Acanthaster benziei, Wörheide & Kaltenbacher & Cowan & Haszprunar, 2022
|
publication ID |
https://doi.org/10.11646/zootaxa.5209.3.7 |
|
publication LSID |
lsid:zoobank.org:pub:0576E9E6-22F1-4A77-ACC8-439662DF53FC |
|
DOI |
https://doi.org/10.5281/zenodo.7334240 |
|
persistent identifier |
https://treatment.plazi.org/id/817B8797-1B48-FF90-AEAE-5E1DFE68F8C6 |
|
treatment provided by |
Plazi (2022-11-17 07:18:50, last updated 2022-11-18 11:58:57) |
|
scientific name |
Acanthaster benziei |
| status |
sp. nov. |
Acanthaster benziei sp. nov. Wörheide & Kaltenbacher
Zoobank LSID: urn:lsid:zoobank.org:act:4C462EF3-39AF-4767-96DF-C3B8CC5D9388
Formal name. Acanthaster benziei Wörheide & Kaltenbacher in Wörheide, Kaltenbacher, Cowan & Haszprunar 2022
Etymology. The species name pays tribute to Professor John Benzie, who has decisively promoted research on CoTS, with numerous publications and his own collection. He was among the first scientists to genetically analyse Acanthaster spp. and his collection was the basis of the work of Vogler et al. (2008), which represents a milestone in the species identification of these sea stars.
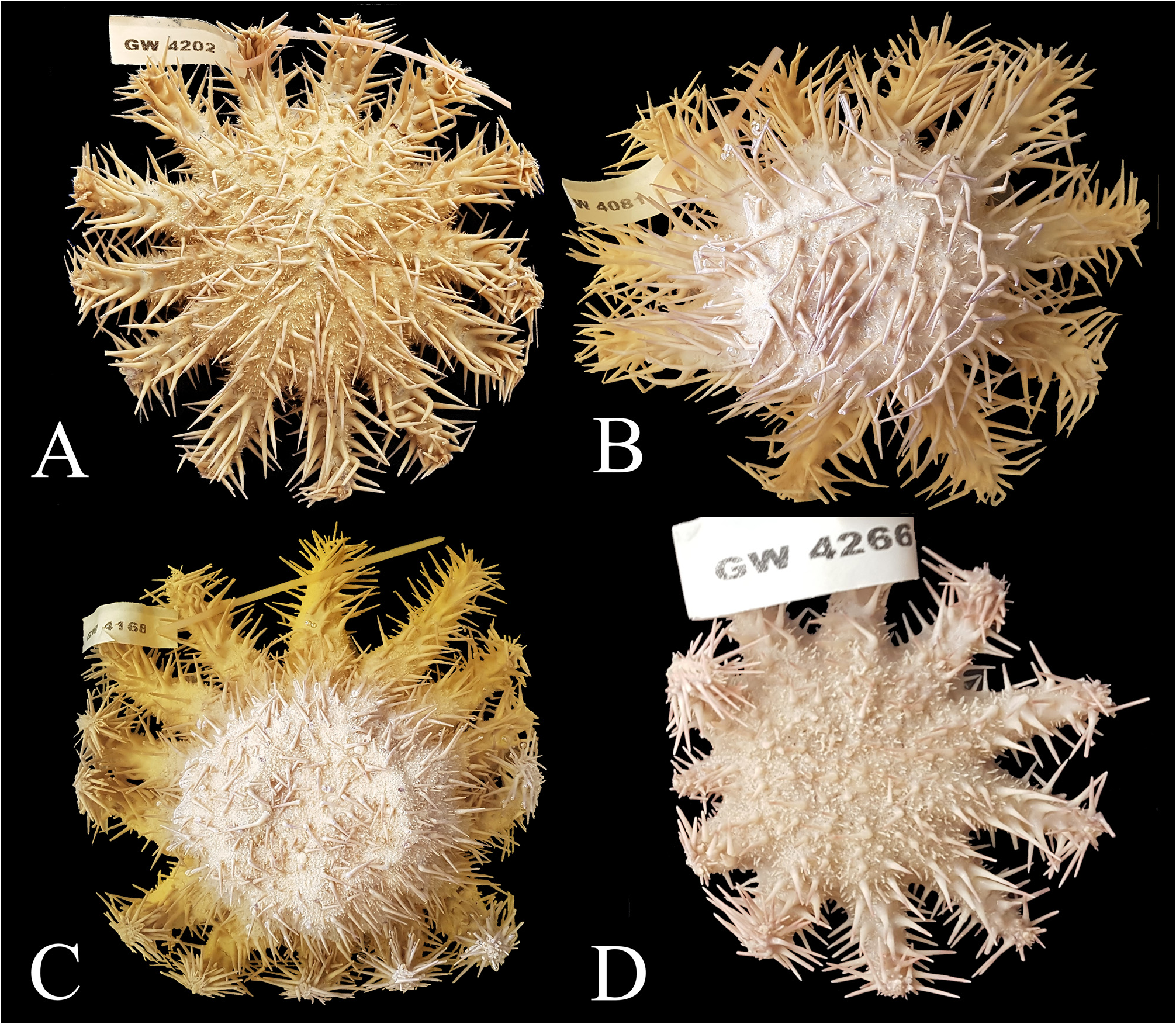
Holotype. SNSB-BSPG.GW.4202, adult individual ( Fig. 2A View FIGURE 2 ), collected in 2017 by Sara Campana and Oliver Voigt at Miskah, Farasan, Saudi Arabia (18.84166667 / 40.78138889) in a water depth of 10 m. The sea star was narcotized with menthol, fixed in 4% formaldehyde, and preserved in 70% EtOH. Some tube feet for DNA analyses were preserved in 95% EtOH and are stored at SNSB-BSPG together with the specimens. GoogleMaps
Paratypes. SNSB-BSPG.GW.4081, adult individual ( Fig. 2B View FIGURE 2 ), collected from Coast guard reef, near AlLith, Saudi Arabia (20.124560 / 40.258746) in a water depth of <10 m, narcotized with menthol, fixed in 4% formaldehyde, and preserved in 70% EtOH. Some tube feet for DNA analyses were preserved in 95% EtOH and are stored at SNSB-BSPG together with the specimens GoogleMaps .
SNSB-BSPG.GW.4168, adult individual ( Fig. 2C View FIGURE 2 ), collected from Mubarak , Farasan Banks, Saudi Arabia (19.09444444 / 40.37916667) in a water depth of <10 m, narcotized with menthol, fixed in 4% formaldehyde, and preserved in 70% EtOH. Some tube feet for DNA analyses were preserved in 95% EtOH and are stored at SNSBBSPG together with the specimens GoogleMaps .
SNSB-BSPG.GW.4266, juvenile individual ( Fig. 2D View FIGURE 2 ), collected from Tidhkar Island , Farasan Banks, Saudi Arabia (19.12777778 / 40.6694444) in a water depth of <10 m, narcotized with menthol, fixed in 4% formaldehyde, and preserved in 70% EtOH. Some tube feet for DNA analyses were preserved in 95% EtOH and are stored at SNSB-BSPG together with the specimens GoogleMaps .
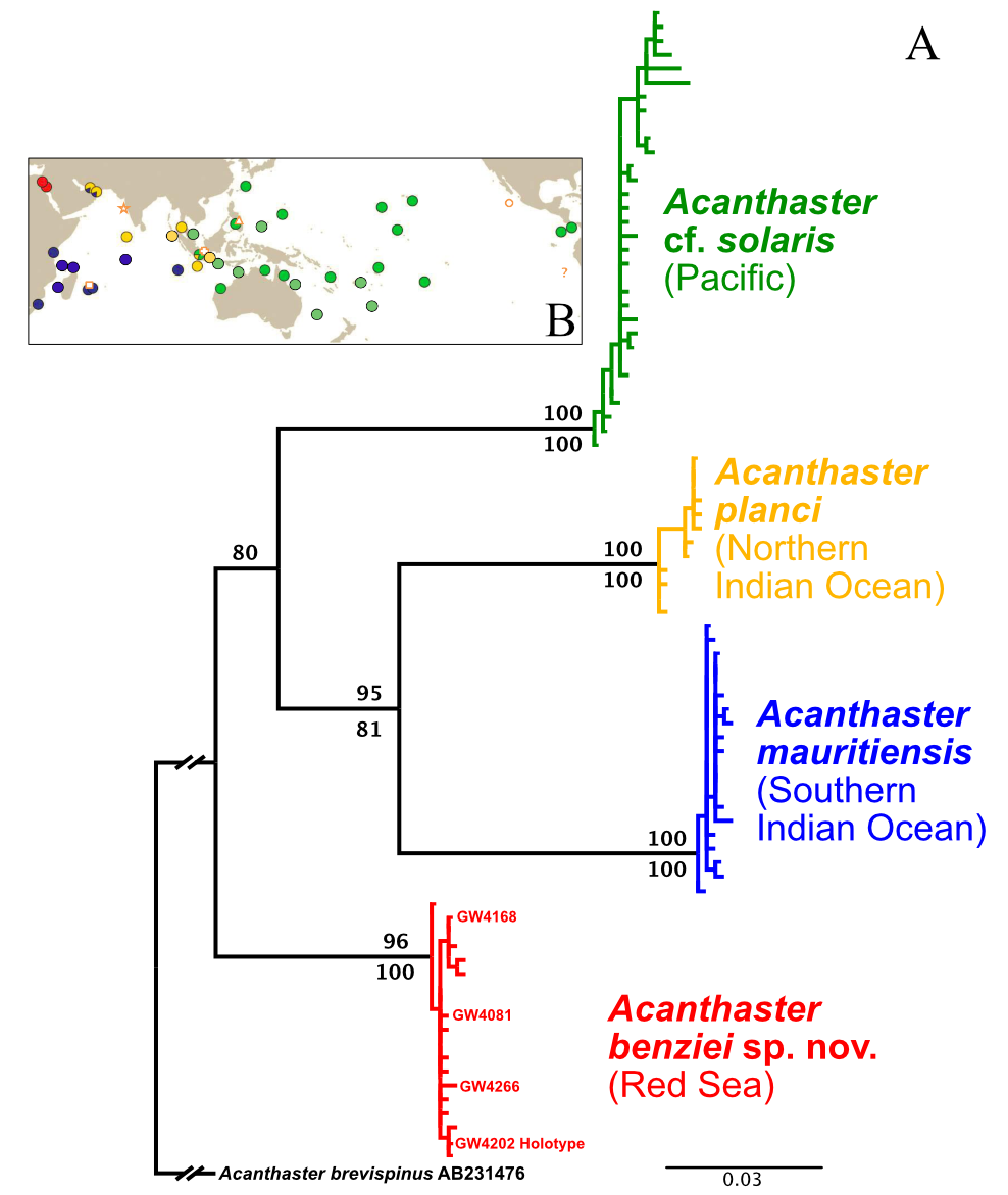
Diagnosis. DNA barcoding analysis of partial COI sequences reasserted Acanthaster benziei as a deeply divergent clade ( Fig. 3 View FIGURE 3 , see also Vogler et al. [2008]) with distinct geographic distribution (Red Sea). Acanthaster benziei possesses diagnostic mutations in its partial mitochondrial COI gene sequenced here that are unique for the Red Sea and not shared with any other species of the species’ complex, i.e., A. planci , A. mauritiensis and A. cf. solaris . Specifically, mutations in the following positions are diagnostic for A. benziei (the position refers to the position in the COI gene extracted from the mitochondrial genome of a specimen from Israel [GenBank accession number LC566218 View Materials ]; the first nucleotide is the one in A. benziei , the second one in the other three species): 150 (T / C), 426 (G / A), 495 (T / C), 504 (G / A), 555 (T / C), 585 (G / A), 588 (T / C), 612 (G / A), 711 (T / C), 714 (C / T). All these are silent third-codon mutations.
The following diagnostic morphological characters differentiate A. benziei from its congeners, and were assessed from the type series at size measured (see Table 3 View TABLE 3 ): fanned spine tips in primary and latero-oral spines; a wider tip or tapering shape in circumoral spines; and rhombus-shaped oral pedicellariae. Additionally, A. benziei has fewer arms than its congeners (up to 14 in A. benziei vs. up to 23 in A. cf. solaris from the Pacific).
Morphological description. Applies to the holotype, except otherwise noted. Large sea star with a convex disk and 13 arms (number of arms across type series 11-14, Table 3 View TABLE 3 ), which have a subcylindrical cross section. Each arm tapers to an acute point and the arms are slightly variable in length. The mean disk radius (r) measures 58 mm, the mean length of rays (R) is 91 mm (R/r ratio = 1.57).
There are two rows of ambulacral tube feet (approximately 1–3 mm in diameter, with flattened tips and no sucker) in the ambulacral groove in the midline of the oral side of each arm. The stereom on both the oral and aboral side consists of a mesh of ossicles, concealed by soft tissue and a large number of spines and pedicellariae, both of which are sheathed in tissue, typically labyrinthic or elongated trabeculated. The aboral disc surface has many papulae with no clear arrangement. The tissue here is relatively soft and compressible. The anus – in the centre of the disc – has no papulae, is harder, and is spaciously encircled by six madreporites on the disk.
Six spine types are distinguished: primary and secondary spines on the aboral side, and subambulacral, circumoral, oral, and latero-oral spines on the oral side (see Fig. 1 View FIGURE 1 for definition). The primary and the secondary spines, that cover the aboral surface, differ in size and supporting ossicle (the pedicle), which is shorter and supported by a secondary ossicle in the secondary spines ( Motokawa 1986; Walbran 1987). Subambulacral spines are very short and occur next to the ambulacral groove or furrow. Long latero-oral spines intercross with those of the adjacent arm, while the oral spines are shorter, with a blunt tip, positioned in one or two rows next to the subambulacral spines. Most circumoral spines are longer than the oral spines and are located in a single row at the mouth opening. One group of circumoral spines is part of two adjoining rays. Spines on the oral side may have a bend in the lower quarter of the shaft, are more irregular than the aboral spines, and either lack or have a less dominant pedicle. The oral and circumoral row of spines is continuous throughout all of the arms. They fringe the ambulacral spines from the tip of one arm to the mouth opening, turning to the next arm, where they also fringe the ambulacral spines, remaining symmetrical on both sides of the ambulacral groove. Variations and intermediate forms of one or more of the spine tip shapes within one individual are possible, however, the pointed tip is common in any sea star studied from the type series.
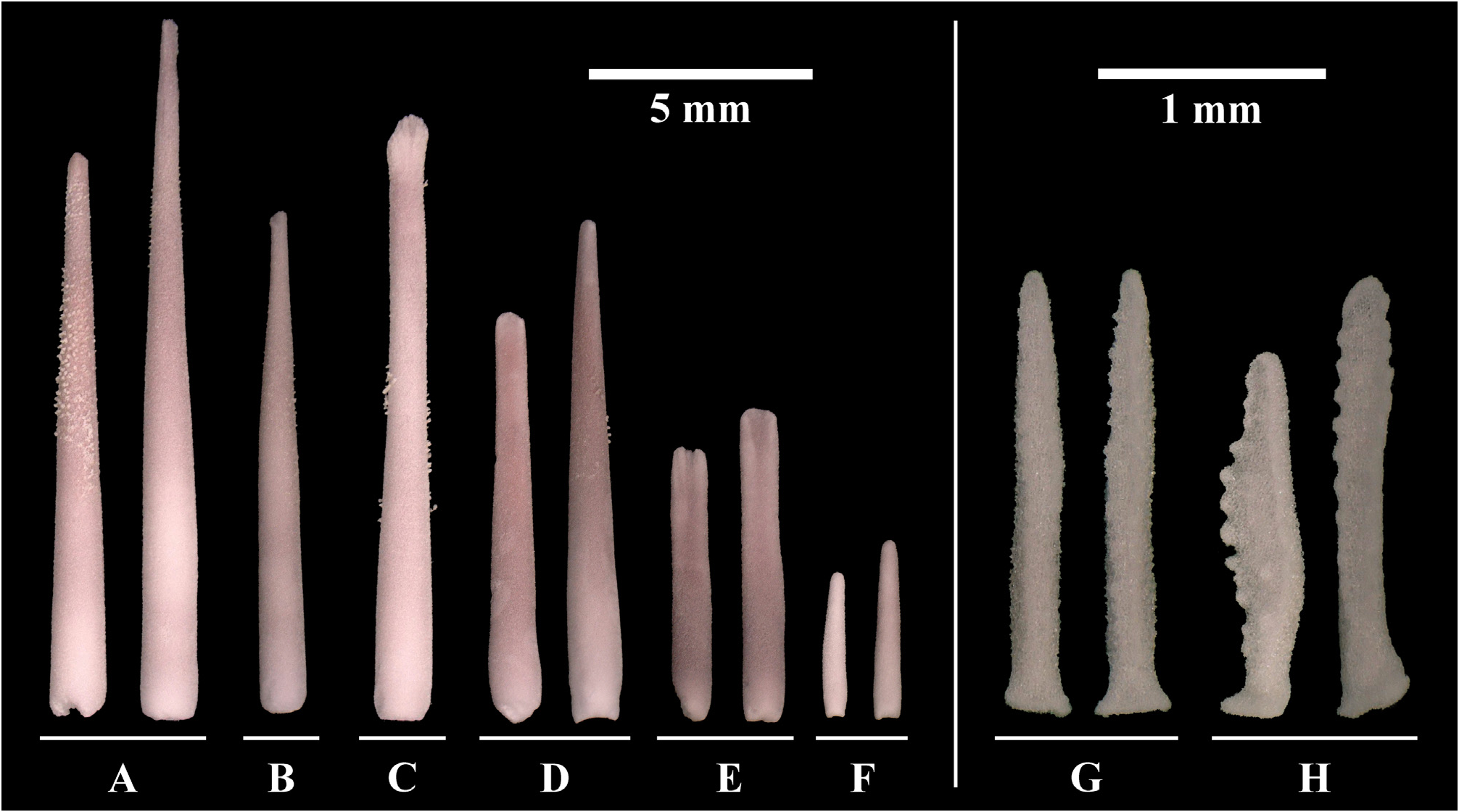
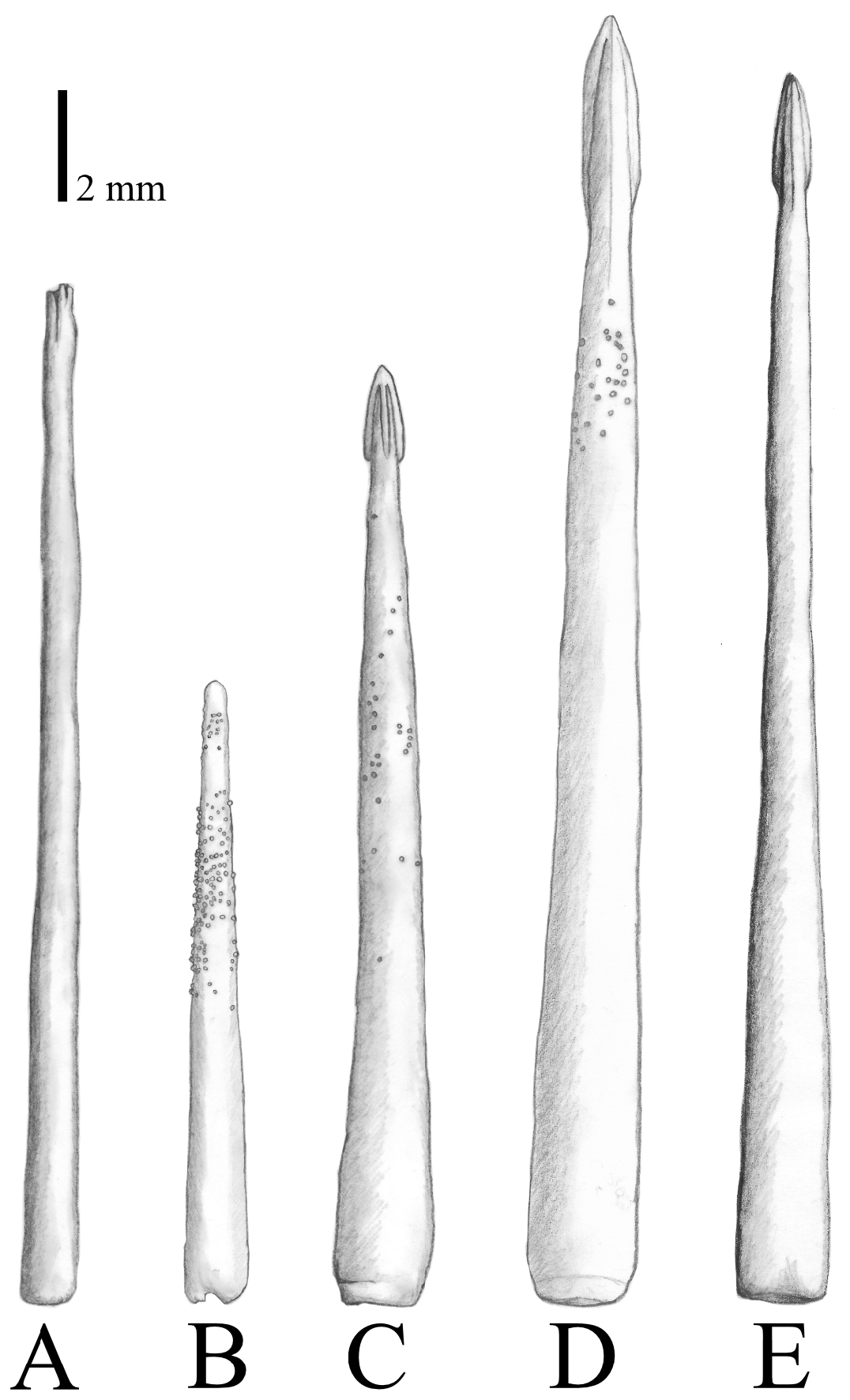
Primary spines ( Figs. 4A View FIGURE 4 , 6 View FIGURE 6 ) are the longest spines on the aboral surface, ranging between approximately 6–33 mm in length. They are straight and slender, slightly shorter (approx. 6–27 mm) on the disc and longer (approx. 27–33 mm) on the arms, consist of one to two parts and are supported by a basal/primary ossicle (= pedicle). The shape of spine tips is variable, but most common is a fanned spine tip with several small furrows ( Fig. 6A View FIGURE 6 ). The spines can be granulated in the upper half.
Secondary spines ( Fig. 4B View FIGURE 4 ) are found mainly on the disc, but also on the arms. They are less numerous and shorter than the primary spines, ranging between approximately 8–11 mm in length. Secondary spines always consist of one part, but otherwise reflect the appearance of primary spines, also regarding granulation.
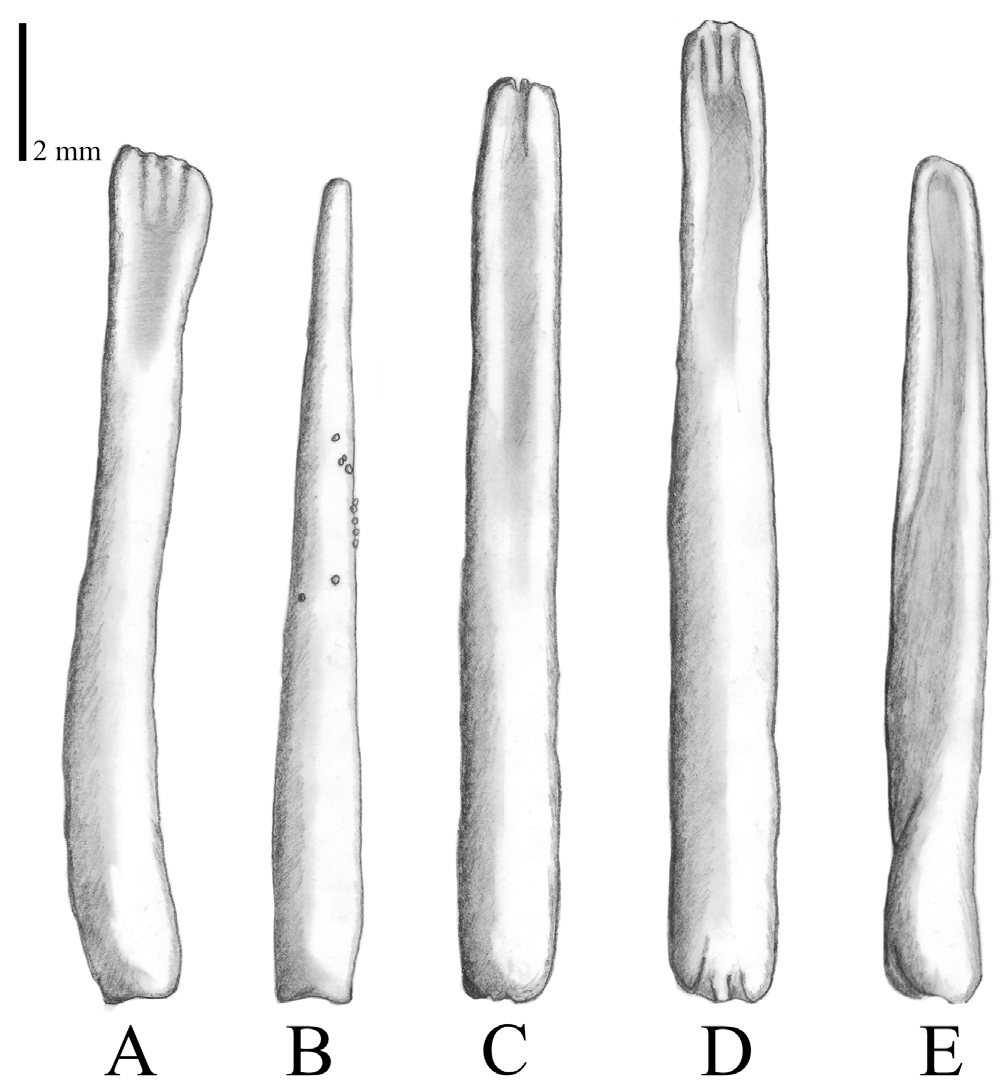
Latero-oral spines ( Figs. 4C View FIGURE 4 , 7 View FIGURE 7 ) are similar in size to the primary spines, ranging between approximately 4–20 mm in length. They are located on marginal ossicles, forming no or short pedicles, compared to primary spines. Spine tips have similar shapes to primary spines: pointed to fanned or flat with a slight furrow in the middle, rounded tip with small furrows, mostly broader than tips of aboral spines, can be asymmetrical, can widen or taper upwards. Granulation may be present.
Circumoral spines ( Figs. 4D View FIGURE 4 , 8 View FIGURE 8 ) form a single row surrounding the mouth, and range in length between approximately 9 and 11 mm. Groups of 8–12 spines are associated with two large oral ossicles and every ray has two oral ossicles (occurring symmetrically, one on each side of a ray), each with the same number of spines. One group of circumoral spines is part of two adjoining rays. Within each group, the spine that is closest to the mouth is the terminal spine. There are always two terminal spines, which are the longest, and the spines become shorter towards the adambulacral ossicles. All spines are wider and mostly flattened towards the tip, with the flat side facing either the ambulacral furrow or, if terminal, the mouth opening. Spine tips are mostly flat and may have furrows or be smooth; a pointed tip is rare. Granulation may be present.
Oral spines ( Fig. 4E View FIGURE 4 ) are very abundant, occurring in one or two rows on the oral-intermediate ossicles and in one row on the adambulacral ossicles, with a flat side facing the ambulacral furrow. They are similar to circumoral spines, but smaller, in the range of 4–7 mm length, and with deeper furrows. The upper outline can show a depression in the middle.
Subambulacral spines ( Fig. 4F View FIGURE 4 ) fringe the margin of the ambulacral grooves. They are the most abundant and smallest, ranging between 2–11 mm in length, becoming shorter towards the tip of the arm. Three to four spines are grouped in an adambulacral comb on one ossicle, which is connected by tissue at the base; the outer spines within the grouped spines are always the smallest. Each group of spines is associated with one tube foot. Most spine tips are pointed; however, larger spines can have a flattened tip with slight furrows. The shaft of the spines can be bulbous on one side, increasing the width at the middle of the spines.
All pedicellariae, aboral and oral, are straight, bivalved, and alveolar, positioned over a small cavity, or cupule in the underlying ossicle (see Gale 2011).
Aboral pedicellariae ( Fig. 4G View FIGURE 4 ) are located among the primary spines, secondary spines and papulae, and are mostly very frequent, giving the aboral side of the sea stars a hairy appearance. They are very common on the disc, but less frequent on the arms; however, abundance differs between individuals. They consist of two equally sized valves, ranging between approximately 2–3 mm in length, are long and slender, and are nearly symmetrical with a tapering tip. The outer rim of the valves has a fine, tooth-like structure that is uniform but can be more prominent on one side.
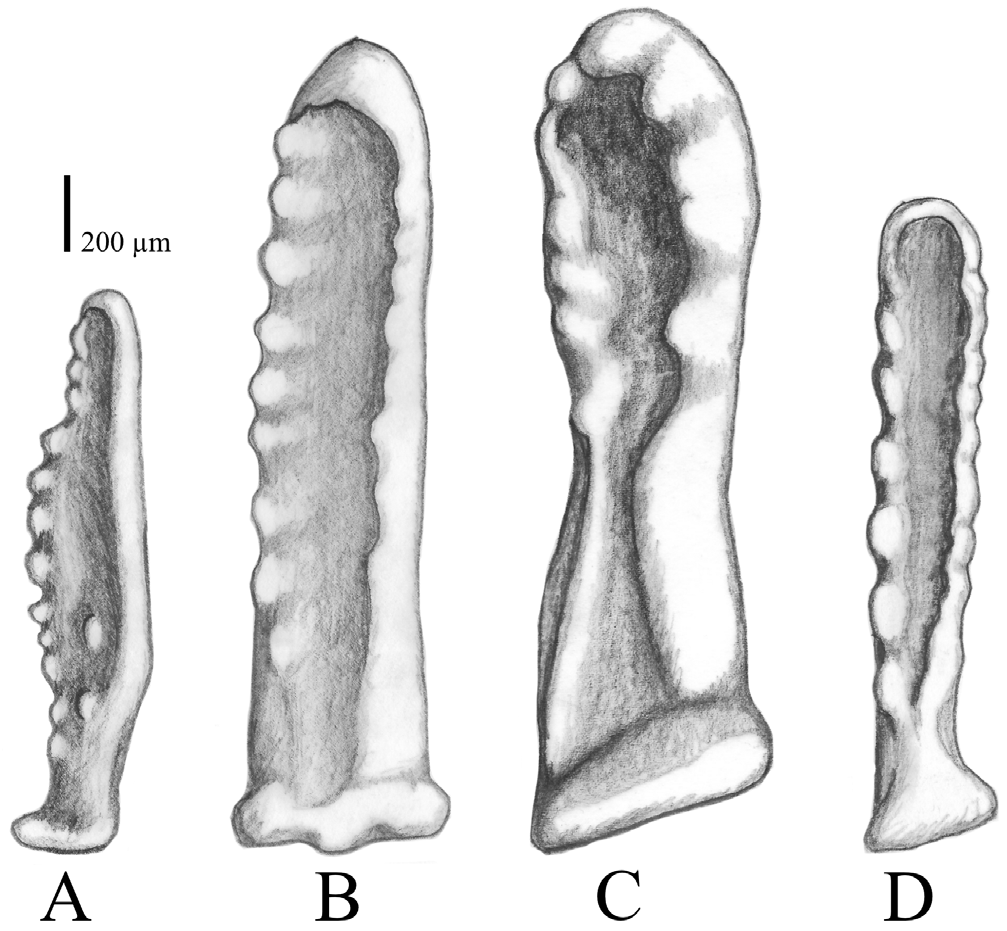
Oral pedicellariae ( Figs. 4H View FIGURE 4 , 8 View FIGURE 8 ) occur at two locations: most commonly next to oral spines or the group of subambulacral spines on the adambulacral ossicle; or more rarely, between the oral spines on the oral ossicles, or exceptionally found on marginal ossicles. The valves are mostly of the same length, ranging between approximately 1.5–2 mm in length. The overall shape is very variable, however there are two main shapes: i) smaller and irregularly formed with a hook-shaped tip, wide from the side, slender from the front, rounded shaft under the “hook” which may have small, asymmetrically-arranged teeth; or ii) flatter, largely resembling the shape of a rhombus due to a widening in the middle part and a pointed tip, with teeth occurring asymmetrically on the outer rim and occasionally on the inner surface, if the area is large enough.
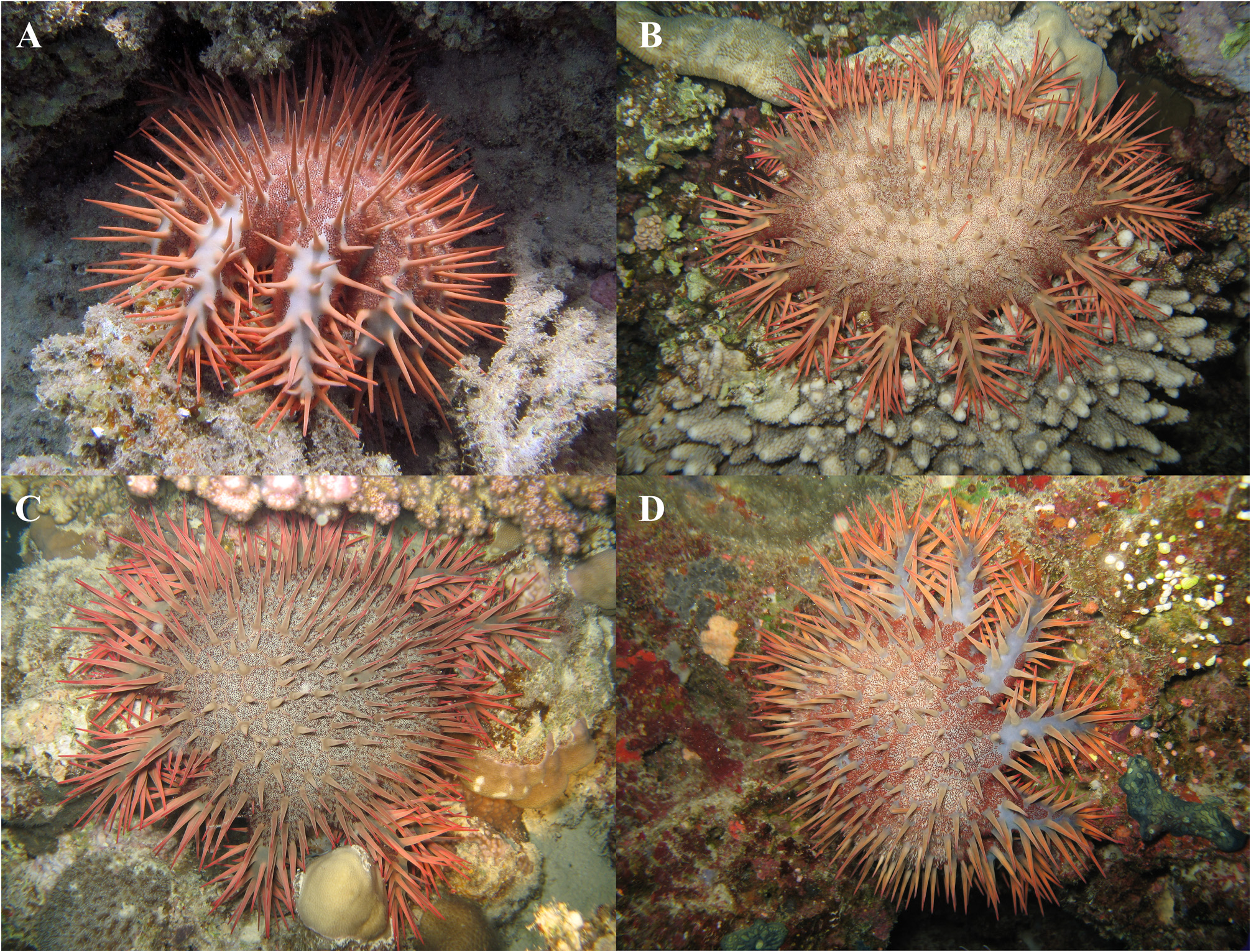
Colour. The colouration of live adult specimens is grey-green to grey-purple, with aboral spines that can be orange to reddish in colour ( Fig. 5 View FIGURE 5 ). Reddish papulae on the aboral surface may also give a bulls-eye appearance due to the formation of two darker rings ( Birkeland & Lucas 1990) ( Fig. 5 B, D View FIGURE 5 ).
Distribution and habitat. So far known Acanthaster benziei is restricted to the Red Sea, where it inhabits coral reefs, predominantly the outer reef surfaces where it mostly hides in crevices during the day and feeds nocturnally.
Differential diagnosis. Acanthaster benziei sp. nov. can clearly be distinguished by diagnostic mutations in the partial COI gene sequences analysed, all material examined fall within the deeply divergent monophyletic group of CoTS from the Red Sea (see Fig. 3 View FIGURE 3 ). There is full agreement with initial results that proposed species distinction of the Red Sea clade based on COI data alone ( Vogler et al. 2008), recently corroborated by nuclear genome analyses ( Yuasa et al. 2021). The molecular-based species distinction of A. benziei sp. nov. is also substantially supported by diagnostic morphological characters.
Acanthaster benziei has fewer arms than congeneric sea stars of comparable size from the other geographic regions/species. Our reported range between 11 and 14 arms in the type series ( Tab. 3 View TABLE 3 ) is consistent with values previously reported for other individuals from the Red Sea (mean of 13 arms [ Campbell and Ormond 1970]; maximum of 13–14 arms [ Haszprunar et al. 2017]). By contrast, the number of arms reported for sea stars from India (= A. planci ) was 15 (Linnaeus 1758) and for A. mauritiensis 13–16 ( de Loriol 1885), with a maximum of 23 arms reported for A. planci , A. mauritiensis and the Pacific species A. cf. solaris ( Haszprunar et al. 2017) (see Supp. Tab. 1 View TABLE 1 ).
The spines and pedicellariae of A. benziei are more variable, and spines are narrower and thinner than in its congeners. The pointy spine type was not found in such high abundance in specimens outside the Red Sea ( Fig. 6 View FIGURE 6 , Suppl. Fig. S2–4 View FIGURE 2 View FIGURE 3 View FIGURE 4 ). Unique for A. benziei are the fanned primary spines ( Fig. 4A View FIGURE 4 , 6A View FIGURE 6 ) and the distally fanned laterooral spines, which can also be granulated ( Fig. 4C View FIGURE 4 , 7A View FIGURE 7 ). The granulation of the latero-oral spines is rare, and may also be found in A. mauritiensis , where the longest spines have an arrow-head tip ( Fig. 7C View FIGURE 7 ; Suppl. Fig. S3C View FIGURE 3 ).
Compared to spines of its congeners, there are some key differences: primary spines of A. planci show only one tip-shape, which resembles an arrowhead ( Fig. 6C View FIGURE 6 ; Suppl. Fig. S2A View FIGURE 2 ) – this was not found in A. benziei ; secondary spines are longer compared to primary spines in A. benziei than in A. mauritiensis (around half to three quarters the size of the primary spines), A. planci and A. cf. solaris (around one quarter to half the size of primary spines); and the second articulation of primary spines is only found in A. benziei . Primary spines in CoTS from the Red Sea are also considered to be less harmful than spines of other regions, which could be connected to the shape, and they seem to have less toxins ( Campbell & Ormond 1970).
Some shapes of the circumoral spines are unique for A. benziei ( Fig. 8A View FIGURE 8 ). While the common shape of these spines in the species complex is straight, flattened, and with a blunt tip (e.g., in A. planci [ Fig. 8C View FIGURE 8 ; Supp. Fig. S2D View FIGURE 2 ] and A. mauritiensis [ Fig. 8D View FIGURE 8 ; Supp. Fig. S3D View FIGURE 3 ]), the spines of A. benziei ( Figs. 4D View FIGURE 4 , 8A View FIGURE 8 ) may have a wider tip or the tip is tapering and more pointed with some granules present on the shaft of the spine ( Fig. 8B View FIGURE 8 ). Both shapes are only found in this spine type of A. benziei . The other oral spines are very similar to the corresponding spines of specimens examined from the other three species (Supp. Figs. S2–4 View FIGURE 2 View FIGURE 3 View FIGURE 4 ).
Aboral pedicellariae are more numerous in A. benziei than in the two Indian Ocean species A. planci and A. mauritiensis . The oral pedicellariae of A. planci ( Fig. 9B View FIGURE 9 ; Supp. Fig. S2H View FIGURE 2 ) are mostly wider and straighter than those of A. benziei ( Figs. 4H View FIGURE 4 , 9A View FIGURE 9 ), which are the smallest among the four species, and are not as deeply curved as the pedicellariae of A. mauritiensis ( Fig. 9C View FIGURE 9 ; Supp. Fig. S3H View FIGURE 3 ). The valves of the flat oral pedicellariae, which resemble the shape of a rhombus, with a widening at the middle, a pointed tip and teeth on the inner surface, were additionally only found in specimens from the Red Sea ( Figs. 4H View FIGURE 4 , 9A View FIGURE 9 ).
In summary, the main distinguishing morphological characters of A. benziei considered to be species-specific are the fanned spine tips in primary ( Figs. 4A View FIGURE 4 , 6 View FIGURE 6 ) and latero-oral spines ( Figs. 4C View FIGURE 4 , 7 View FIGURE 7 ), the wider tip or the taperingpointed shape in circumoral spines ( Figs. 4D View FIGURE 4 , 8 View FIGURE 8 ), as well as the rhombus-shaped oral pedicellariae with occasionally internal teeth ( Figs. 4H View FIGURE 4 , 9 View FIGURE 9 ).
Birkeland, C. & Lucas, J. S. (1990) Acanthaster planci: MaJor Management Problem of Coral Reefs. CRC Press, Boca Raton, Florida, 262 pp.
Campbell, A. C. & Ormond, R. F. G. (1970) The threat of the crown-of-thorns starfish (Acanthaster planci) to coral reefs in the Indo-Pacific area: observations on a normal population in the Red Sea. Biological Conservation, 2 (4), 246 - 251. https: // doi. org / 10.1016 / 0006 - 3207 (70) 90004 - 2
de Loriol, P. (1885) Catalogue raisonne des Echinodermes recueillis par M. V. de Robillard a l'ile Maurice. Memoires de la societe de physique et d'histoire naturelle de Geneve, 29 (1 re Partie), No 4, 84 pp., pls. VII - XXII. [in French] https: // www. biodiversitylibrary. org / page / 36249315 (accessed 24 October 2022)
Gale, A. S. (2011) The phylogeny of post-Palaeozoic Asteroidea (Echinodermata, Neoasteroidea). Special Papers in Palaeontology, 85, 1 - 112. ISBN 978144435029 - 6
Haszprunar, G., Vogler, C. & Worheide, G. (2017) Persistent gaps of knowledge for naming and distinguishing multiple species of crown-of-thorns-seastar in the Acanthaster planci species complex. Diversity, 9 (2), 22. https: // doi. org / 10.3390 / d 9020022
Motokawa, T. (1986) Morphology of spines and spine joint in the crown-of-thorns starfish Acanthaster planci (Echinodermata, Asteroida). Zoomorphology, 106 (4), 247 - 253. https: // doi. org / 10.1007 / BF 00312046
Vogler, C., Benzie, J., Lessios, H., Barber, P. & Worheide, G. (2008) A threat to coral reefs multiplied? Four species of crownof-thorns starfish. Biology Letters, 4 (6), 696 - 699. https: // doi. org / 10.1098 / rsbl. 2008.0454
Walbran, P. (1987) An atlas of the skeletal components of the crown-of-thorns starfish (Acanthaster planci (L )). Technical Memorandum GBRMPA-TM- 11, 1 - 45 (including 13 plates). Available from: http: // www. gbrmpa. gov. au / __ data / assets / pdf _ file / 0005 / 9752 / gbrmpa-tm 11. pdf (accessed 13 March 2022)
Yuasa, H., Kajitani, R., Nakamura, Y., Takahashi, K., Okuno, M., Kobayashi, F., Shinoda, T., Toyoda, A., Suzuki, Y., Thongtham, N., Forsman, Z., Bronstein, O., Seveso, D., Montalbetti, E., Taquet, C., Eyal, G., Yasuda, N. & Itoh, T. (2021) Elucidation of the speciation history of three sister species of crown-of-thorns starfish (Acanthaster spp.) based on genomic analysis. DNA Research, 28 (4), dsab 012. https: // doi. org / 10.1093 / dnares / dsab 012
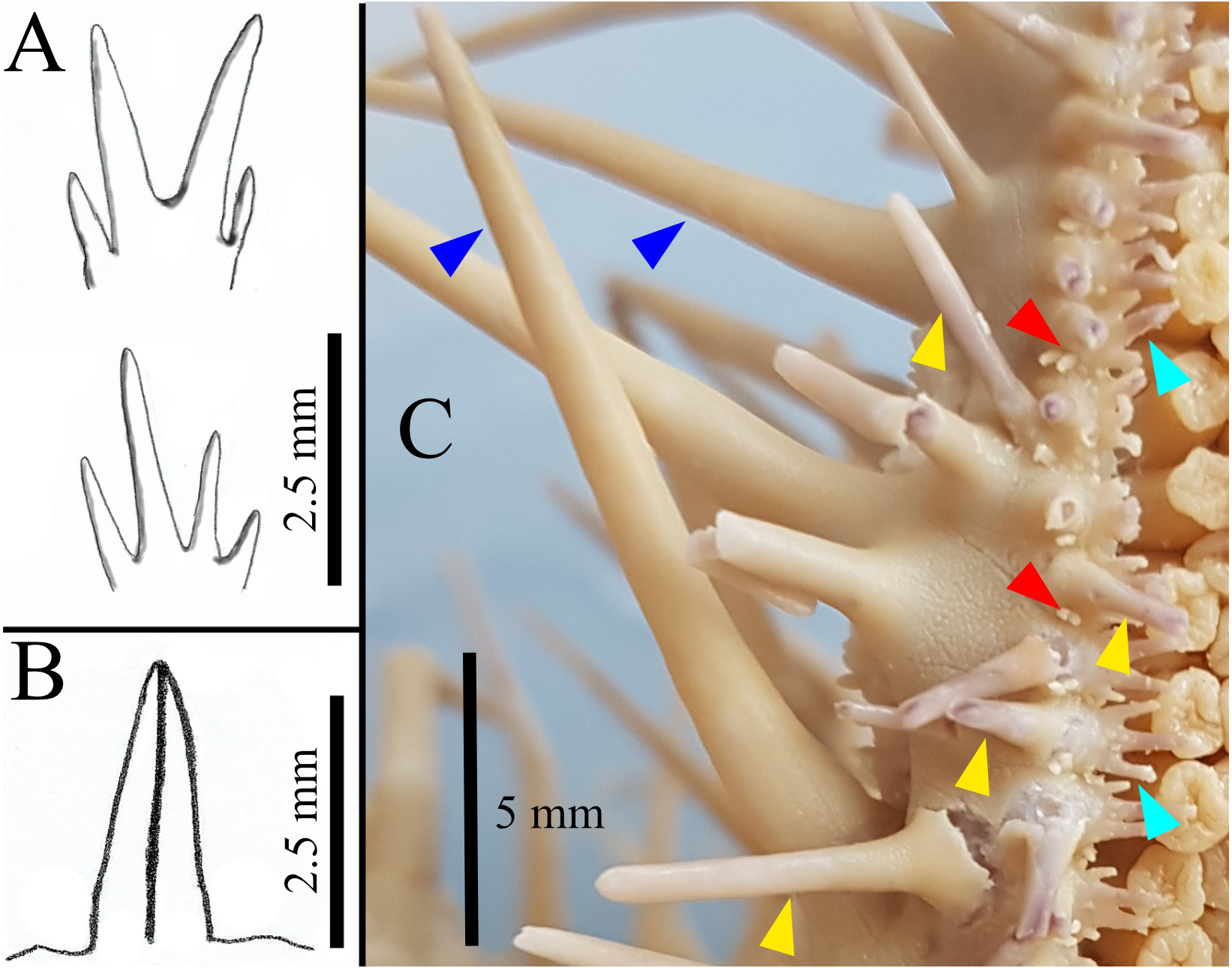
FIGURE 1. Illustrations of pedicellariae and spines (A–C). A: Subambulacral spines, top: bundle with two equally long spines each, bottom: bundle with four unequal long spines; B: Aboral pedicellariae with two valves; C: Close up of part of one arm of paratype GW4081, showing three different spine types indicated by arrows: latero-oral spines (dark blue), oral spines (yellow), subambulacral spines (turquoise). The actinal pedicellariae (red) are associated with the first row of oral spines.
FIGURE 2. Ethanol-preserved specimens of the type series. GW4202 (A) is the holotype, all the others (B–D) are paratypes. Note that individual GW4266 is a juvenile specimen. Size of labels 6 × 2 cm.
FIGURE 3. A: Indo-Pacific ‘Acanthaster planci’ species complex, COI ML tree based on the haplotype alignment of Vogler et al. (2008), supplemented with five samples from Israel (see text for details) extracted from full mitochondrial genome sequences from Yuasa et al. (2021) and four sequences of the type series of Acanthaster benziei sp. nov. (GW4xxx, highlighted in bold). The tree was rooted with Acanthaster brevispinus (accession number AB231476), showing the deep divergence among, and little diversity within, species/geographic clades. ML bootstrap values are above branches, bootstrap values of the NJ clustering of haplotypes below branches. B: Geographic distribution of COI-barcoded clades and of type localities of names (Figure 1 from Haszprunar et al. 2017): red—Red Sea (RS) species; blue—Southern Indian Ocean (SIO) species (A. mauritiensis); yellow—Northern Indian Ocean (NIO) species (A. planci); green—Pacific Ocean (PO) species (A. cf. solaris). Location of type localities of nominal Acanthaster species: asterisk—A. planci; cross—A. echinites; triangle—A. solaris, square—A. mauritiensis; circle—A. ellisii pseudoplanci; “?” - the type locality of A. ellisii was not specified: in South American waters of the East Pacific.
FIGURE 4. Acanthaster benziei sp. nov. (holotype GW4202) (A-B) Aboral spines, (C-F) oral spines and (G-H) pedicellariae of four adult specimens of Acanthaster benziei sp. nov.: (A) Primary spines, (B) Secondary spines, (C) Latero-oral spines, (D) Circumoral spines, (E) Oral spines, (F) Subambulacral spines, (G) Aboral pedicellariae, (H) Oral pedicellariae.
FIGURE 5. Typical colouration of Acanthaster benziei sp. nov. (A) GW4081 (Paratype, hiding during the day under a crevice), Al-Lith, Saudi Arabia, (photo credit: Oliver Voigt), (B–D) Thuwal Reefs, Saudi Arabia (photo credit: Gert Wörheide). Approximate diameter of specimens is 25–30 cm.
FIGURE 6. Typical primary spines, showing species-specific variation among (A, B) Acanthaster benziei sp. nov., (C) A. planci, (D) A. mauritiensis, and (E) A. cf. solaris.
FIGURE 7. Typical latero-oral spines, showing species-specific variation among (A) Acanthaster benziei sp. nov., (B) A. planci, (C) A. mauritiensis, and (D) A. cf. solaris.
FIGURE 8. Circumoral spines, showing species-specific variation among (A, B) Acanthaster benziei sp. nov., (C) A. planci, (D) A. mauritiensis, and (E) A. cf. solaris.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |