Stellera chamaejasme, L.
|
publication ID |
https://doi.org/10.1016/j.phytochem.2014.07.013 |
|
DOI |
https://doi.org/10.5281/zenodo.10561838 |
|
persistent identifier |
https://treatment.plazi.org/id/7B1D87B4-335D-FF90-FFD5-F922FE65901F |
|
treatment provided by |
Felipe |
|
scientific name |
Stellera chamaejasme |
| status |
|
2.2. Phytotoxic activity of flavonoids from S. chamaejasme View in CoL
Flavonoids are known to exhibit a wide range of functions in plant physiology, biochemistry and ecology ( Taylor and Grotewold, 2005). They have been reported to be involved in allelopathic effects ( Treutter, 2006). Thus, two flavone O -glycosides isolated from rice seedlings showed strong inhibition on the growth of Echinochloa crus -galli and Cyperus difformis ( Kong et al., 2007) . The flavone luteolin from Chrysanthemum morifolium L. significantly reduced the frond number and chlorophyll content of Lemna gibba plants ( Beninger and Hall, 2005).
In the study herein, compounds 1–8 were assayed for phenotypic and growth effects on seven-day-old A. thaliana seedlings . Results indicated that all of the compounds isolated by the bioassay-guided fractionation of S. chamaejasme decreased the fresh weight of A. thaliana seedlings ( Table 1 View Table 1 ). Among these compounds, 1–3 and 6–8 showed significant inhibitory effects, and the concentrations required for 50% inhibition in the assay (defined as IC 50) were 6.9, 12.1, 43.2, 74.8, 7.1 and 27.3 µg/mL, respectively. Compounds 4 and 5 only showed slight inhibitory effects. After the administration of compounds 1–3 and 6–8 for 7 days, the treated plants showed wilting symptoms prior to senescence with morphological alteration of roots (data not shown). The fresh weights of the A. thaliana seedlings treated with compounds 1–3 and 6–8 at 25 µg/mL were reduced by 94.1%, 59.8%, 25.9%, 6.2%, 85.7% and 38%, respectively, compared to that of the control. Compounds 1, 2 and 7 showed significant activity on A. thaliana seedlings at low concentrations ( 6 50 µg/mL), and this resulted in arrested growth of treated plants at high concentrations ( À 100 µg/mL), but without mortality. Compounds 3, 6 and 8 were not as phytotoxic as compounds 1, 2 and 7 at low concentrations, but were effective at high concentrations; compound 3 even killed the plants at 100 µg/mL (data not shown).
In contrast, compound 4 showed only a slight inhibitory activity and compound 5 inhibited the growth of A. thaliana only at high concentrations ( À 100 µg/mL). The phytotoxicity of all eight compounds was dose-dependent.
Previous studies showed that neochamaejasmin B ( 1) inhibited cellular 3 H-thymidine incorporation and subsequent proliferation of human prostate cancer LNCaP cells ( Liu et al., 2008), and also had antifungal activity ( Yang et al., 2005). Genkwanol A ( 6) and daphnodorin B ( 7) exhibited OE- glucosidase inhibitory activity ( Zhou et al., 2010) and antitumor activity ( Sakaguchi et al., 2001; Zheng et al., 2007a,b; Shu et al., 2009). Daphnodorin B ( 7) was moderately active against HIV- 1 in vitro ( Hu et al., 2000).
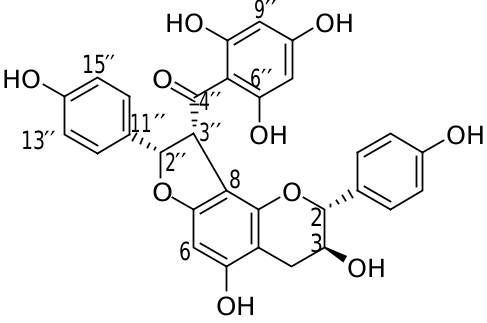
Parvez et al. (2004) showed that the presence of a methyl group in the flavonoid nucleus enhances growth inhibition on A. thaliana and Neurospora crassa . Our results are consistent with this report as compound 3 was a strong inhibitor of A. thaliana development, but compound 4 was not. However, compounds 1, 2 and 6–8, which have no methyl groups, also arrested the growth of A. thaliana seedlings . Therefore, the number and location of methyl groups and/or other substituents such as hydroxyl groups may influence the phytotoxicity of these compounds. Compounds 1 and 2 had strong phytotoxic activities on A. thaliana seedlings despite the stereochemical configuration difference of the hydrogen at the C- 200 position ( Fig. 2 View Fig ), indicating that the spatial configuration is not a critical factor.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |