Asphondylia monacha, OSTEN SACKEN, 1869
|
publication ID |
https://doi.org/ 10.1111/zoj.12234 |
|
persistent identifier |
https://treatment.plazi.org/id/81198784-FF8F-FFED-92ED-8E1AF932FDC2 |
|
treatment provided by |
Felipe |
|
scientific name |
Asphondylia monacha |
| status |
|
ASPHONDYLIA MONACHA OSTEN SACKEN, 1869 View in CoL
Asphondylia monacha Osten Sacken, 1869: 299 View in CoL .
Hosts plants
Solidago juncea , S. erecta , S. uliginosa (summer generation), and S. altissima (spring generation).
Gall and biology
This species has two generations a year that induce distinct bud galls on different Solidago species. The early-spring generation was found only on S. altissima in April–May. Galls were discovered accidentally in early April while digging out rhizomes, as they developed in buds that grew from the rhizomes and were barely visible above ground ( Fig. 1 View Figures 1–6 ). Galled buds were wider and felt harder to the touch than normal buds, were 5 cm long and 2 cm wide, and contained a single chamber, the internal walls of which were lined by a thick layer of white mycelium. Each gall contained a single larva or pupa. In May, some galls were found in much longer sprouts (∼ 15 cm long) that still appeared stunted and somewhat thicker than normal sprouts ( Fig. 2 View Figures 1–6 ). The larval chamber in these galls was situated at the very tip of the shoot. Adults of the spring generation emerged in May. The much more conspicuous summer-generation gall of this species on S. juncea (the host was incorrectly identified as S. canadensis in the original description) is a rosette bud gall that is found in great numbers ( Fig. 3 View Figures 1–6 ). The galls become apparent in mid-June and reach their final size while the larvae inside them are still tiny first instars. They are usually composed of 15–30 individual units, each with a single larval chamber that is surrounded by shortened leaves and lined internally by white mycelium. These units form a spherical structure on shoot tips that is 4–7 cm in diameter and can be spotted from a distance. Adults of the summer generation emerged in late August to mid-September. Although it was not observed, we assume that adults of the autumn generation lay their eggs in plant tissue close to the ground and the hatching first-instar larvae overwinter next to dormant buds in the rhizomes. Old galls turn black and may remain on dry shoots of S. juncea throughout winter and into the next spring. Galls of similar structure were found on S. erecta ( Fig. 4 View Figures 1–6 ) and S. uliginosa , and our molecular analysis indicates that they are all induced by A. monacha . No morphological differences were found among adults from these three host plants. Asphondylia monacha galls superficially resemble those of Rhopalomyia solidaginis on S. altissima , but they are not as wide and flat as the Rhopalomyia galls, are never found on S. altissima , and their structure is different, as indicated by Osten Sacken (1869) in his original description of A. monacha .
Adult
General colour black.
Head: Eye facets round. Palpus three-segmented, segments successively longer, with several strong setae and otherwise covered by microtrichia. Labella slightly pointed, with numerous strong setae on lateral surface.
Antenna: Scape and pedicel with long, dark setae. Male flagellomeres cylindrical, flagellomere 1 slightly longer than succeeding flagellomere, apical flagellomere slightly shorter than preceding flagellomere, all covered by anastomosing loops of circumfila, numerous strong setae, and microtrichia ( Fig. 23 View Figures 23–27 ); flagellomere 1/ flagellomere 5 ratio = 1.13–1.34 (N = 23). Female flagellomeres 1–9 cylindrical with well-developed circumfila, with two transverse connections, numerous strong setae, and otherwise covered by microtrichia ( Fig. 24 View Figures 23–27 ); flagellomere 1 conspicuously longer than succeeding flagellomere, flagellomere 1/flagellomere 5 ratio = 1.41–1.62 (N = 38); flagellomeres 7 and onwards successively shorter; flagellomeres 10–12 with two whorls of circumfila and several longitudinal connections, numerous strong setae, and otherwise covered by microtrichia ( Fig. 25 View Figures 23–27 ); flagellomere 10 slightly longer than wide; flagellomere 11 slightly wider than long; flagellomere 12 rudimentary.
Thorax: Legs: densely covered by black scales other than a patch of white scales from apical part of femur to base of tibia, and from base of tarsomere 1 to first third of tarsomere 2; ventral part with silvery hairlike scales, coxae with long black setae. Tarsal claws thick, evenly curved; empodia longer than bend in claw. Wing: dark grey, densely covered by dark hair-like microtrichia ( Fig. 18 View Figures 15–22 ); length 2.00– 2.80 mm in males (N = 41) and 2.01–3.30 mm in females (N = 57) of summer generation, 2.91–3.11 mm in males (N = 2) and 3.29–3.59 mm in females (N = 5) of spring generation; R1 joins C proximal to mid-length of wing, R5 joins C posterior to wing apex, M weak, CuA forked into CuA1 and CuA2.
Female abdomen ( Fig. 26 View Figures 23–27 ): Dorsum covered by black scales, pleuron and venter with silvery hair-like scales. Tergites 1–7 rectangular, with posterior one or two rows of strong setae and otherwise evenly covered by scales; tergite 8 narrower than preceding tergite, saddlelike, without setae. Sternites 2–6 with posterior row of setae and several setae on mid part; sternite 7 much longer than preceding sternite, narrowed posteriorly, with group of strong setae on posterior half. Ovipositor relatively long: sclerotized part 1.96–3.04 times as long as sternite 7 (N = 55) in summer-generation females, 2.62–3.25 times as long in spring-generation females (N = 6).
Male abdomen ( Fig. 27 View Figures 23–27 ): Colour pattern as in female. Tergite 1 narrow, band-like, without setae; tergites 2–7 rectangular, with posterior row of strong setae, few setae on basal area, and evenly scattered scales; tergite 7 more setose than preceding tergite; tergite 8 narrow, band-like, without setae. Sternites 2–6 rectangular, with posterior row of strong setae and several strong setae medially, otherwise evenly covered by scales. Sternite 7 more setose than preceding sternite. Sternite 8 with small but strongly setose sclerotized area.
Male terminalia ( Figs 28–30 View Figures 28–30 ): Gonocoxite compact, wide, and short, with short apical projection extending medially; bearing numerous strong setae and evenly setulose. Gonocoxal apodeme extending on both sides of aedeagus to form complex, strongly sclerotized structure ( Figs 28, 30 View Figures 28–30 ). Gonostylus round–ovoid, with numerous strong setae and otherwise evenly setulose, bearing crescent-shaped apical tooth. Aedeagus wide at base, tapered towards rounded apex, curved anteriorly in lateral view ( Fig. 30 View Figures 28–30 ). Hypoproct wide at base, deeply divided into two lobes apically, setose and setulose, with two longer setae apically on each lobe. Cerci completely or almost completely separated, bulbous, strongly setose and setulose throughout.
Larva (third instar) ( Fig. 31 View Figures 31–37 )
Orange; integument covered by spicules. Length 2.06– 2.84 mm (N = 6). Antennae about 1.5 times as long as wide; cephalic apodeme as long as head capsule. Spatula shape variable ( Figs 32–37 View Figures 31–37 ): lateral teeth slightly or conspicuously longer and more pointed than median teeth, gap between median teeth slightly or clearly deeper than gaps between lateral and median teeth, shaft thick and well-sclerotized in summer-generation larvae ( Figs 35–37 View Figures 31–37 ), thinner and less sclerotized in spring-generation larvae ( Figs 32–34 View Figures 31–37 ).
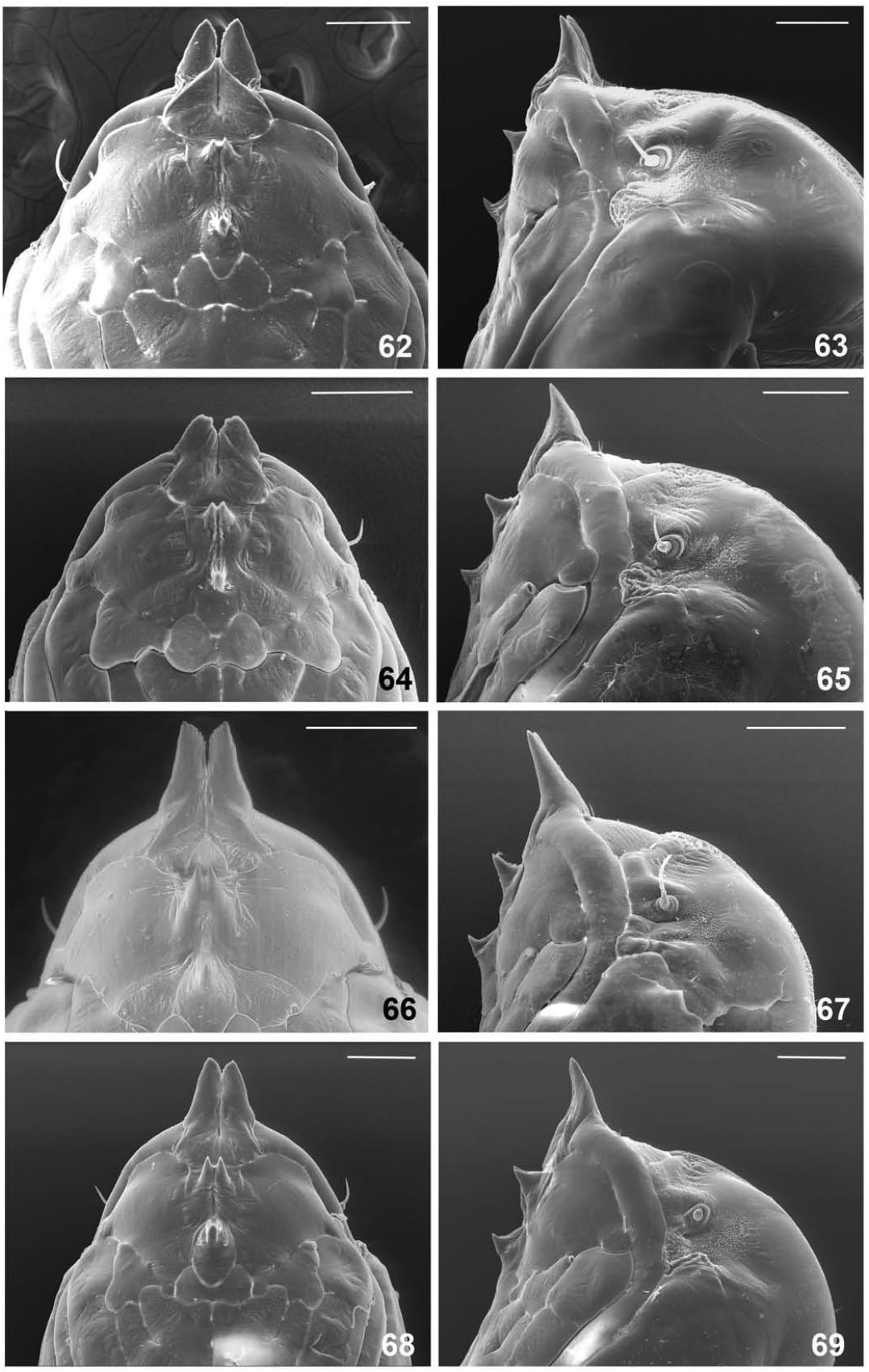
Pupa ( Figs 62–65 View Figures 62–69 )
The pupae of the summer and spring generations differ from each other in the shape of the antennal horns. In summer-generation pupae antennal horns are robust, wide at base, slightly arched ( Fig. 65 View Figures 62–69 ), with apices flat and finely serrated in frontal view ( Fig. 64 View Figures 62–69 ); in springgeneration pupae antennal horns are longer, more slender ( Fig. 63 View Figures 62–69 ), and are tapered at apex in frontal view ( Fig. 62 View Figures 62–69 ). Other attributes are similar in pupae of both generations, as follows. Cephalic seta minute. Upper facial horn divided into two apices separated by shallow, curved notch. Lower facial horn curved dorsally at apex, on each side with two papillae, one bearing a relatively long seta. Frons on each side with three lateral papillae: one setose and two asetose. Prothoracic spiracle long and slender, with widened base; trachea ends at apex. Abdominal segments, except for first, each with posterior straight row and two or three anterior less ordered rows of spikes.
Notes
We could not find any substantial morphological differences among populations from S. juncea , S. erecta , and S. uliginosa , and our molecular analysis indicates that all belong to A. monacha . The galls on S. uliginosa are somewhat smaller than those on the other two host plants, and were also found in lateral buds, whereas galls on S. juncea and S. erecta almost always develop in apical buds. Adults reared from galls on S. uliginosa were likewise smaller than those from the two other hosts. Additional molecular work on the S. uliginosa population may show that it represents a separate species.
Adults of the spring generation that develop on S. altissima are clearly bigger than those of the summer generation. Based on the collection date, one individual from the Felt collection represents the spring generation of A. monacha , but the host from which it was reared is not indicated. It is possible that the spring generation of A. monacha induces galls on other Solidago species in areas where S. altissima is uncommon, but such galls have not been found in the present study. Aggregated bud galls that are very similar to those of A. monacha are also found on S. sempervirens ( Fig. 5 View Figures 1–6 ) and S. bicolor ( Fig. 6 View Figures 1–6 ), and although we could not find morphological differences between A. monacha and individuals from these populations, our molecular analysis indicates that the latter belong to one or more undescribed species. There are additional Solidago species on which similar composite galls have been observed, and further molecular study will probably be necessary to determine whether they belong to A. monacha or to undescribed species. Felt (1908, 1916) attributed rosette and inflorescence galls on Euthamia ‘ lanceolata ’, as well as leaf galls on S. gigantea or S. Canadensis , to A. monacha , but these galls belong to different species discussed in the present paper.
Material examined
Spring generation (from S. altissima bud galls): 4♀, 1 exuviae, 3 larvae, USA, PA, Route 642 (40°59.114′N 76°38.567′W), 28 April 2005, N. Dorchin GoogleMaps ; 3♀, 2 exuviae, USA, PA, Montour Environmental Preserve , 25 May 2007, N. Dorchin ; 1 larva, USA, PA, Bucknell University Chillisquaque Creek Natural Area , 1 May 2005, N. Dorchin ; 1♂, 2 exuviae, USA, PA, Bucknell University Chillisquaque Creek Natural Area , 13 May 2005, N. Dorchin ; 5 pupae, USA, PA, multiple localities, May 2005 –2007, N. Dorchin (on SEM stubs) .
Material from Felt collection with no indication of host: 1♀, USA, NJ , Orange Mountain , May 1907? (Felt no. 813; USNM) .
From S. juncea: Gall (syntype), USA, NY, near Brooklyn , 1867 ( USNM) ; 2 larvae, USA, MD , Wheaton Park , 22 August 1976, R . J. Gagné ( USNM) ; 1 larva, USA, PA , Pittsburgh , 13 August 1991, J. Plakidas ( USNM) ; 1♂, USA, PA , Warrendale , 30 August 1991, J. Plakidas ( USNM) ; 1♂, 1♀, 3 larvae, USA, PA , Lewisburg , 22 August 2005, N. Dorchin ; 1♂, 2♀, USA, PA , Route 642 (40°59.114′N 76°38.567′W), 16 Spetember 2005, N. Dorchin GoogleMaps ; 1♂, USA, PA , Mauses Creek , 16 September 2005, N. Dorchin ; 3♂, 12♀, 7 exuviae, USA, PA , Lewisburg , 23 August 2007, N. Dorchin ; 1♂, USA, PA , Lewisburg , 30 August 2007, N. Dorchin ; 12♂, 11♀, USA, VA , Bedford , Sharp-top, 15 Spetember 2012, M.J. Wise ; 9 pupae, USA, PA , multiple localities, August– September 2005 –2007, N. Dorchin (on SEM stubs).
From S. erecta : 4♂, 3♀, 2 exuviae, 1 larva, USA, VA, Roanoke , 28 August 2010, N. Dorchin and M.J. Wise ; 3♂, 5♀, USA, VA, Roanoke, Spetember 2010, M.J. Wise ; 3♂, 9♀, USA, VA, Roanoke , Havens, 5 September 2012, M.J. Wise ; 3♂, 3♀, USA, VA, Roanoke , Forest Acre Trail, 9 September 2012, M.J. Wise ; 12 pupae, USA, VA, Roanoke , August–September 2010, 2012, M.J. Wise (on SEM stubs) .
From S. uliginosa : 7♂, 6♀, USA, ME , Winter Harbor, 7 September 2007, R . J. Gagné ( USNM) .
Material from Felt collection with no indication of host and collector (recognized by Felt as A. monacha ; all in
USNM): 1♀ , USA, NY, Albany , 11 June 1906 (Felt no. 208); 1♀ , USA, NY, Albany , 20 July 1906 (Felt no. 650a); 1♀, 1♂ , USA, NY, Karner, 5 August 1906 (Felt no. 1583); 1 exuviae , USA, NY, Albany , 4 September 1906 (Felt no. 1200); 1♀ , USA, NY, Albany , 21 August 1906 (Felt no. 761); 1♂ , USA, NY, Nassau , 17 September 1906 (Felt no. 1336); 1♀ , USA NY, Albany , 20 July 1907 (Felt no. 1568a); 1♂ , USA, NY, Bath , 24 July 1907 (Felt no. 1568a); 1 larva , USA, NY, Albany , 24 July 1907 (Felt no. 1583); 1♀ , USA, NY, Bath , 16 July 1907 (Felt no. 1568a); 1♂ , USA, NY, Nassau , 7 August 1907 (Felt no. 1583a); 1♀ , 1 exuviae, USA NY, Bath , 18 July 1907 (Felt no. 1268); 1♂ , USA, MA, Magnolia, 11 August 1908 (Felt no. 1879) .
| PA |
Universidade Federal do Oeste do Pará |
| NJ |
Njala University College |
| USNM |
Smithsonian Institution, National Museum of Natural History |
| MD |
Museum Donaueschingen |
| R |
Departamento de Geologia, Universidad de Chile |
| VA |
University of Virginia |
| NY |
William and Lynda Steere Herbarium of the New York Botanical Garden |
| MA |
Real Jardín Botánico |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Asphondylia monacha
| Dorchin, Netta, Joy, Jeffrey B., Hilke, Lukas K., Wise, Michael J. & Abrahamson, Warren G. 2015 |
Asphondylia monacha
| Osten Sacken CR 1869: 299 |