 View TABLE 1 . In dorsal view ( Fig. 6A View FIGURE 6 ), snout rounded, nearly rounded, or semi-circular; some of the rounded or nearly rounded snouts are also slightly mucronate or slightly truncate. In lateral view, snout rounded or slightly protruding. Vomerine teeth in two fairly separated, barely separated or juxtaposed transverse or oblique series. Number of vomerine teeth ranges between 3–9 on right and 4–8 on left processes. Tongue shape ovoid, lanceolate (MZUESC 20410), or cordiform (MZUESC 20422). Tongue texture granular or smooth. The degree of the medial constriction in males’ vocal sacs varies from slight to conspicuously evident among specimens ( Fig. 6B View FIGURE 6 ).
Overall skin texture similar to holotype, with some variation concerning the development and density of dorsal granules and tubercles, which are more (e.g., MZUESC 10214 View Materials ) or less (e.g., MZUESC 20417 View Materials ) protuberant in some specimens. Ulnar and tarsal tubercles protuberant (e.g., MHNJCH 1257 , MZUESC 17933 View Materials , 20412 View Materials , 20450 View Materials ) or inconspicuous (e.g., MZUESC 20194 View Materials , 20417 View Materials , 20421 View Materials ).
In preservative, dorsal color pattern varies from a gray to brown background, with small, irregular, dark blotches and a pair of continuous or discontinuous longitudinal ones ( Fig. 6A View FIGURE 6 ). In most specimens, both or, at least, one of the longitudinal blotches extending continuously from the post-orbital to sacral regions; both blotches are discontinu-ous in some specimens; when discontinuous, the anterior portion is longer than the posterior one. Male specimens from Bahia usually have longer, continuous, or discontinuous dorsal blotches, whereas specimens from Ceará have shorter and discontinuous dorsal blotches. Dorsal longitudinal blotches also can vary in width and outline, being straight or sinuous, with lateral projections (e.g., MHNJCH 1257) and coalescing with the interocular mark in some specimens. When present, small light spots on dorsum vary in number and size and are usually coincident with the skin granules and tubercles. Other dark brown smaller blotches on dorsum are present in some specimens (e.g., MZUESC 20437, 20450).
Interocular marking can be inverted triangle, trapezium, pentagon (MZUESC 20438), T-shaped (MHNJCH 1257, MZUESC 20409), V-shaped (MZUESC 20439), W-shaped (MZUESC 10214), or irregular (MZUESC 20193). Most of inverted triangle and trapezium markings are anteriorly mucronate, resembling the outline of a duck’s foot; the mucronate projection’s length varies from short (not surpassing the anterior edge of the eyes) to long (almost reaching the nostrils). Posterior projection of the triangle, T-, and V-shaped markings varies in length, reaching the level of tympanum, arms insertion, or posterior edge of eyes. Interocular markings sometimes coalesce with one or both dorsal longitudinal blotches. Canthal dark stripe is present in almost all specimens but can be inconspicuous (MZUESC 10214, 20450) in some individuals. Dorsolateral and post-orbital longitudinal blotches are poorly defined in one specimen (MZUESC 20450). Infra-orbital blotch is inconspicuous or diffuse in three specimens (MZUESC 10214, 20419, 20450). Dark brown oblique bars on dorsal surface of shanks (e.g., MZUESC 20234, 20408, 20433) and transversal bars on tarsi (e.g., MZUESC 20410, 20414, 20435) are present in some specimens. In some individuals, dark coloration predominates on posterior portion of thighs, with small light blotches generally present (e.g., MZUESC 20414, 20433, 20436, 20443).
Palms, soles, gular region, chest, and belly cream white to light beige, immaculate, or finely or conspicuously covered with brown pigmented spots ( Fig. 6B View FIGURE 6 ). Mental region finely pigmented. Gular region (vocal sac) lacks pigmentation in some individuals. Chest and belly conspicuously pigmented in most specimens (e.g., MZUESC 20412, 20435), finely pigmented (MZUESC 20421), or immaculate in some specimens (CFBH 25423, 25425, MZUESC 20437). Webbing formulae in males varies as follows: I (21/2 –2 -)–(21/2 –2 -) II (11/2 –1)–(2 + –2 -) III (11/2 –1)–(21/2 –2) IV (21/2 –2 -)–(11/2 –1) V.
Females are larger than males ( Fig. 6 View FIGURE 6 ; Table 1 View TABLE 1 ) and lack vocal slits, vocal sacs, and nuptial pads. Dorsal color pattern varies from gray to brown, being usually grayish than males—that is specially noticed when they are in amplexus. Gular region, chest, and belly are white or light gray, conspicuously covered with brown spots. Axillae, inguinal region, and hidden surfaces of thighs and shanks have light blue or purple background. Webbing is slightly more developed in females than males (with exception of webbing between toes II and III) and varies as follows: I (2 + –2 -)–(2 + –2) II (2–1)–(2–2 -) III (1 + –1)–(2 + –2) IV (2 + –2)–(1 + –1) V.
In life, paratypes’ dorsal color pattern is generally similar to that of holotype but varies from gray to brown, with light to dark blotches ( Fig. 7 View FIGURE 7 ). Throat region and abdomen of males vary from light yellow to beige ( Fig. 7 View FIGURE 7 A–C), while in females they are whitish ( Fig. 7D View FIGURE 7 ). Axillae, inguinal region, and hidden surfaces of hindlimbs are colored with light tones of blue, green, yellowish-green, and dark brown to black blotches; flanks are light yellow ( Fig. 7 View FIGURE 7 A–C). Females are usually more grayish than males ( Fig. 7D View FIGURE 7 ), and background coloration of axillae, inguinal region, and hidden surfaces of hindlimbs are white, light blue, or light purple.
There are no photos of living paratype specimens ( CFBH 25423 View Materials , 25425 View Materials ) from municipality of Guaramiranga, state of Ceará. We included some photos of unvouchered specimens from Pacoti and Guaramiranga in Fig. 8 View FIGURE 8 . In general, the pattern of coloration of these specimens agrees with that of paratypes, as described above.
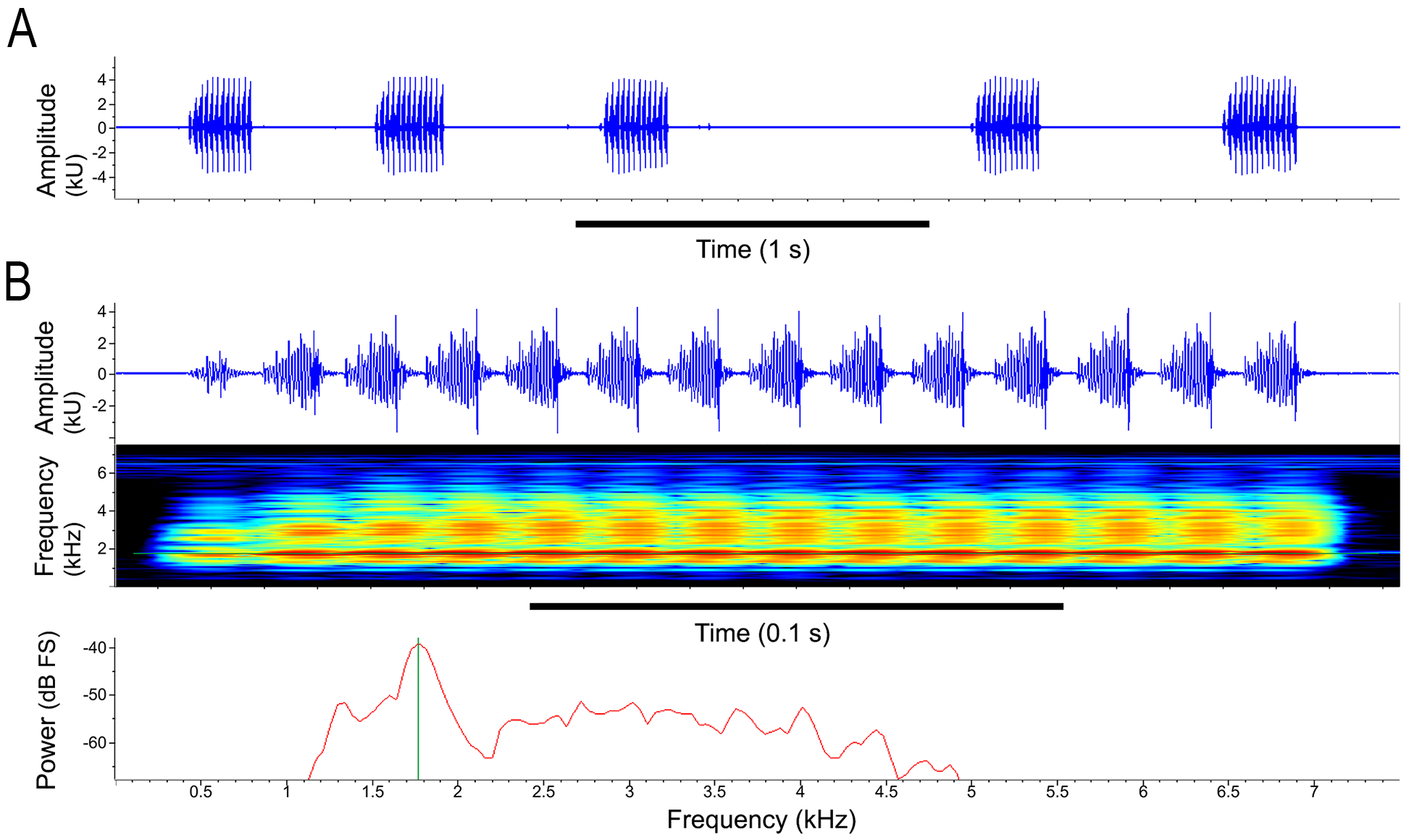
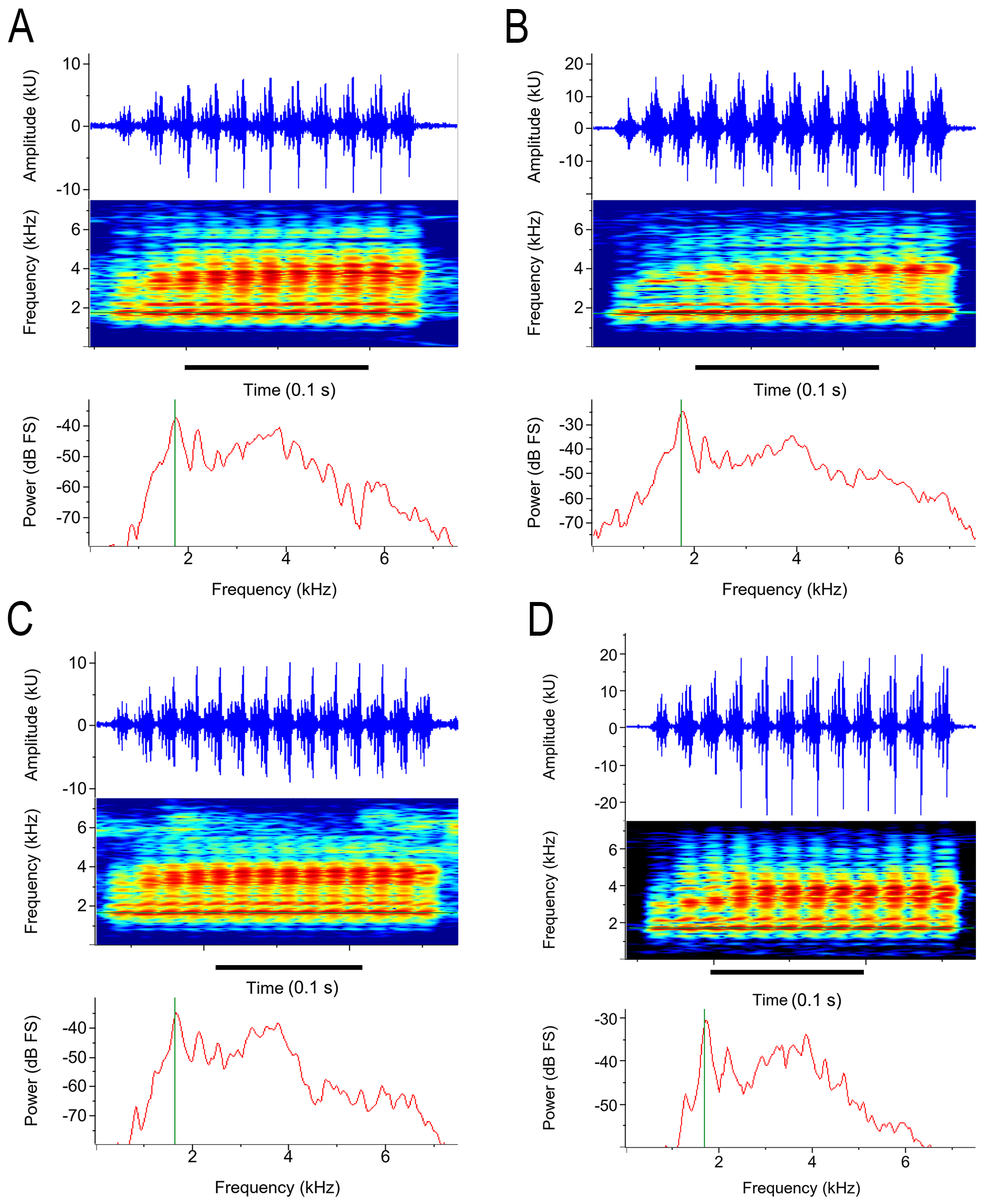
Bioacoustic repertoire. The vocal repertoire of Scinax tropicalia is composed of at least three types of calls. The advertisement call (sensu Wells 2007) is the most commonly emitted by males ( Fig. 9 View FIGURE 9 , Table 3 View TABLE 3 ). It consists of a single stereotyped pulsed short note (i.e., amplitude-modulated; 0.114 –0.310 s) emitted repeatedly at irregular intervals (0.291 –1.336 s), not forming a coherent series ( Fig. 9 View FIGURE 9 , Table 3 View TABLE 3 ). Notes begin with one or two initial pulses with lower amplitude frequency and duration than the following pulses; from the second or third pulse, the maximum amplitude is similar ( Figs. 9 View FIGURE 9 , 10 View FIGURE 10 ). The number of pulses per note ranges from 8–20, which are emitted at a rate of 56–71 pulses/s with a pulse period of 0.014 –0.016 s ( Table 3 View TABLE 3 ). The pulse with maximum amplitude frequency is at the middle or at the ending of the note (see “Note shape” column in Table 3 View TABLE 3 ), and in the second pulse in one single call. Pulses are amplitude-modulated with five pulse sub-units of discrete amplitude, except for the first pulse with fewer, inconspicuous sub-units ( Figs. 9 View FIGURE 9 , 10 View FIGURE 10 ). Pulses increase in amplitude from beginning to the end, with the pulse sub-unit of maximum amplitude at the end of the pulse ( Figs. 9B View FIGURE 9 , 10 View FIGURE 10 ).
Note energy is distributed through a broad spectrum of frequencies, from 1.51–1.77 kHz at the bottom (frequency 5%) up to 3.06–5.12 kHz at the top (frequency 95%), comprising a mean bandwidth of 2.44 kHz ( Table 3 View TABLE 3 ). The frequency 75% is the most variable spectral parameter ranging from 1.76–4.56 kHz. The dominant frequency varies between 1.59–1.85 kHz ( Figs. 9 View FIGURE 9 , 10 View FIGURE 10 , Table 3 View TABLE 3 ), near or equal to the frequency 25% (1.59–1.89 kHz). A second energy peak around 3.0–4.0 kHz that corresponds to the frequency 75% was observed in 14 individuals; however, the dominant frequency remained between 1.59–1.85 kHz ( Figs. 9 View FIGURE 9 , 10 View FIGURE 10 ).
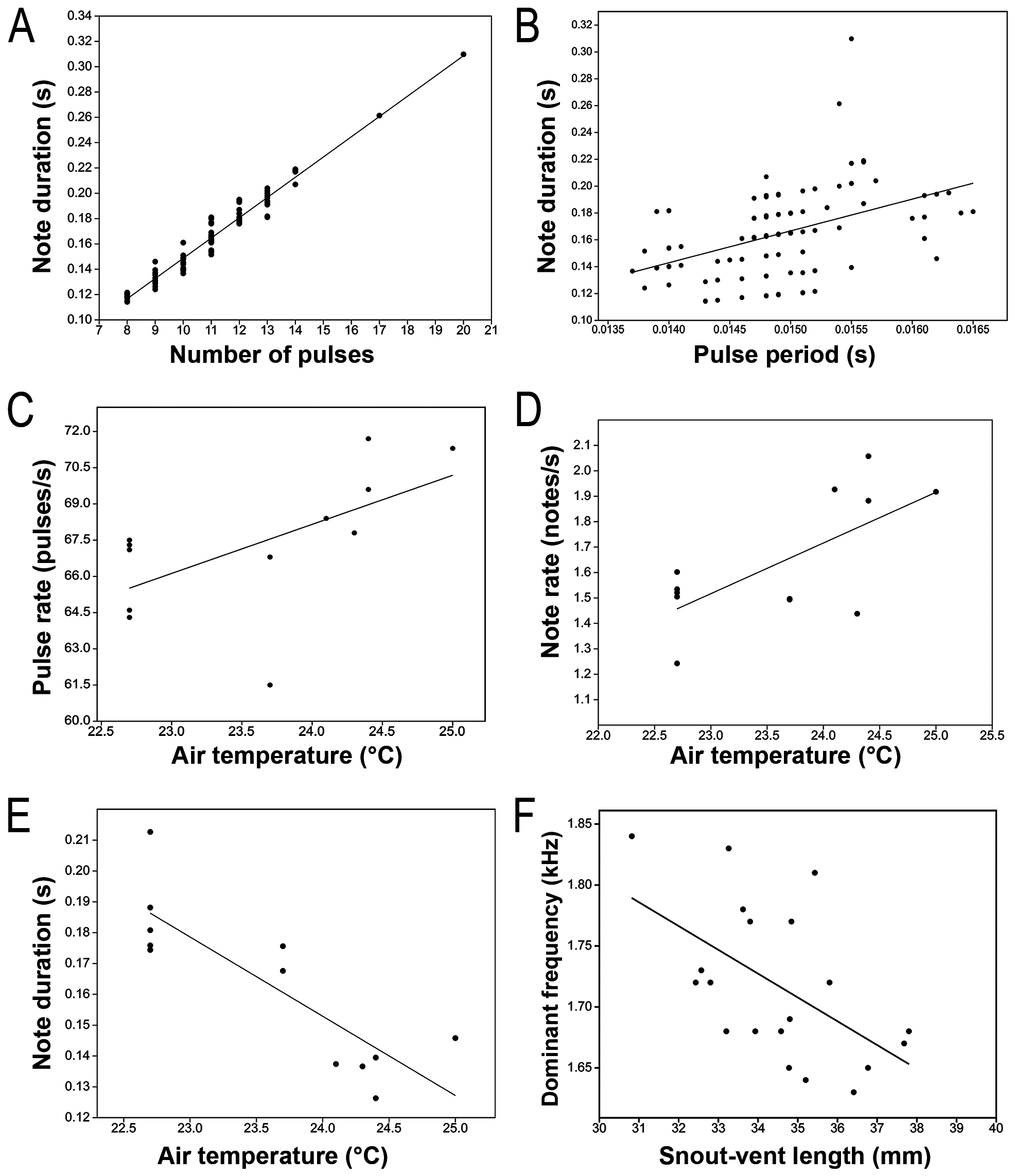
The correlation between note duration and pulse period was weak (r = 0.45, r² = 0.20, p <0.01, n = 116; Fig. 11 View FIGURE 11 ), whereas the correlation between note duration and number of pulses was strong (r = 0.98, r² = 0.96, p <0.01, n = 116; Fig.11 View FIGURE 11 ). This could indicate the duration of notes is modulated mostly by increasing or decreasing of the number of pulses instead of lengthening or shortening of the pulse period. There was a moderate positive correlation between air temperature and mean pulse rate (r = 0.60, r² = 0.36, p <0.05, n = 12; Fig. 11 View FIGURE 11 ); excluding an outlier with lower pulse rate (61.5 pulse/s at 23.7 °C), the correlation between these parameters was strong (r = 0.81, r² = 0.65, p <0.05, n = 12). Air temperature was positively correlated to mean note rate (r = 0.69, r² = 0.47, p <0.01, n = 12; Fig. 11 View FIGURE 11 ) and negatively correlated to mean note duration (r = –0.85, r² = 0.72, p <0.01, n = 12; Fig. 11 View FIGURE 11 ). The dominant frequency was negatively correlated with the SVL (r= –0.55, r²=0.31, p<0.05, n = 20; Fig. 11 View FIGURE 11 ).
The two other call types were recorded from six males, including three paratypes (MZUESC 20193, 20195, 20209) and three unvouchered individuals ( Table 4 View TABLE 4 ). These calls were classified in short and long agonistic calls and had aggressive or release functions depending on the social context in which they were emitted (sensu Wells 2007; Fig. 12 View FIGURE 12 ). The short and long agonistic calls are pulsed and differ from the advertisement call by having undefined and juxtaposed pulses—therefore, the number of pulses per note reported is an approximation—and notes with some degree of modulation of the dominant frequency, often increasing in amplitude.
The short agonistic call is a squeak-like sound with repetitive amplitude modulation that generates well-defined sidebands in the spectrogram ( Fig. 12A View FIGURE 12 , Table 4 View TABLE 4 ). The dominant frequency (1.55–3.88 kHz) reaches higher amplitude than that of the advertisement call (1.59–1.85 kHz). In some notes, the frequency of the amplitude modulation (i.e., the pulse rate) increases from the beginning to the middle of the note and then decreases or increases from the beginning to the end of the note ( Fig. 12A View FIGURE 12 , Table 4 View TABLE 4 ). The short agonistic call was observed in two social contexts. The first and most commonly observed occurred when two or more males called nearby or when a male came close to a calling male ( Fig. 12A View FIGURE 12 ). In this context, commonly observed in high-density choruses, the call seems to have an aggressive function (sensu Wells 2007). On a single occasion inside a plastic bag, the second social context was observed when a male amplected by another male emitted repeatedly this short agonistic call of apparent release function (sensu Wells 2007; Fig. 12C View FIGURE 12 ).
The long agonistic call is an amplitude-modulated, broad-spectrum, harsh trill-like sound ( Fig. 12B View FIGURE 12 , Table 4 View TABLE 4 ). The frequency of the amplitude modulation (i.e., pulse rate) increases from the beginning to the note’s ending. The power spectrum distinguishes from those of the advertisement call and the short agonistic call in having an energy plateau through a broad range of frequencies around 1–4 kHz (discrete narrow peaks in the advertisement call and short agonistic call). The long agonistic call seems to have an aggressive function (sensu Wells 2007). This long agonistic call was observed on a single occasion inside a plastic bag. The male of a couple in axillary amplexus was partially displaced by another male that amplected the female on its inguinal region (see red arrow in Fig. 12B View FIGURE 12 ). Males interacted acoustically emitting several short agonistic calls antiphonally for a few seconds, then one of the males—we could not distinguish which of the males emitted the sound—responded to this interaction emitting the long agonistic call, twice ( Fig. 12B View FIGURE 12 ).
Diagnosis. The new species is diagnosed by the following combination of characters: (1) male SVL 30.8–39.7 mm, n = 48; (2) snout rounded, nearly rounded, or semi-circular in dorsal view and rounded to slightly protruding in profile; (3) tympanum diameter about half size of eye diameter (TD/ED = 0.50 ± 0.054); (4) pointed tubercles on heel and lower jaw absent; (5) vocal sac bilobate, subgular; (6) slightly thick nuptial pad covering the Metacarpal II dorsomedially, and obscuring the outer margin of the thenar tubercle ventrally; (7) spicule-shaped papillary epidermal projections absent on the nuptial pad; (8) pectoral glands absent in males; (9) moderately developed pre- and postaxial webbing of Toe IV, reaching the proximal half of the penultimate phalanx; (10) dorsal pattern consisting of a brown background with two continuous or discontinuous longitudinal darker brown blotches, and an interocular marking; (11) in living specimens, color pattern of hidden surfaces of thighs, shanks, and tarsi consisting of medium to large size, rounded or irregular, dark brown blotches in a white, light blue, light green, light purple, or light yellowish-green background; (12) iris bronze with dark reticulations in living specimens; (13) physiological chlorosis absent; (14) advertisement call composed of a multipulsed note of duration 0.11– 0.31 s, 8–20 pulses per note, pulse rate of 61–73 pulses/s, and dominant frequency of 1.59–1.85 kHz.
......continued on the next page
Comparisons with other species of the Scinax ruber clade. Scinax tropicalia can be distinguished from species of the S. uruguayus group by having bilobate, subgular vocal sac; more developed webbing of Toe IV that reaches the proximal half of the penultimate phalanx; dorsal pattern color consisting of brown background with two continuous or discontinuous, longitudinal, darker brown blotches, and an interocular marking; and bronze iris
in adults (single, subgular vocal sac; less developed webbing of Toe IV that reaches the proximal half of antepenultimate phalanx; dorsal pattern color consisting of brown-black background with a light gray or white reticulated pattern, and anterior cephalic blotch; and bicolored iris, golden upper half and dark brown to black lower half—a putative synapomorphy of this group; Schmidt 1944; Bokermann & Sazima 1973; Faivovich et al. 2005; Baldo et al. 2019). The new species also differentiates from species assigned to the S. rostratus group ( Duellman 1972a; Faivovich et al. 2005) by lacking pointed tubercles on the heel and lower jaw and having rounded to slightly protruding snout in profile (presence of pointed tubercles on heel—a putative synapomorphy of this group—and lower jaw, and elongate pointed snout in profile; e.g., Duellman 1972a; Faivovich 2002; Lima et al. 2005; Faivovich et al. 2005).
Scinax tropicalia distinguishes in adult morphology and advertisement call parameters from the remaining 62 species of the S. ruber clade unassigned to any species group ( Faivovich et al. 2005). We arranged the comparison first based on noticeable size differences in adult males (no overlapping between size ranges), continued by detailed comparisons with species based on conspicuous external morphological characters, and finally by bioacoustic characters of the advertisement call.
Adult morphology comparisons. The SVL (in mm) in males of Scinax tropicalia (30.8–39.7, n = 48) distinguishes it from the larger species S. castroviejoi De la Riva (male holotype 45.0; De la Riva 1993), S. eurydice (Bokermann) (44.0–52.0, n = 9; Bokermann 1968), and S. fuscovarius (Lutz) (41.0–47.3, n = 9 in this study; see also Lutz 1973 and Cei 1980), and from the smaller species S. altae (Dunn) (21.7–26.0, n = 72; Duellman 1970), S. auratus (Wied-Neuwied) (21.4–24.7, n = 21; Duellman & Wiens 1992; Nunes & Pombal 2011), S. cabralensis Drummond, Baêta, and Pires (22.5–25.0, n = 4; Drummond et al. 2007), S. caldarum (Lutz) (23.0–27.0, n = 19; Lutz 1973), S. cruentomma (Duellman) (24.8–27.1, n = 25; Duellman & Wiens 1993), S. danae (Duellman) (24.5–27.4, n = 20; Duellman 1986), S. exiguus (Duellman) (18.0–20.8, n = 25; Duellman 1986), S. fuscomarginatus (Lutz) (15.7–26.7, n = 467; Brusquetti et al. 2014), S. juncae Nunes and Pombal (23.0–27.1, n = 15; Nunes & Pombal 2010), S. madeirae (Bokermann) (18.0–23.5, n = 22; Brusquetti et al. 2014), S. ruberoculatus Ferr „o, Fraga, Moravec, Kaefer, and Lima (22.6–25.9, n = 28; Ferr„o et al. 2018a), S. rupestris Araujo-Vieira , Brand„o, and Faria (21.9–27.7, n = 27; Araujo-Vieira et al. 2015), S. strussmannae Ferr „o, Moravec, Kaefer, Fraga, and Lima (20.2–22.5, n = 5; Ferr„o et al. 2018b), S. tymbamirim Nunes, Kwet, and Pombal (20.6–27.4, n = 148; Nunes et al. 2012), S. villasboasi Brusquetti, Jansen, Barrio-Amorós, Segalla, and Haddad (16.7–20.0, n = 15; Brusquetti et al. 2014), and S. wandae (Pyburn and Fouquette) (19.5–26.9, n = 22; Pyburn & Fouquette 1971).
The snout rounded, nearly rounded, or semi-circular in dorsal view, and rounded to slightly protruding in profile distinguishes Scinax tropicalia from S. caldarum , S. curicica Pugliese, Pombal, and Sazima , S. duartei (Lutz) , S. maracaya (Cardoso and Sazima) , S. rossaferesae Conte, Araujo-Vieira, Crivellari, and Berneck , and S. tigrinus Nunes, Carvalho, and Pereira (sub-elliptical or subovoid in dorsal view and slightly acuminate in profile; e.g., Cardoso & Sazima 1980; Pugliese et al. 2004; Nunes et al. 2010; Conte et al. 2016), S. squalirostris (Lutz) (pointed in dorsal view and acuminate in profile; Pinheiro et al. 2014), and S. alter (Lutz) , S. imbegue , and S. tymbamirim (sub-elliptical with a pointed tip in dorsal view and protruding in profile; Nunes et al. 2012).
The tympanum diameter about 50% of eye diameter (TD/ED = 0.50 ± 0.054, n = 65) distinguishes Scinax tropicalia from S. manriquei Barrio-Amorós , Orellana, and Chacón-Ortiz (tympanum diameter about 28% of eye diameter; see Barrio-Amorós et al. 2004) and S. x-signatus (tympanum diameter about 71% of eye diameter, TD/ED = 0.71 ± 0.073, n = 13; Araujo-Vieira et al. 2020).
The bilobate, subgular vocal sac (weakly bilobate vocal sac sensu Araujo-Vieira et al. 2020) differentiates Scinax tropicalia from S. camposseabrai (Bokermann) [paired subgular vocal sac (bilobate vocal sac sensu Araujo-Vieira et al. 2020); see also Caramaschi & Cardoso 2006: fig. 1] and from the remaining species of the S. ruber clade that have single, subgular vocal sacs; exceptions are S. acuminatus (Cope) , S. dolloi (Werner) , S. funereus (Cope) , S. fuscovarius , S. hayii , S. karenanneae (Pyburn) , S. lindsayi Pyburn , S. montivagus Juncá, Napoli, Nunes, Mercȇs, and Abreu , S. onca Ferr „o, Moravec, Fraga, Pinheiro de Almeida, Kaefer, and Lima, S. oreites , S. pachycrus (Miranda-Ribeiro) , S. perereca , S. ruberoculatus , S. tsachila Ron, Duellman, Caminer, and Pazmiño , and S. x-signatus , whose vocal sacs are also externally bilobate and subgular (weakly bilobate vocal sac sensu Araujo-Vieira et al. 2020; see also Cei 1980; Pyburn 1992, 1993; Duellman & Wiens 1993; Ferr„o et al. 2017, 2018a).
The slightly thick nuptial pad, covering the Metacarpal II dorsomedially and obscuring only the base of thenar tubercle ventrally differentiates Scinax tropicalia from S. dolloi , S. granulatus (Peters) , S. hayii , and S. perereca (thicker and wider nuptial pad that covers almost the entire dorsal surface of Metacarpal II and obscures nearly half of the thenar metacarpal tubercle). The absence of spicule-shaped papillary epidermal projections on the nuptial pad differentiates the new species from S. fuscovarius and S. x-signatus (nuptial pad with spicule-shaped papillary epidermal projections; Luna et al. 2018; Araujo-Vieira et al. 2020). The absence of pectoral glands in males differentiates S. tropicalia from S. funereus , S. fuscovarius , S. nasicus (Cope) , S. onca , S. similis (Cochran) , and S. x-signatus (present in males of these species; e.g., M̹ller & Hellmich 1936; Lutz 1973; Cei 1980; Araujo-Vieira et al. 2020).
The moderately developed pre- and postaxial webbing of Toe IV that reaches the proximal half of the penultimate phalanx distinguishes the new species from S. caldarum , S. curicica , S. duartei , S. maracaya , S. rossaferesae , S. similis , S. squalirostris , S. tigrinus , and S. villasboasi (not surpassing anteriorly the proximal half of antepenultimate phalanx in Toe IV; Bokermann & Sazima 1973; Cardoso & Sazima 1980; Carvalho-e-Silva & Peixoto 1991; Pugliese et al. 2004; Nunes et al. 2010; Brusquetti et al. 2014).
The dorsal color pattern of the body consisting of a brown background with two continuous or discontinuous, longitudinal, darker brown blotches, and an interocular marking distinguishes Scinax tropicalia from S. altae , S. fuscomarginatus , S. madeirae , S. pachycrus , S. quinquefasciatus (Fowler) , S. ruber , S. squalirostris , S. staufferi (Cope) , and S. villasboasi (variable number of dorsal and/or lateral stripes; Duellman 1970, 1972b; Duellman & Wiens 1993; Lutz 1973; Heyer et al. 1990; Brusquetti et al. 2014), S. alter , S. auratus , S. cretatus Nunes and Pombal , S. crospedospilus (Lutz) , S. cuspidatus (Lutz) , S. imbegue Nunes, Kwet, and Pombal , S. juncae , and S. tymbamirim (light or dark dorsal continuous or broken stripes, sometimes delimiting a central darker area; Bokermann 1969; Lutz 1973; Nunes & Pombal 2010, 2011; Nunes et al. 2012), S. blairi (Fouquette and Pyburn) (few brown markings and blotches, or small scattered dark dots; Fouquette & Pyburn 1972), S. boesemani (Goin) (dorsum with or without small white and brown dots; Lescure & Marty 2000), S. cabralensis (small dark spots homogeneously distributed; Drummond et al. 2007), S. caldarum , S. curicica , and S. duartei (pair of longitudinal stripes which converge and often coalesce in front, between, or just behind the eyes; Lutz 1973; Pugliese et al. 2004), S. chiquitanus (small and scattered grayish dots and marks; De la Riva 1990), S. danae (brown with small scattered dark brown flecks; Duellman 1986), S. dolloi and S. perereca (indistinct light pattern or dark spots, a pair of inverted dorsolateral parentheses, and interocular marking; Pombal et al. 1995a), S. exiguus (brown with dark brown dorsolateral and lateral stripes with intervening creamy tan stripe; Duellman 1986), S. haddadorum Araujo-Vieira, Valdujo, and Faivovich (light and dark gray to dark brown, with round and irregular dark blotches; Araujo-Vieira et al. 2016), S. hayii (olive-green, olive-yellow or brown, often devoid of pattern or with only a pale gray spot between the shoulders; Barbour 1909, Lutz 1973), S. ictericus Duellman and Wiens (dorsum with or without dark brown interorbital bar and irregular mostly transverse marks; Duellman & Wiens 1993), S. iquitorum Moravec, Tuanama, Pérez-Peña, and Lehr (small and scattered dark brown dots and blotches; Moravec et al. 2009), S. lindsayi (irregular, dark brown spots and blotches; Pyburn 1992), S. maracaya (many dark blotches with light rims; Cardoso & Sazima 1980), S. onca (round spots and chevron-like markings; Ferr„o et al. 2017), S. oreites Duellman and Wiens (creamy white dorsolateral stripe extending from eye to groin; Duellman & Wiens 1993), S. rossaferesae and S. tigrinus (beige to black with larger, darker, irregular blotches; Nunes et al. 2010; Conte et al. 2016), S. ruberoculatus (light grey or light brown with a large brown or grey spot on the head and scapular region shaped like the moth of the species Copiopteryx semiramis , a human molar in lateral view, or a triangle; Ferr„o et al. 2018a), S. strussmannae (yellowish-bronze with light-brown spots, darker over the snout and eyelids, and an irregularly shaped light-brown spot on the interorbital region; Ferr„o et al. 2018b), and S. tsachila (creamy tan to reddish brown without markings or with faint mid-dorsal and paravertebral stripes from occipital to sacral region; Ron et al. 2018).
The hidden surfaces of thighs consisting of a medium to large size, rounded or irregular, dark brown blotches over a white, light blue, light green, light purple, or light yellowish-green background in living specimens distinguish Scinax tropicalia ( Fig. 13F, G View FIGURE 13 ) from S. acuminatus (somewhat marbled with dark brown blotches on a yellowish background), S. blairi and S. manriquei (densely pigmented with dark dots on a light background; Fouquette & Pyburn 1972), S. boesemani (light-colored, without any markings; Goin 1966), S. chiquitanus (De la Riva) (with or without a broad, dark brown longitudinal stripe or lightly pigmented spots on a uniform tan background; De la Riva 1990), S. cretatus (dark brown colored, without markings; Nunes & Pombal 2011), S. danae (brown colored, without markings; Duellman 1986), S. dolloi (brown colored or with few small yellow spots over a brown background; Lucas R. Santos pers. comm.; Fig. 13E View FIGURE 13 ), S. duartei (lemon yellow or chrome yellow ocelli on a dark brown background; Lutz 1973), S. elaeochroa (Cope) (pale yellow colored or with suffuse olive-tan mottling; Duellman 1970), S. eurydice (dense brown reticulations on a yellowish background), S. granulatus , S. nasicus , and S. similis (minute light alveoli or a tapestry-like pattern of light intervals and darker bars or spots; light parts in life brownish yellow, honey yellow or orange ochraceous), S. hayii (marbling of black and bright deep/chrome/orange yellow blotches; Barbour 1909; Heyer et al.,1990; Fig. 13A, B View FIGURE 13 ), S. ictericus (brown to black colored, without markings; Duellman & Wiens 1993), S. iquitorum (black colored, without markings; Moravec et al. 2009), S. montivagus (beige colored, without markings and yellow flash coloration; Juncá et al. 2015), S. perereca (marbling of black and yellow flash coloration; Pombal et al. 1995a; Fig. 13C, D View FIGURE 13 ), S. quinquefasciatus (pale brown to yellowish cream markings/small blotches on a brown and pale brown background; Ron et al. 2018), S. ruber (bold dark brown or black reticulations enclosing bright yellow spots; Duellman 1970), S. sateremawe (large, orange, black-bordered blotches; Sturaro & Peloso 2014), S. strussmannae (brown colored, without markings; Ferr„o et al. 2018b), S. tigrinus (brown stripes on a flash orange-yellowish background; Nunes et al. 2010), and S. tsachila (pale cream to white or reddish brown colored, without markings; Ron et al. 2018).
Furthermore, the color pattern of the hidden surfaces of shanks and tarsi with dark brown blotches over a white, light blue, light green, light purple, or light yellowish-green background (similar to the hidden surfaces of thighs mentioned above) in living specimens of Scinax tropicalia differentiates it from S. x-signatus (hidden surface of shanks and tarsi immaculate or with a diffuse pattern of light brown dots and light yellow small blotches; Araujo-Vieira et al. 2020).
The bronze iris in living specimens distinguishes Scinax tropicalia from S. cruentomma (silvery bronze with a median horizontal red streak; Duellman 1972b), S. funereus (greenish bronze; Duellman 1971), S. onca (bright orange; Ferr„o et al. 2017), S. quinquefasciatus (reddish bronze; Duellman 1971), S. ruberoculatus (bicolored iris, red above and grey below; Ferr„o et al. 2018a), S. sateremawe (golden and silver; Sturaro & Peloso 2014), S. strussmannae (golden, with a broad medial horizontal red stripe; Ferr„o et al. 2018b), and S. tsachila (brown with orange flecks to orange-yellow with brown reticulations; Ron et al. 2018).
The absence of physiological chlorosis distinguishes Scinax tropicalia from at least 13 species for which the presence of physiological chlorosis was reported in previous studies: S. boesemani , S. caprarius , S. cruentomma , S. cuspidatus , S. elaeochroa , S. funereus , S. ictericus , S. iquitorum , S. karenanneae , S. manriquei , S. onca , S. strussmannae , and S. tsachila ( León, 1969; Lutz, 1973; Pyburn, 1993; La Marca, 2004; Moravec et al., 2009; Cole et al., 2013; Melo-Sampaio & Souza, 2015; Ferr„o et al., 2017, 2018b; Acosta-Galvis, 2018; Ron et al., 2018; Taboada et al., 2020).
Advertisement call comparisons. The advertisement call ( Table 3 View TABLE 3 ) composed of a multipulsed note of duration 0.11– 0.31 s distinguishes Scinax tropicalia from S. ictericus (note duration 0.07– 0.09 s, n = 7; Duellman & Wiens 1993), S. curicica , S. exiguus , S. madeirae , S. quinquefasciatus , S. tymbamirim , and S. wandae (note duration of 0.44– 4.5 s, n = 3–11; Pyburn & Fouquette 1971; Duellman 1986; Pugliese et al. 2004; Kwet 2001; Pombal et al. 2011; Nunes et al. 2012; Brusquetti et al. 2014; Ron et al. 2018).
The number of pulses per note (8–20 pulses) distinguishes the new species from Scinax juncae (number of pulses 2–5, n = 2; Nunes & Pombal 2010), S. alter , S. curicica , and S. wandae (number of pulses 29–152, n = 4–15; Pyburn & Fouquette, 1971; Pombal et al. 1995a, 2011; Nunes et al. 2012; Pugliese et al. 2004). The pulse rate of 61–73 pulses/s distinguishes S. tropicalia from S. cruentomma , S. fuscomarginatus , S. ictericus , S. madeirae , S. staufferi , and S. strussmannae (100–230 pulses/s, n = 1–56; León 1969; Duellman 1972b; Duellman & Wiens 1993; Brusquetti et al. 2014; Carvalho et al. 2015; Ferr„o et al. 2018b).
The dominant frequency of 1.59–1.85 kHz distinguishes Scinax tropicalia from S. cretatus , S. dolloi , S. nasicus , S. oreites , and S. x-signatus (dominant frequency of 0.90–1.45 kHz, n = 1–8; Duellman & Wiens 1993; De la Riva et al. 1994; Nunes & Pombal 2011; Novaes & Zina 2016; Santos et al. in press; Araujo-Vieira et al. 2020), S. auratus , S. baumgardneri (Rivero) , S. cabralensis , S. exiguus , S. fuscomarginatus , S. madeirae , S. quinquefasciatus , S. rossaferesae , S. similis , S. tigrinus , and S. wandae (dominant frequency of 2.70–5.05 kHz, n = 1–56; Pyburn & Fouquette 1971; Duellman 1972b, 1986; Duellman & Pyles 1983; Drummond et al. 2007; Nunes et al. 2007, 2010; Nunes & Pombal 2010; Bilate & Lack 2011; Brusquetti et al. 2014; Bang & Giaretta 2016; Conte et al. 2016; Ron et al. 2018).
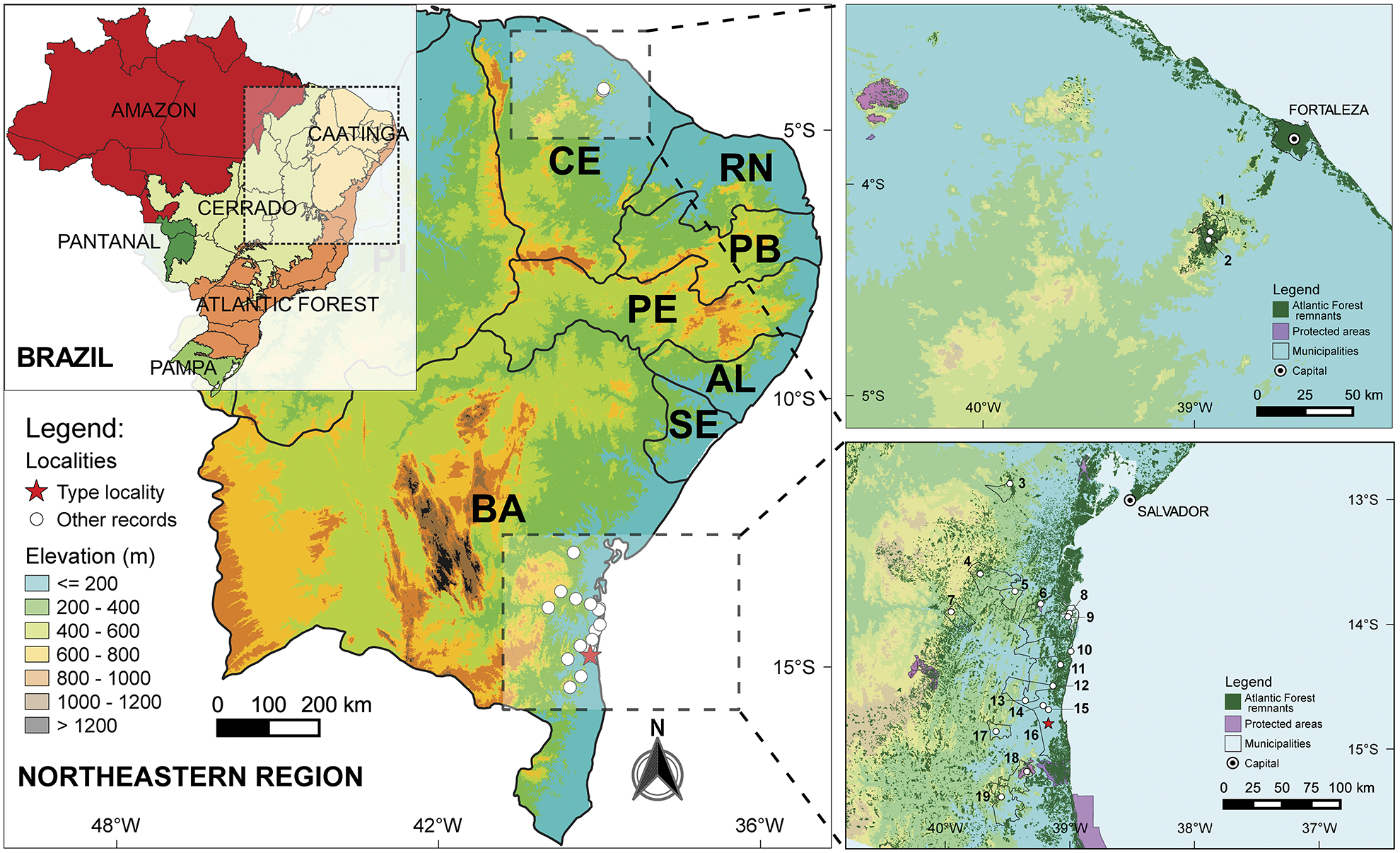
Geographic distribution and natural history. Scinax tropicalia has a disjunct distribution in Brazil, occurring in several localities in the Atlantic Forest of southern Bahia state and Guaramiranga and Pacoti—nearby localities, Pacoti distances approximately 4.5 km N from Guaramiranga—in the Serra de Baturité , northern Ceará state. The locality of Parque das Trilhas in Guaramiranga is approximately 980 km N distant from the northernmost locality of Elísio Medrado in the state of Bahia ( Fig. 14 View FIGURE 14 : localities 2 and 3). In Bahia, S. tropicalia can be found in several localities inside protected areas, such as the Parque Nacional das Lontras and the Reserva Serra Bonita ( Fig. 14 View FIGURE 14 ). In Ceará, Serra de Baturité has an environmental conservation unit (“ Área de Proteç „o Ambiental ” in Portuguese ), being one of the named “Brejos Nordestinos” or “Brejos de Altitude”: high elevation enclaves of humid forest surrounded by dry forests of the Caatinga biome. These enclaves encompass 43 disjunct areas of complex and distinct vegetation type, located in mountain ranges of at least 600 m elevation. Annual rainfall of more than 1200 mm and mean temperatures around 22ºC differentiate these enclaves from the surrounding dry-Caatinga areas. These key biodiversity enclaves are also characterized by their unique endemism and high species richness (Tabarelli & Santos 2004; Roberto & Loebmann 2016).
The new species’ distribution covers a wide altitudinal range, from sea level (e.g., municipality of Maraú, Bahia) to over 600 meters a.s.l. in the state of Bahia (e.g., Morro do Mara, municipality of Jitaúna; Serra da Jibóia, municipality of Elísio Medrado; and Serra Bonita, municipality of Camacan). In the state of Ceará, it is restricted to altitudes above 600 m a.s.l. (Serra de Baturité, municipalities of Guaramiranga and Pacoti; Figs. 14 View FIGURE 14 , 15A, B View FIGURE 15 ). According to K̂ppen’s classification ( Peel et al. 2007; Alvares et al. 2013), most localities where the new species occurs in the state of Bahia have a climate type Af (tropical without dry season); the exception is Serra da Jibóia, municipality of Elísio Medrado that is Aw (tropical with dry winter). The climate of Serra de Baturité in the state of Ceará is As (tropical with dry summer).
Scinax tropicalia inhabits preferentially humid forests ( Fig. 15 View FIGURE 15 ), but was also found in secondary forests, agroforestry systems, forest edges, and near urban centers, always with some degree of forest cover—we did not find it in open areas, such as savannas, fields or pastures. The type locality of the new species is inside the campus of the Universidade Estadual de Santa Cruz, Ilhéus, Bahia, an area with some degree of urbanization and strong anthropic presence. The campus has small forested areas, and although surrounded by some secondary ombrophilous forest, the “cabruca”—a local agroforestry system of cocoa plantation shaded by native and exotic trees—dominates the landscape.
Scinax tropicalia is mainly arboreal, and is usually found perched on vegetation or rarely on the ground among the leaf litter ( Fig. 16I, J View FIGURE 16 ). Males call in the horizontal or vertical position, with head upwards, over branches, leaves, vines, and tree trunks ( Fig. 16 View FIGURE 16 A–H) at heights from close to the ground up to two meters high. Terrestrial and epiphytic bromeliads are used as a shelter during day, but also as calling site, and probably for foraging as well ( Fig. 16K View FIGURE 16 ). Males often acquire a yellow hue skin color when in calling activity or in amplexus ( Fig. 16 View FIGURE 16 A–C, L–N).
The amplexus type of Scinax tropicalia is axillary (sensu Duellman & Trueb 1986; Fig. 16 View FIGURE 16 L–P), and the reproductive mode is Type 1 (eggs and exotrophic tadpoles in lentic water, sensu Haddad & Prado 2005). The new species seems to be an explosive breeder (sensu Wells 2007) since it was observed in mating activity during or up to a few days after heavy rains. Scinax tropicalia reproduces in permanent and temporary lentic water bodies ( Fig. 15 View FIGURE 15 B–D) and occasionally uses artificial structures, such as water tanks, for reproduction ( Fig. 16O View FIGURE 16 ). We observed some behaviors typical of explosive breeders, such as male-male amplexus and males trying to dislodge competitors in amplexus to take the female ( Fig. 12B, C View FIGURE 12 ). Males produce agonistic calls in these contexts or, when other males es approach the calling site (see the Bioacoustic repertoire section for the agonistic calls description). We also observed the passive defensive behavior of contracting (sensu Toledo et al. 2010) in one male specimen (CFBH 44691) after it had been handled ( Fig. 16S, T View FIGURE 16 ). In the state of Bahia, other species of Scinax syntopic with S. tropicalia are S. argyreornatus (Miranda-Ribeiro) ( S. catharinae clade), S. alter , S. eurydice , S. juncae , S. similis , and S. x-signatus ( S. ruber clade).
Conservation status. We suggest assigning Scinax tropicalia to the Least Concern (LC) category of IUCN based on its abundant occurrence in several localities in the state of Bahia, including two localities in the state of Ceará, as well as its presence in conservation units and diverse types of environments, such as anthropic areas, primary and secondary forests, agroforestry systems, and forest edges.
Etymology. The specific epithet, a noun in apposition, is in allusion to the tropical habitat where the new species occurs, and also in homage to the Brazilian revolutionary artistic movement known as Tropicália, or Tropicalismo. This cultural movement arose in the late 1960s and had among its famous members the musicians from Bahia, Caetano Veloso, Gilberto Gil, Gal Costa, and Tom Zé, among other artists. The “tropicalists” introduced many aesthetical innovations and mixed a myriad of elements and rhythms from diverse origins (national and foreign, traditional and avant-garde, popular and erudite). This movement was formally disintegrated in 1968 with the prison and exile of Caetano Veloso and Gilberto Gil by the authoritarian military dictatorship that ruled Brazil from 1964 to 1985. Tropicália certainly has marked and influenced the Brazilian popular culture over the years from its genesis. For more details about Tropicália, see Veloso & Dunn (1996), Favaretto (2000), Dunn (2001, 2014), Veloso (2017), and Oliveira (2020).
Suggested common name. Tropicalia’s Snouted Treefrog.
| UESC |
Universidade Estadual de Santa Cruz |
| R |
Departamento de Geologia, Universidad de Chile |
| V |
Royal British Columbia Museum - Herbarium |
| T |
Tavera, Department of Geology and Geophysics |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Scinax tropicalia
| Novaes-E-Fagundes, Gabriel, Araujo-Vieira, Katyuscia, Entiauspe-Neto, Omar M., Roberto, Igor J., Orrico, Victor G. D., Solé, Mirco, Haddad, Célio F. B. & Loebmann, Daniel 2021 |
Scinax
| Araujo-Vieira, K. & Pombal Jr., J. P. & Caramaschi, U. & Novaes-e-Fagundes, G. & Orrico, V. G. & Faivovich, J. 2020: 18 |
| Roberto, I. J. & Loebmann, D. 2016: 136 |