Elysia bangtawaensis Swennen, 1998
|
publication ID |
https://doi.org/ 10.5281/zenodo.4508360 |
|
persistent identifier |
https://treatment.plazi.org/id/807CB709-B50C-5E1D-FF67-46AEBE69FB3A |
|
treatment provided by |
Carolina |
|
scientific name |
Elysia bangtawaensis Swennen, 1998 |
| status |
|
Elysia bangtawaensis Swennen, 1998 View in CoL
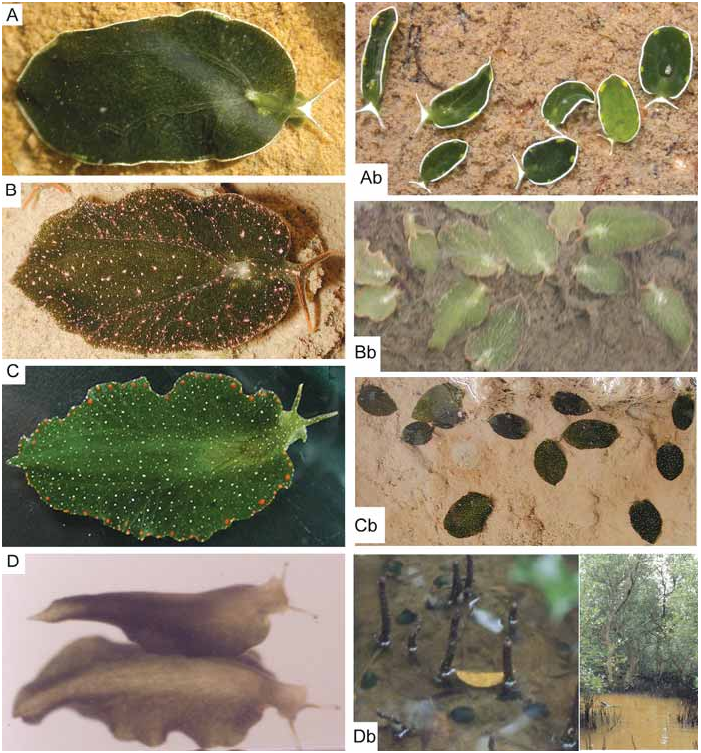
( Figs. 1-7 View Fig View Fig View Fig View Fig View Fig View Fig View Fig )
Syn.: Elysia bangtawaensis Swennen, 1997 . ( Rudman, 2007; Coleman, 2008). Incorrect year of publication due to a difference of the year printed on the volume and the date of distribution of the last issue which is stated as 18 April 1998.
Material examined. – Gulf of Thailand. Pak Phanang Bay about 08°36'10"N, 99°58'16"E and 8°29'19"N, 100°10'58"E, 2 respectively 4 specimens near hundreds of Elysia leucolegnote , 28-30 Sep.2007; Bang Tawa, about 06°51'28"N, 101°09'31"E, type locality, yearly between Mar.1997 and Oct.2010; Inland ditch near Ban Di, about 6°52'17"N, 101°18'48"E, 5-10 during several visits between 1999 and 2002 (site now converted into shrimp ponds); Inner Pattani Bay, about 6°53'50"N, 101°20'49"E, 2 to 30 individuals during several visits between 1997 and 2007; Straits of Malacca, Johor State, 01°21'30"N, 103°30'37"E, 3 specimens, 17 Oct.2009; Andaman Sea, Thailand, mangrove east of Krabi at about 08°03'17"N, 098 53'57"E, one, 3 Oct, 2010. Arabian Sea, estuary Mandovi River, Goa, India, material seen. [See photo: Jagtap et al., 2009]. Bay of Bengal, Andhra Pradesh, about 20 km south of Kãkinãda, 16°51'40"N, 82°15'10"E, some specimens, 4 Dec.2002 [specimens sent to me by Dr S. Bouillon]; South Pacific Ocean, Australia, Queensland, near Coolangatta, about 28°10'S, 153°32'E, 28 Jul.2007 (photos by G. Cobb in Coleman, 2008).
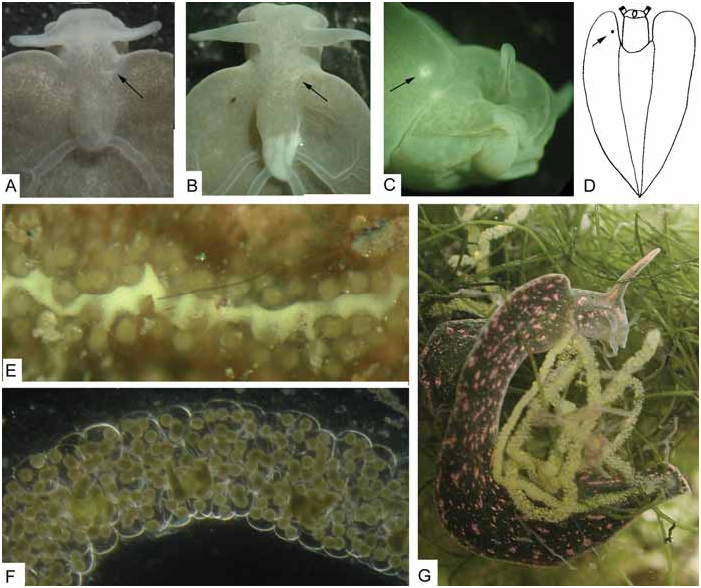
Live colouration. – Prominent reddish to orange, glandular warts along parapodial borders. White spots of different sizes and fine reddish specks dispersed on dorsal and ventral sides, including foot sole. Green ductules of digestive gland cover renal part in most specimens; they do not reach tips of rhinophores. Tips pale or coloured by white and orange spots ( Fig. 1C View Fig ). Individuals with digestive gland yellowish starting in the central parts have not fed for some months as confirmed in captivity.
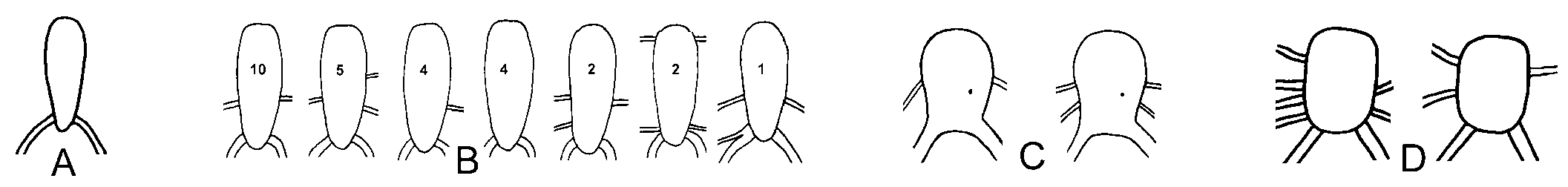
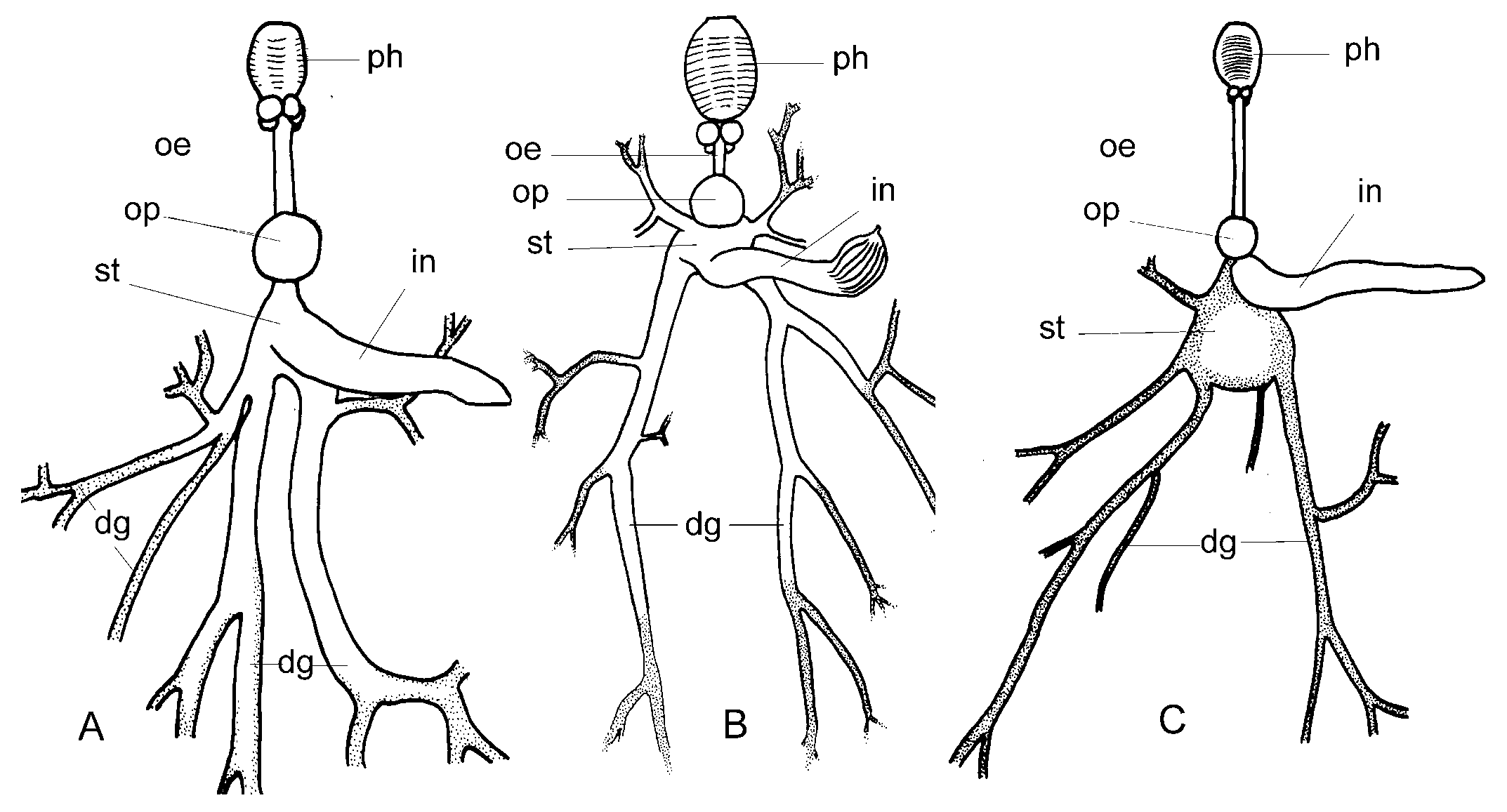
External characters. – Length alive up to 52 mm. Renopericardial prominence oval, length less than twice width. Two major dorsal vessels on posterior side and one,
sometimes two, thinner vessels on both lateral sides ( Fig. 2C View Fig ). Renopore on dorsal side of pericardium in posterior part between heart and renal part ( Fig. 2C View Fig ). Vaginal aperture on ventral side of right parapodium near anterior border ( Fig. 7C View Fig ).
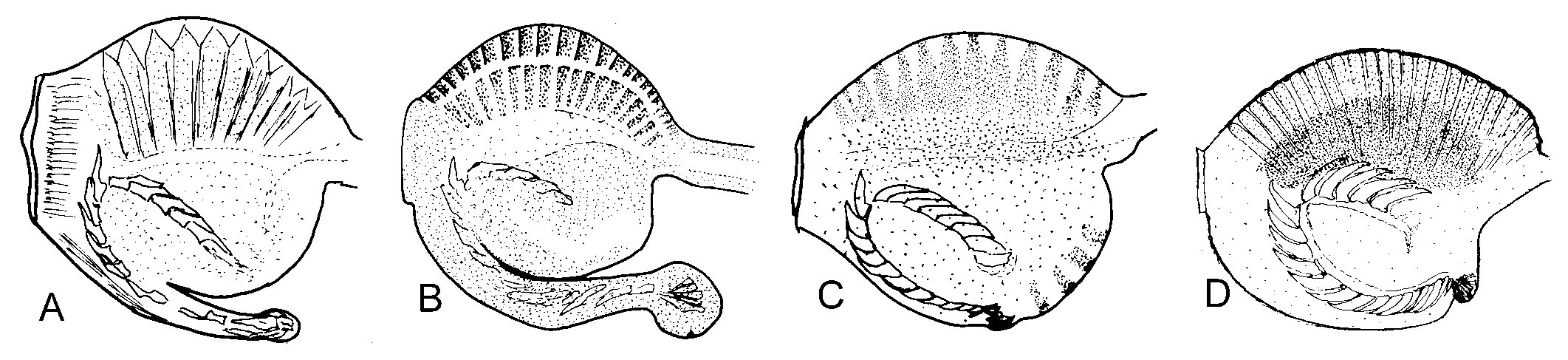
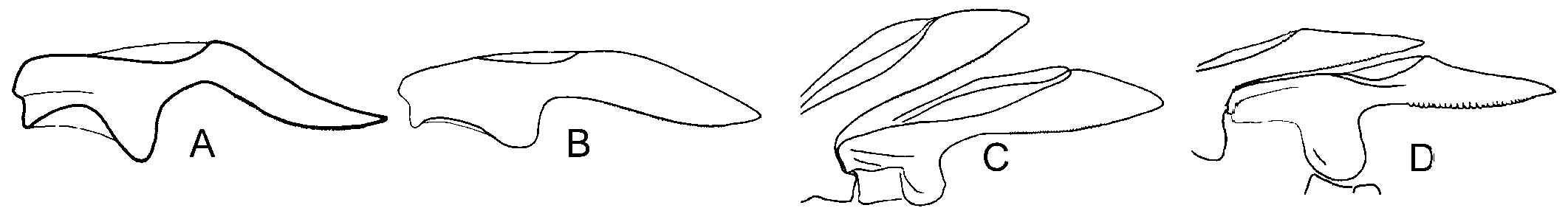
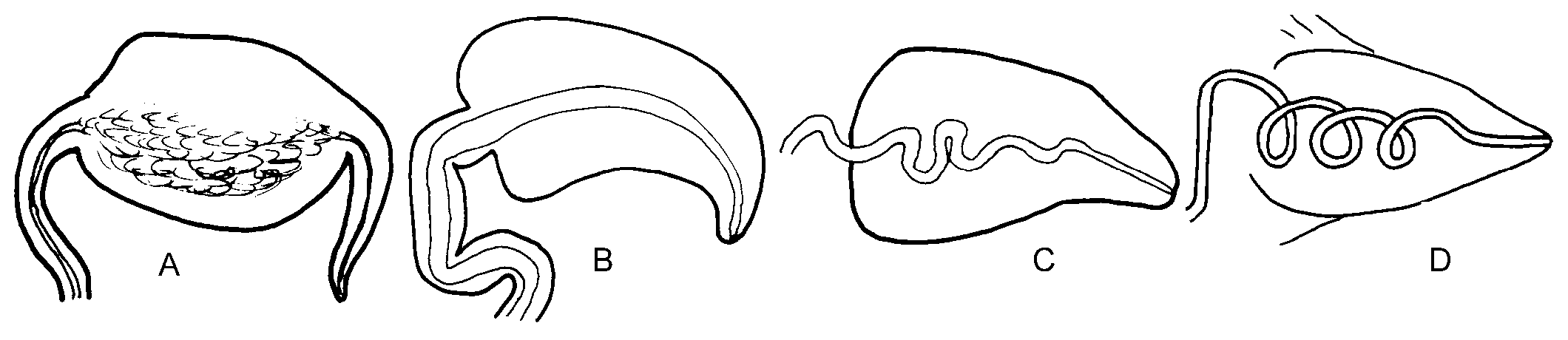
Internal characteristics. – Pharynx small, length about 400 µm, dark pigment band over sides, ascus vestigial ( Fig. 3C View Fig ). Radula with 7 to 9 teeth in ascending limb and same number in descending limbs. Teeth blade-shaped, more than 80 very fine denticles along cutting edge, tooth length in specimens of 23 to 49 mm length alive similar, about 67 µm ( Fig. 4C View Fig ). Oesophagus narrow, length about 2.5 times length of pharynx. Stomach wide, green, glandular tissue of digestive gland starts in stomach. Intestine pale, wide, runs upward from anterior part stomach ( Fig. 5C View Fig ). Penis unarmed, conical ( Fig. 6C View Fig ). Male follicles more central, larger and less numerous than female follicles.
Biology. – Copulation by lying with the right side of the frontal part of the body against the partner. Penis goes into the underside of the right parapodium. At the end they may form a ball when penises withdraw. Egg strings without extra-capsular yolk, up to 720 mm long, irregularly coiled over substrate; in captivity irregular spiral on glass.
During low tide, when there is no water current, the slugs were often found in dense aggregations resembling fallen leaves on bare mud in shallow, water-filled depressions or gullies in mangrove forests. Depression can be as small as a footprint of a water buffalo or human. When a sudden movement in the water occurred (e.g., a foot step), the slugs closed their parapodia and become detached from the floor. Their feet clearly did not strongly adhere to any substrate. The slugs rolled around across the substrate usually to slightly deeper parts from where they can be easily swept out by another foot step. I was unable to determine what happened when the tide moved in. The movements of the tidal currents are strong and longer lasting than a step; I would expect that they be dislodged from the substrate. However, when the flood came in, the water turbidity was high due to the heavy load of mud particles and visibility immediately decreased to zero. Although the slugs are likely dislocated by the tidal currents, I have no idea how they could select depressions that contain sufficient water for survival during the next low tide.
When discovering E. bangtawaensis in March 1997, I had not dealt with sacoglossans since 1959 ( Swennen, 1961) and had missed the developments in knowledge since then. Therefore, I wondered what was the meaning of their behaviour and what was their food, because there were no visible algae in the shallow pools with a soft mud bottom. Specimens were put in trays in the laboratory with the local mud and seawater. They soon became immobile and stretched out their parapodia as seen in the field. When undisturbed, they kept the parapodia expanded also during the night. Closing the parapodia over the body occurred when the tray was touched, but also when exposed to direct sunlight that even stimulated them to move to a shaded position such as below other specimens ( Swennen, 1998). This made it unlikely that resembling a leaf was procryptic. Feeding trials with algae from outside the forest had no success.
I noticed during a subsequent visit that tiny green algae were present on the dry mud surface between mangrove roots above mean high tide level. Mud and algae could not be separated and were placed in-situ in the trays with the slugs. This time, the slugs were attracted by the algae and appeared to feed on them. At my request, Mr. M. Lavaleye (Royal Netherlands Institute for Sea Research, Texel) conducted phytopigment analyses of an E. bangtawaensis and the algae. That showed that the spectra of both organisms were almost identical. Mr. K. A. Sjollema (Laboratory for Electron Microscopy, University of Groningen) made images of a piece of slug tissue and the alga. They showed that in the cells of the digestive gland of E. bangtawaensis several chloroplasts occurred that were similar to the ones in the algae. Then, Dr. W. Stolte (Royal Netherlands Institute for Sea Research) placed a live slug in a respirometer and measured the oxygen from incoming and outgoing seawater. The specimen had a length of 3.4 cm, and had no contact with any algae for 20 days. The space was too small for allowing the slug to fully expand its parapodia in the measuring chamber, but still the measurements in various light conditions showed a gross photosynthesis of 4.2 micromole O 2 /h and a respiration of 1.3 micromole O 2 /h illustrating that E. bangtawaensis can indeed act as a plant with real photosynthesis taking place in dimmed light (117 micromole photons/m 2 /s) producing more oxygen than used. This is known under such names as chloroplast retention, kleptoplasty, chloroplast symbiosis, solar-powered slugs. Further investigations were stopped due to lack of time and funding.
Discussion. – Specimens from India, Malaysia, Thailand and Australia show the same colour pattern.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |