Chiropotes sagulatus (Traill, 1821)
|
publication ID |
https://doi.org/ 10.5281/zenodo.6632289 |
|
DOI |
https://doi.org/10.5281/zenodo.6632267 |
|
persistent identifier |
https://treatment.plazi.org/id/8477905E-8652-C340-282A-A3FD1965F85C |
|
treatment provided by |
Carolina |
|
scientific name |
Chiropotes sagulatus |
| status |
|
41. View Plate 30: Pitheciidae
Guianan Bearded Saki
Chiropotes sagulatus View in CoL
French: Saki a gilet / German: Guayana-Rotrlickensaki / Spanish: Saki barbudo de dorso pardo
Other common names: Northern Bearded Saki Monkey, Reddish-brown Bearded Saki
Taxonomy. Simia sagulata Traill, 1821 View in CoL ,
Demerara (= Guiana).
Prior to the splitting of the bearded sakis north of the Rio Amazonas into two species, C. sagulatus was referred to as C. chiropotes . Monotypic.
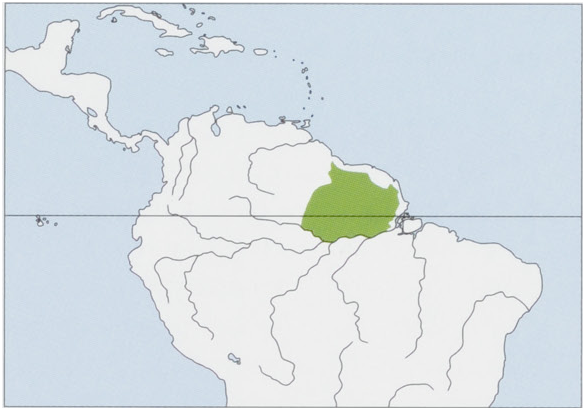
Distribution. The Guianas and N Brazil (N of the Rio Amazonas and E of the Rio Branco). View Figure
Descriptive notes. Head-body 36-46 cm (males) and 33-43 cm (females), tail 39-46 cm; weight 3 kg. Dorsum and upper limbs of the Guianan Bearded Saki are orange to reddish-brown. Head, nape, lower arms, and legs are blackish.
Habitat. High rainforest, terra firma primary forest, high mountain savanna forest, and occasionally seasonally flooded riparian forest. The Guianan Bearded Saki prefers the upperstrata of the forest canopy.
Food and Feeding. Guianan Bearded Sakis prefer soft immature seeds that are typically protected by hard pericarps. They can open fruits with puncture resistance of their pericarps greater than 38 kg/mm? The feeding ecology of the Guianan Bearded Saki was studied in continuous forest and forest fragments in Suriname, Guyana, and Brazil. Seeds made up a relatively large proportion of diets (63-86%), complemented with fleshy fruits (9-28%), flowers (1-11%), leaves (0-11%), and arthropods (0-10%). Long-term studies in continuous forest show a high diversity of plant foods, up to 112 species in the studies of E. Frazao (north of Manaus, Brazil) and L. Gregory (Brownsberg Nature Park, Suriname), and over 175 species in C. Shaffer’s study (Guyana). Among the top-ranked plant families in diets of Guianan Bearded Sakis, Sapotaceae , Lecythidaceae , and Moraceae were very important in all studies. Studies of the stomach contents of Guianan Bearded Sakis shot by hunters have revealed that they are more than occasional insectivores. One stomach alone (of an adult male) was found to contain 133 insects of eight different taxonomic orders and at least ten families. Prominent were the larvae of moths ( Pyralidae ) and beetles ( Curculionidae ), and ants ( Formicidae ), including Cephalotes , Crematogaster , and Pseudomyrmex . Analyses of a further four stomachs in a separate study identified insects ofsix orders, that included also hemipteran nymphs, and the larvae and adults of flies ( Diptera , Phoridae ).
Breeding. Reproduction of the Guianan Bearded Sakis appears to be seasonal, with birthing periods in the early wet season; the period of maximum fruit abundance. Courtship behaviors have not been documented.
Activity patterns. Guianan Bearded Sakis are diurnal and arboreal. In a study in fragments and continuous forest in northern Manaus by S. Boyle,resting represented 47% of the activity budget, feeding 25%), and traveling 21%. L. Gregory found that most of the time was spent resting (49%), followed by traveling (32%) and feeding and foraging (21%). In Shaffer’s study, feeding (40%) and traveling (35%) were the most common activities, and resting accounted for only 20% of the activity budget. Seasonality influences activity patterns of Guianan Bearded Sakis. They spend more time feeding when seed consumption increases, but travel remains constant. On the other hand, when feeding on seeds decreases, there is an increase in the time spent resting and a decrease in group size.
Movements, Home range and Social organization. Guianan Bearded Sakis live in multimale-multifemale groups that can have from two (in a 10ha fragment north of Manaus) to 65 individuals (Guyana, continuous forest). Groups have a fission-fusion dynamic in which group memberssplit up in subgroups ofdifferent sizes. Gregory and Shaffer documented affiliative relationships between males and no signs of hierarchical relationships, with relatively few interactions among females and between males and females. Male affiliative behaviors included mainly sitting in contact with one another, reciprocal grooming, and lining up. In Brazil, the two-individual group studied by J. M. Ayres north of Manaus used all the available area of a 10ha forest fragment, with an average daily movement of 1300 m. In a continuous forest near Manaus, the group studied by Frazao had 30 individuals, and its daily movement was 1100 m. In the same region, S. Boyle recorded an average home range of 429 ha in continuous forest, an average group size of 23 individuals, and an average daily movement of 3000 m. In Suriname, M. van Roosmalen and coworkers estimated a daily movement of 2500 m and a home range of 200-250 ha. At the same site, M. Norconk and W. Kinzey estimated average daily movements of 3200 m for two groups of nine and 13 individuals. At Brownsberg Nature Park, Gregory reported a large home range of 742 ha for his 45member group, and an average daily movement of 2362 m. In Guyana, Shaffer estimated a home range of 1000 ha and an average day range of 4000 m for a 65member group. Guianan Bearded Sakis studied by van Roosmalen and R. A. Mittermeier were associated with other primates 31% of the time, including Guianan Brown Capuchins (Sapajus apella), Guianan Squirrel Monkeys (Saimiri sciureus), and Guianan Weeper Capuchins (Cebus olivaceus).
Status and Conservation. CITES Appendix II. The Guianan Bearded Saki has not been assessed on The IUCN Red List. It is relatively well protected by the isolation of its geographic distribution. In Brazil, it occurs in Tumucumaque and Cabo Orange national parks; Rio Trombetas Biological Reserve; Anavilhanas, Jari, and Balbina ecological stations; and Saraca-Taquera National Forest. In Suriname, it occurs in Brownsberg Nature Park and Raleigh Falls-Voltzberg Nature Reserve, In Guyana, it occurs in the Kanuku Mountains protected area, Conservation International’s Upper Essequibo Conservation Concession, and the Konashen Community-Owned Conservation Area.
Bibliography. Ayres (1981), Ayres & Nessimian (1982), Boyle (2008), Boyle & Smith (2010), Boyle et al. (2009), Frazao (1991, 1992), Gregory (2011), Hershkovitz (1985), Kinzey & Norconk (1990, 1993), Mittermeier (1977), Mittermeier & van Roosmalen (1981, 1982), Mittermeier et al. (1983), Norconk (1996, 2011), Norconk & Kinzey (1994), Norconk et al. (2003), Peetz (2001), van Roosmalen, Mittermeier & Fleagle (1988), van Roosmalen, Mittermeier & Milton (1981), Shaffer (2012, 2013), Silva & Figueiredo (2002), Silva et al. (2013).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
SubOrder |
Haplorrhini |
|
ParvOrder |
Platyrrhini |
|
Family |
|
|
Genus |
Chiropotes sagulatus
| Russell A. Mittermeier, Anthony B. Rylands & Don E. Wilson 2013 |
Simia sagulata
| Traill 1821 |