Prosadenoporus floridensis, Maslakova & Norenburg, 2008
|
publication ID |
https://doi.org/ 10.1080/00222930802130286 |
|
persistent identifier |
https://treatment.plazi.org/id/816E8F49-220F-FFAB-329A-FC1DFB9A238C |
|
treatment provided by |
Carolina |
|
scientific name |
Prosadenoporus floridensis |
| status |
sp. nov. |
Prosadenoporus floridensis , new species
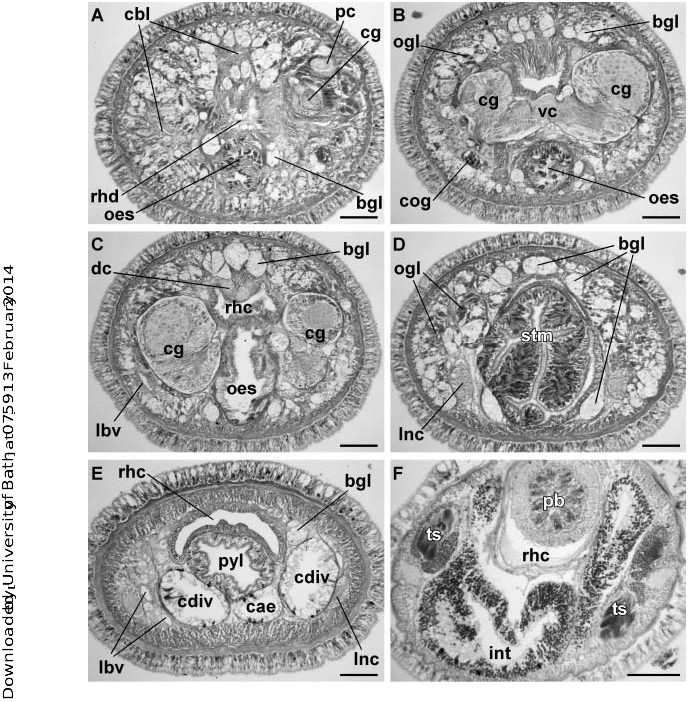
( Figures 1A View Figure 1 , 2A, 2B View Figure 2 , 3–6 View Figure 3 View Figure 4 and 10I View Figure 10 ; Tables 1–4)
Etymology
The species is named after Florida, its type locality .
Type material
Type specimens are deposited at the US National Museum of Natural History ( NMNH), Smithsonian Institution, Washington, DC , USA. The holotype – mature female USNM 1098692 About USNM consists of two series of transverse sections of anterior and posterior body regions in addition to unsectioned proboscis and a fragment of midbody in 70% ethanol (coll. JLN, May 1992, type locality in FL, USA). Paratypes – mature male USNM 1098693 About USNM (two series of transverse sections of anterior and posterior, coll. JLN, May 1992, Link Port , FL, USA) and mature female USNM 1098696 About USNM (a series of longitudinal frontal sections of the anterior and a series of transverse sections of the posterior, coll. C. Glasby, 1986, Twin Cays, Belize) . Additional voucher specimens (sectioned and unsectioned) are stored at the NMNH and assigned the following catalog numbers USNM 1098701 About USNM , 1098702 About USNM , 1098713 About USNM , 1098714 About USNM , 1014302–1014306 About USNM , 1098694 About USNM , 1098695 About USNM , 1098697–10987000 About USNM , 1098703– 1098712 About USNM .
Material examined
Holotype and paratypes USNM 1098692 About USNM , 1098693 About USNM and 1098696. Sections of voucher specimens from Belize and Florida USNM 1098701 About USNM , 1098702 About USNM , 1098713 About USNM , 1098714 About USNM , 1098697–109869700 About USNM , 1098704 About USNM , 1098706 About USNM , 1098708–1098710 About USNM and 1098712 .
Diagnosis
Prosadenoporus floridensis sp. nov. is not known to possess any morphological apomorphies. It differs from P. arenarius in being gonochoric and from P. agricola in being gonochoric and oviparous. It differs from P. winsori by paler colouration (compare Figures 1A and 1B View Figure 1 ) and by lacking neurochords ( Figures 4G View Figure 4 and 7J View Figure 7 ). It differs from both P. winsori and P. fujianensis by having but a single pair of accessory stylet pouches ( Table 3), and from P. mooreae and P. mortoni by lacking characteristic greenish striped colouration (compare Figures 1A and 1C View Figure 1 ) and by having a truncated basis of central stylet ( Figures 10A, 10B and 10I View Figure 10 ). It differs from P. enalios by having neurochord cells ( Figures 5E and 5F) and fewer proboscis nerves (11–14 compared with 14–16), and from P. spectaculum by lacking a distinct spectacle head colour pattern and by having 11–14 proboscis nerves (compared with 22–24). Two accessory stylet pouches. Central stylet (S) 92–165 mm long, average 5130.7 mm, basis (B) truncated 140–372 mm long, 247.9 mm average, S:B ratio 0.39–0.7, average50.55. All three stylet metrics are significantly different from those of P. winsori (p 50.05) and the S:B ratio is significantly different from that of P. mooreae (p 50.05). Data for other species are insufficient to make the statistical comparisons. The average sequence divergence between P. floridensis and other Prosadenoporus species is 9.15% (16S) and 10.65% (COI) ( Table 4).
Type locality, habitat and distribution
Prosadenoporus floridensis is common underneath and on the under surface of large rocks partially embedded in coarse moist sand in the supra-littoral zone in the type locality, locally known as Link Port, near Harbor Branch Oceanographic Institution in Fort Pierce , Florida, USA on the shore of the Indian River Lagoon (27 ° 3296.180N, 80 ° 20947.140W). Nemerteans were found from the top of oligochaete zone up into wrack zone (a vertical distance of about 20 cm). Additional specimens were collected from Twin Cays, Belize (16 ° 49927.930N, 88 ° 6912.630W) from a similar habitat, underneath large logs embedded in moist sand in the supra-littoral zone at the edge of mangroves .
Description
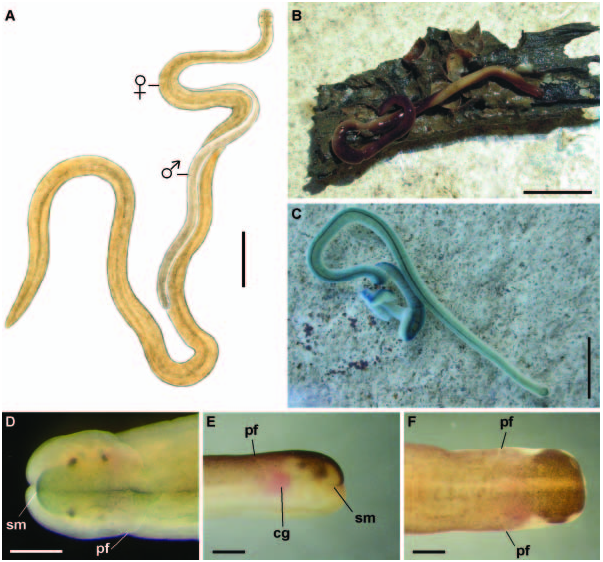
External appearance. Prosadenoporus floridensis is a relatively large species with maximum recorded length of reproductive specimens 70–75 mm and width up to 1.2 mm. Females tend to be larger than males. The colour in life ranges from light yellowish, orange-brown to olive tan or greenish-brown dorsally and from off-white to deeper yellowish-cream ventrally. The colour is darkest in the dorsal midline, sometimes creating an appearance of a very faint median stripe ( Figure 1A View Figure 1 ). There is a rather sharp lateral transition from darker dorsal to paler ventral colouration. The body is broadest in the middle part, slightly narrower at the anterior and gradually tapering toward the posterior to end in a bluntly rounded tip. The dorsal surface is vaulted, so that the body is oval to round in cross-section, similar to other semiterrestrial members of the genus – for example, P. winsori .
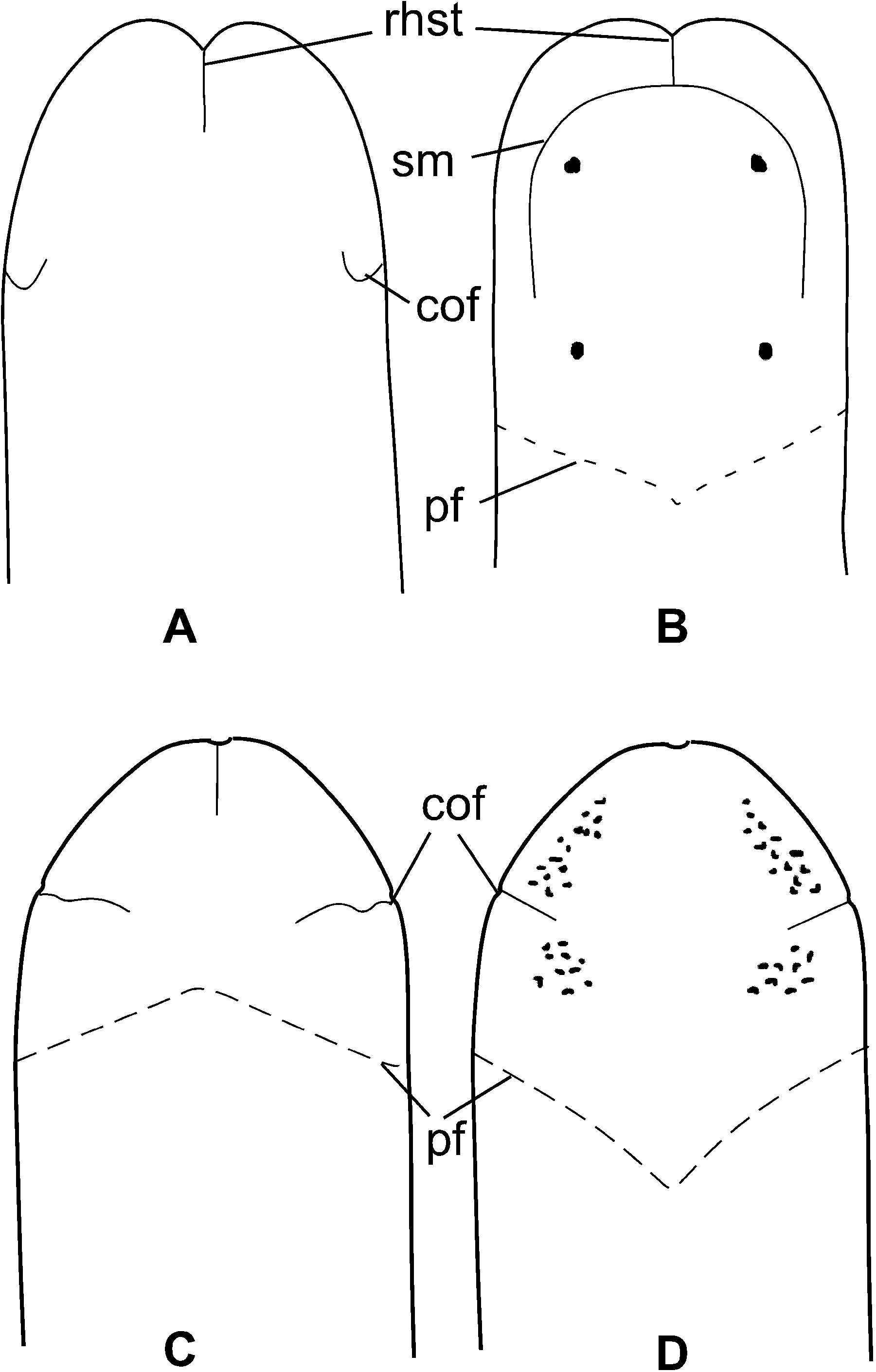
The head is blunt with a characteristic vertical anterior notch giving it a somewhat bilobed appearance, otherwise not demarcated from the body ( Figures 2A and 2B View Figure 2 ). A dorsal horizontal epidermal fold anterior to the eyes separates two ventral apical lobes from a median dorsal lobe, forming the characteristic prosorhochmid ‘‘smile’’ ( Figure 2B View Figure 2 ). The four large dark-brown to black eyes are situated at the anterior margin of the brain in a rectangle; the anterior pair is slightly larger than the posterior. The distance between the eyes of the anterior pair and those of the posterior pair is about the same as the distance between the two pairs ( Figure 2B View Figure 2 ). The rudimentary cerebral organ furrows appear as a pair of inconspicuous lateroventral, whitish, semi-circular grooves between the anterior and posterior pairs of eyes; these are not visible from the dorsal side ( Figures 2A and B View Figure 2 ). The faint posterior cephalic furrow forms a shallow, dorsal, posteriorly directed ‘‘V’’ immediately behind the brain ( Figure 2B View Figure 2 ). The rhynchostome opening is subterminal.
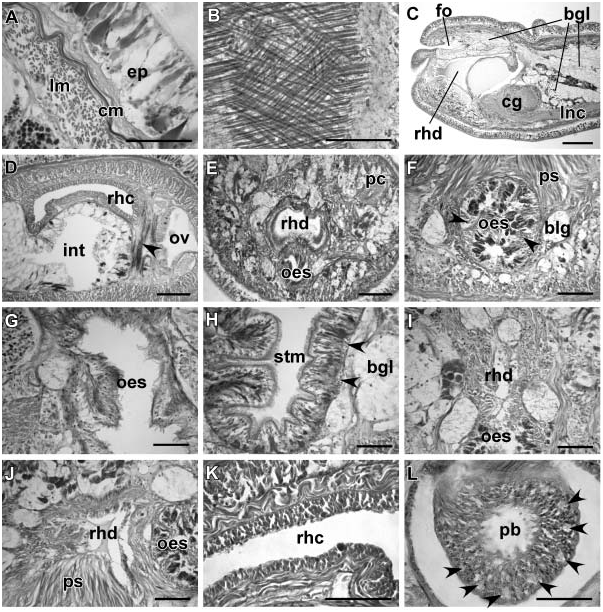
Body wall, musculature and parenchyma. Epidermis is of typical hoplonemertean structure ( Figure 3A View Figure 3 ). Dermis is represented by a thin layer of extracellular matrix. Body-wall musculature consists of an outer circular layer and an inner longitudinal layer. Diagonal (oblique) muscle fibres situated between the circular and longitudinal musculature of the body wall form a very delicate but distinct layer best visualized in longitudinal tangential sections ( Figure 3B View Figure 3 ).
The proboscis insertion comprises mostly longitudinal and oblique muscle fibres, which form a more or less separate inner layer of longitudinal muscles that joins the outer longitudinal muscle layer behind the brain. A radial component of the proboscis insertion is weakly developed or absent. In other words, precerebral septum is not well defined and the longitudinal musculature is anteriorly split into an inner layer composed of proboscis insertion muscles and outer layer – the body-wall longitudinal muscles proper ( Figures 3C View Figure 3 and 4I View Figure 4 ). Individual fibres from the inner portion of the longitudinal musculature continue into the head as cephalic retractors.
Dorso-ventral muscles are well developed ( Figure 3D View Figure 3 ). Isolated muscle fibres oriented dorso-ventrally, obliquely and horizontally are found in the precerebral region ( Figure 3E View Figure 3 ). The longitudinal muscle fibres surrounding rhynchodeum continue posteriorly as an almost continuous thin sheet of muscles surrounding foregut ( Figure 3F View Figure 3 ); these are often referred to as the ‘‘splanchnic musculature’’. At the brain level the foregut muscles are reinforced by longitudinal muscle fibres originating at the proboscis insertion. Foregut or splanchnic muscles continue as a delicate, sometimes indistinct layer of fibres surrounding the stomach ( Figure 3H View Figure 3 ). The amorphous extracellular matrix, the so-called ‘‘parenchyma’’, is very sparse but is otherwise unremarkable.
Proboscis apparatus. The proboscis pore opens at the tip of the head ( Figures 2A and 2B View Figure 2 ) and leads into a short, thin-walled rhynchodeum lined with squamous epithelium. It was not possible with light microscopy to determine whether rhynchodeal epithelial cells are ciliated. The rhynchodeal musculature comprises a delicate layer of longitudinal and circular muscle fibres. There is no localized concentration of circular muscle fibers representing a distinct rhynchodeal sphincter. The rhynchocoel reaches 75–100% of body length. Its wall is of typical distromatonemertean ( Thollesson and Norenburg 2003) structure and contains separate outer circular and inner longitudinal muscle layers ( Figure 3K View Figure 3 ). The thickness of the layers changes dramatically with the state of contraction of the animal.
The proboscis is thin, longer than the body, cream-coloured, and used in escape reaction. Anteriorly the proboscis wall consists of a non-glandular epithelium, a thin layer of circular muscles and a thick layer of longitudinal muscles supplied with proboscis nerves ( Figures 3L View Figure 3 and 4A View Figure 4 ). Further posteriad, the wall of the anterior chamber is composed of a tall glandular epithelium, arranged into conical papillae, a thin layer of extracellular matrix, an outer circular muscle layer, a longitudinal muscle layer divided into two concentric layers by the neural sheath, a delicate layer of inner circular muscles, and a thin endothelium ( Figures 4B and 4C View Figure 4 ). The neural sheath bears 11–14 proboscis nerves ( Figure 4B View Figure 4 ).
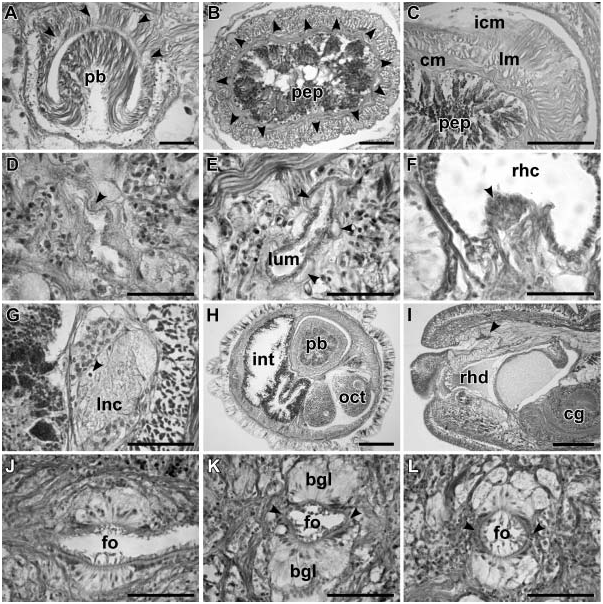
The proboscis armature consists of a central stylet, mounted on a characteristically truncated basis ( Figure 10I View Figure 10 ) and two pouches each containing 2 or 3 accessory stylets. The length of the central stylet (S) varies from 98 to 165 mm, average 5130.7 mm; basis length (B) ranges from 140 to 372 mm, average 5247.9 mm (n 513). S:B ratio varies from 0.39 to 0.7, average50.55 ( Tables 2–3). The wall of the posterior chamber of the proboscis consists of strongly basophilic glandular epithelium organized into papillae, outer longitudinal muscle layer, thin inner circular muscle layer, and a delicate endothelium.
Alimentary canal. The oesophagus opens into the rhynchodeum in front of the proboscis insertion ( Figure 3E View Figure 3 ). It is enclosed by longitudinal muscle fibres, which are confluent with the rhynchodeal musculature and continue posteriorly as the musculature of the stomach ( Figures 3F and 3H View Figure 3 ). As in all other species of Prosadenoporus , the anterior portion of oesophageal epithelium is richly supplied with finely granular acidophilic gland cells, staining deep red with Crandall’s trichrome technique ( Figures 3F, 3J View Figure 3 , 5J, 6A and 6B View Figure 6 ). Posteriorly the oesophageal epithelium is ciliated and conspicuously lacks acidophilic or basophilic glands ( Figures 3G View Figure 3 and 6C View Figure 6 ). This part sometimes is referred to in the literature as the anterior stomach (see, for example, Moore and Gibson 1981, p. 184); however, it more resembles oesophagus in morphology.
The stomach is of typical hoplonemertean structure with densely ciliated, deeply folded epithelium, containing numerous basophilic and acidophilic glands ( Figures 3H View Figure 3 and 6D View Figure 6 ). The intestinal caecum is well developed, anteriorly bifid and bears numerous lateral diverticula throughout its length ( Figure 6E View Figure 6 ). The anterior caecal diverticula reach the posterior portion of the dorsal cerebral ganglia. Intestinal diverticula are lobed.
Blood system. The blood system comprises paired lateral and an unpaired mid-dorsal blood vessel, joined precerebrally via a suprarhynchodeal loop and posteriorly via a supra-anal loop. As is characteristic of Prosadenoporus , the suprarhynchodeal (cephalic) loop in P. floridensis sp. nov. is recurved: i.e., the lateral blood vessels run forward into the tip of the head before curving up and backward to anastomose in the midline just behind the posterior chamber of the frontal organ. The mid-dorsal blood vessel originates near the ventral cerebral commissure from the right cephalic vessel (traced in two specimens) and immediately penetrates the rhynchocoel floor to form a single vascular plug. The wall of the vascular plug consists of thickened endothelium of the blood vessel, a thin layer of extracellular matrix and a modified rhynchocoel endothelium ( Figure 4F View Figure 4 ). No transverse connectives linking mid-dorsal and lateral blood vessels in the intestinal region were found by us. The blood vessels are thin-walled with few ‘‘valves’’ and ‘‘pouches’’ ( Figures 4D and 4E View Figure 4 ).
Nervous system. As in other nemerteans, the brain consists of two ventral and two dorsal ganglia, joined by ventral (subrhynchocoelic) and dorsal (suprarhynchocoelic) commissures, respectively ( Figures 3C View Figure 3 , 4I View Figure 4 , 5E, 6B and 6C View Figure 6 ). The dorsal ganglia are more widely separated than the ventral. A thin, but distinct outer neurilemma encloses the brain as a whole, but there is no inner neurilemma dividing the fibrous and ganglionic tissues. Notably, there is a pair of large and conspicuous neurochord cells close to the inner side of the ventral cerebral ganglia in vicinity of the ventral cerebral commissure ( Figures 5E and 5F). However, no neurochords are found in the lateral nerve cords ( Figure 4G View Figure 4 ).
The lateral nerve cords contain a single fibrous core throughout their length, i.e. there are no accessory nerve cords. As observed in most monostiliferans studied in the last three decades, each lateral nerve cord contains a single delicate muscle bundle, consisting of 3–7 fibres and running within or adjacent to the fibrous core, near its dorsal border. In addition, there are several less conspicuous muscle fibres running along the inner lateral side of the fibrous core ( Figure 4G View Figure 4 ). Muscle fibres associated with the lateral nerve cords can be traced to their origin near the proboscis insertion. Numerous cephalic nerves lead anteriorly from the brain lobes to supply various structures of the head. A pair of stout nerves originating from the ventral brain lobes supplies the cerebral sensory organs. Paired proboscis nerve trunks originate from the ventral brain lobes near the ventral cerebral commissure and branch before entering the proboscis ( Figure 4A View Figure 4 ).
Frontal organ. The well-developed frontal organ opens at the tip of the head. Its elongated ciliated canal, about 200–400 mm long, extends about half way to brain ( Figures 3C View Figure 3 and 4I View Figure 4 ) and is lined by regionally differentiated epithelium. The ventral and dorsal walls as well as the posterior extremity of the canal, through which the basophilic mucus cephalic glands discharge, have a vacuolated appearance and bear long, sparsely distributed cilia. The lateral walls of the canal, sometimes bearing a more or less distinct groove, comprise strongly acidophilic epithelium clad in short densely arranged cilia ( Figures 4L View Figure 4 and 5 G–I). The acidophilic appearance results from the densely arranged elongated nuclei of the ciliated cells. Unlike some other species of the genus, such as P. winsori , P. mooreae and P. spectaculum , the frontal organ canal does not exhibit any noticeable ‘‘twisting’’ (compare Figures 4 View Figure 4 J–L, 5G–I to 7A–C, 7F–I, 8A–D and 9C–F).
Cephalic glands. Cephalic glands are extremely well developed. As in other Prosadenoporus , they include three types of cells: basophilic lobules with vacuolated appearance (mucus glands), coarsely granular proteinaceous gland cells staining golden-yellow to brown with Mallory trichrome or orange with Crandall’s method (orange-G glands), and finely granular proteinaceous acidophilic cells, staining red with Mallory or Crandall’s technique (acidophilic or red glands).
Mucus glands open into the frontal organ ( Figures 3C View Figure 3 and 4I View Figure 4 ), as well as through the epidermis via multiple improvised ducts. Mucus glands are found precerebrally around the frontal organ: organized into more compact lobes dorsally and ventrally and interspersed with the orange-G glands laterally ( Figures 3C, 3F, 3 View Figure 3 H–J, 4I–L and 5H–J). Two major ventro-lateral lobes of the mucus gland descend closely on both sides of the rhynchodeum and run posteriad ventro-lateral to the foregut ( Figures 3F, 3I View Figure 3 , 5J and 6 View Figure 6 A–D). At the level of proboscis insertion dorsal lobes of mucus glands abound above the rhynchodeum ( Figures 5J and 6A View Figure 6 ). Numerous smaller lobes are found between the body wall and internal organs laterally. In the cerebral region, mucus glands occupy the dorso-medial region between the dorsal body wall and rhynchocoel, as well as the region ventro-lateral to the brain ganglia. Ventro-lateral mucus tracts are very prominent postcerebrally ( Figure 6D View Figure 6 ). Further posteriad, ventro-lateral lobes gradually become displaced by the caecal diverticula, while dorso-lateral lobes remain prominent ( Figure 6E View Figure 6 ). Mucus glands gradually decrease in number toward the posterior and disappear at the end of pylorus.
Acidophilic glands are well developed and, for the most part, restricted to the ventro-lateral precerebral region ( Figures 5H and 5I). Individual acidophilic gland cells are found dorsally in the precerebral region directly underneath the body wall interspersed with the mucus cephalic glands ( Figures 5 H–J). Ventral acidophilic glands largely disappear posterior to the proboscis insertion, while the scattered dorsal gland cells can be found in the cerebral region.
Orange-G glands are extremely well developed. Overall, they present a distribution typical for the genus. Two dorso-lateral tracts of orange-G glands first appear at the level of the frontal organ and reach as far back as the end of the pylorus. In the precerebral region, orange-G glands are found laterally between the ventral acidophilic glands and dorsal mucus glands, and dorso-laterally, where they intersperse with mucus glands ( Figure 5J). The orange-G glands are very abundant postcerebrally above the lateral nerve cords where they are interspersed with the mucus glands ( Figure 6D View Figure 6 ).
Cerebral organs. The compact paired cerebral organs are situated in front of the brain between the anterior and posterior pairs of eyes. Each organ opens ventro-laterally into a reduced anterior cephalic furrow ( Figure 2A View Figure 2 ). The latter are shallow semi-circular grooves lined by strongly acidophilic ciliated epithelium ( Figure 5A). Cerebral organ canals are not branched. Two types of gland cells can be distinguished in the cerebral organs. The coarsely granular cells, staining dark reddish-brown with Crandall’s method are found at anterior face of the cerebral organ ( Figure 5A). The second type of cell is finely granular, stains less intensively red to brownish purple and forms a single lobe on the posterior face of each organ ( Figure 5B). This posterior glandular lobe of cerebral organs may extend under the brain.
Excretory system. The collecting tubules of the excretory system are thin-walled and inconspicuous. Terminal flame cells are small, binucleate and reinforced by five to seven very thin and indistinct transverse support bars. Flame cells are typically observed embedded in the extracellular matrix in the vicinity of blood vessels, and are particularly numerous around the cephalic blood vessels ( Figures 5C and 5D). Collecting tubules open to the outside via numerous inconspicuous thin-walled nephridioducts. With effort, few flame cells and nephridioducts can be detected past foregut region, but it is impossible to determine the full extent of the nephridial system in this species with light microscopy.
Reproductive system and life history. Sexes are separate. All sectioned sexually mature individuals possessed either ovaries ( Figure 4H View Figure 4 ) or testes ( Figure 6F View Figure 6 ) and never mixed gonads. Reproductive males and females were observed from January to May in Florida. Males and females can be distinguished by the colour of mature gonads: testes are whitish, while the ovaries are pinkish-orange, due to the colour of mature oocytes. Males also tend to be smaller than females. Mature ovaries contain three to seven oocytes each. Mature egg is about 380 mm in diameter, very yolky, pinkish-orange to brownish-orange and enclosed within an egg envelop approximately 430 mm in diameter. In captivity, several females laid clutches of 100– 150 eggs on walls of glass or plastic containers above the water line.
Development is encapsulated. Gastrulation occurs on the second day of development (approximately 36 h) and ciliation is obvious on the third day. Large and yolky cells of epidermis, possibly corresponding to the transitory larval epidermis, described in some other hoplonemertean larvae ( Maslakova and Malakhov 1999; Maslakova and von Döhren 2007), are apparent on the sixth day of development. Yolky, teardrop-shaped to vermiform juveniles about 2 mm long hatch from the egg envelopes in about 6–10 days, after which they may remain crawling within the mucus clutch for a few more days. Twoweek old juveniles have brain, ocelli and proboscis with a central stylet on a basis.
| US |
University of Stellenbosch |
| NMNH |
Smithsonian Institution, National Museum of Natural History |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.