Copidozoum obliquum, Souto, Javier, Berning, Björn & Ostrovsky, Andrew N., 2016
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4067.4.1 |
|
publication LSID |
lsid:zoobank.org:pub:1CC5D0E7-0B60-4E62-BACD-9775931ED7F9 |
|
DOI |
https://doi.org/10.5281/zenodo.4547319 |
|
persistent identifier |
https://treatment.plazi.org/id/821CCCC5-6F61-47D5-94DA-8C0DDABA0BE9 |
|
taxon LSID |
lsid:zoobank.org:act:821CCCC5-6F61-47D5-94DA-8C0DDABA0BE9 |
|
treatment provided by |
Plazi |
|
scientific name |
Copidozoum obliquum |
| status |
sp. nov. |
Copidozoum obliquum n. sp.
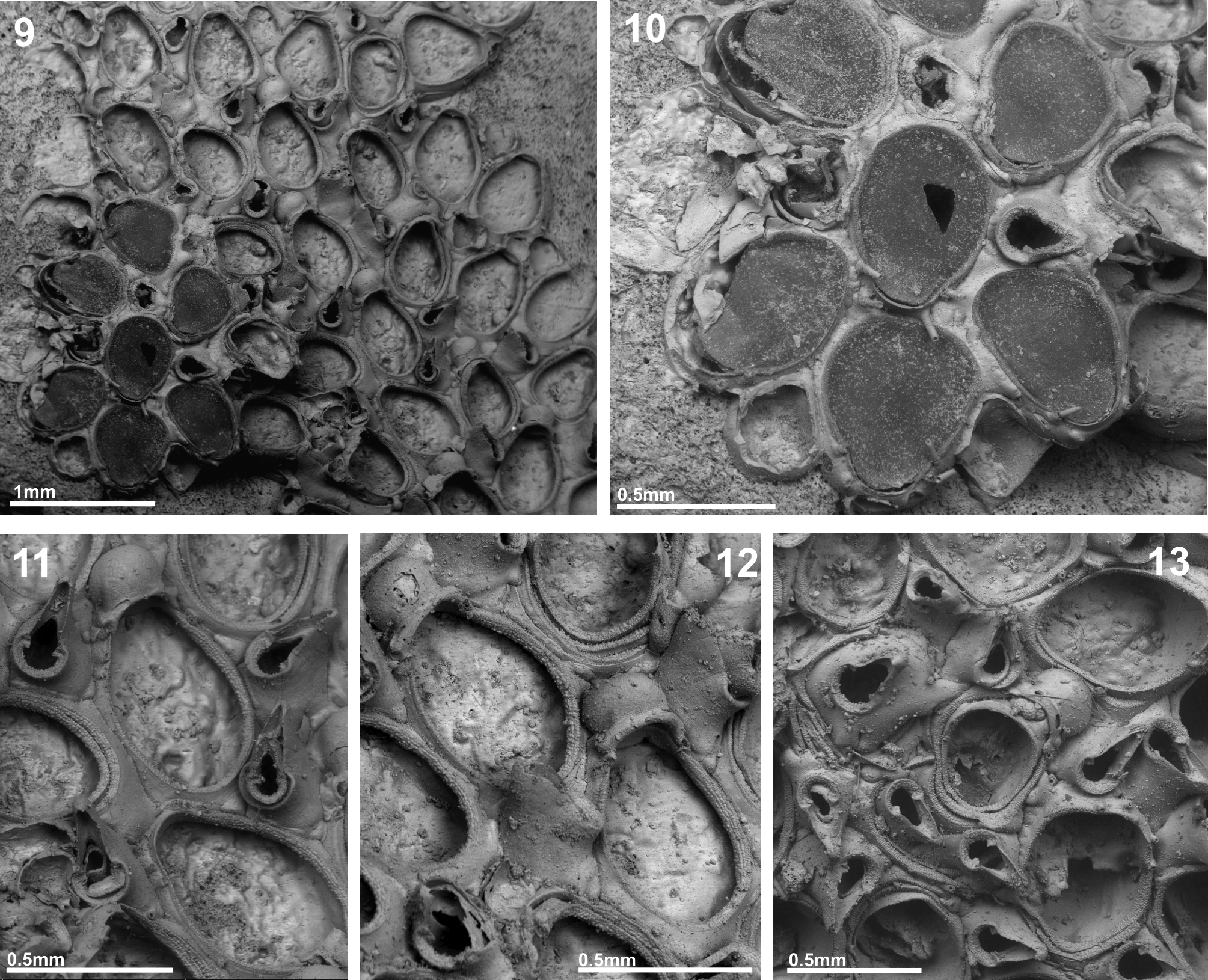
( Figs 9–13 View FIGURES 9 – 13 , Table 4 View TABLE 4 )
Material examined. Holotype: MNCN 25.03/3929, locality DR02. Paratypes: MNCN 25.03/3930, locality DR05; MNHN IB- 2013-620, 621, locality DW117; OLL 2015/895, locality DR05.
Etymology. Latin obliquus, oblique, alluding to the steeply inclined avicularium.
Description. Colony encrusting, unilaminar, multiserial. Zooids relatively broad, either oval or pyriform with greatest width midlength or in proximal third or quarter, opercular region slightly constricted, separated by narrow linear grooves; gymnocyst smooth, variably developed proximally, reduced to narrow band distolaterally; cryptocyst very narrow, coarsely granular band of usually constant width extending distally towards opercular region; opesia extensive, operculum not well sclerotised and almost indistinguishable from frontal membrane; 2, rarely 3, pairs of oral spines, a pair of distal spines situated below mural rim. Basal and mural pore-chambers present.
Ovicells hyperstomial, ooecium globular, wider than long, formed by distal kenozooid concealed from frontal view; ectooecium smooth, entirely calcified apart from 1–2 small oval areas (sometimes missing) near proximal margin, proximal rim variably thickened, sometimes prominent, its lateral parts adjoining oral zooidal spines.
Avicularia interzooidal, distolateral to almost every zooid; cystid fairly erect, with gymnocystal walls variably developed proximally, distally extending vertically towards frontal area; rostrum inclined at varying angles to colony surface (sometimes>45°), pointing distolaterally, triangular, elongated, distally hooked; palatal foramen triangular, with round or sometimes pointed distal end, distally bordered by smooth cryptocyst; postmandibular opesia semicircular, bordered by well-developed pustulose cryptocystal shelf of constant width, terminated by pair of short, stout, triangular condyles with straight proximal margin and central depression.
Ancestrula not observed.
Remarks. Three other species of the genus Copidozoum are present in the bathyal NE Atlantic— Copidozoum magnum López-Fé, 2006, Copidozoum balgimae Reverter-Gil & Fernández-Pulpeiro, 1999b and Copidozoum exiguum ( Barroso, 1920) .
Copidozoum obliquum n. sp. can be distinguished from all of these species by the presence of two to three pairs of oral spines, and by its entirely calcified ectooecium. In C. balgimae from the Strait of Gibraltar, mural spines are absent and the ectooecium is uncalcified to a large extent, exposing granular endooecium. Copidozoum magnum , described from the Canary Islands, is characterised by its larger zooid size and only a single pair of oral spines. While C. obliquum n. sp. is closely related to C. exiguum based on the overall morphology of autozooids, ovicells and avicularia, the latter species has 9–14 mural spines and a crescentic membranous area on the proximal margin of the ectooecium.
Ovicells in the genus Copidozoum are described as having an ectooecium that is partly or largely membranous. Nevertheless, C. obliquum n. sp. and C. magnum have the ectooecium totally calcified, and in C. exiguum the membranous area is much reduced. It may be noted that the ectoooecium in the new species often has small, oval, non-calcified areas near its proximal margin that are the remnants of the membranous window presumably existing early in ovicellogenesis. The outline of such a window is seen in the two distalmost ooecia in Fig. 4 View FIGURES 2 – 5 A. Nevertheless, an analogous situation has been previously described in the genus Callopora ( Ostrovsky et al. 2009) .
SD, standard deviation; N, number of measurements The entirely calcified ectooecium in two species makes it necessary to amend the description of the ectooecium in the generic diagnosis as follows: ovicell with the ectooecium partly or largely membranous, or sometimes completely calcified.
We should also add that, despite most of the ooecia in our material being formed by the distal kenozooid, it is still possible that some of them are formed by the distal autozooid. For example, both of these variants can be simultaneously present in the calloporid Callopora craticula ( Ostrovsky et al. 2009; Ostrovsky 2013). The tight apposition of the ooecium to the distal autozooid in the central part of the colony of C. obliquum n. sp. makes it impossible to determine the precise type of ooecial formation without sectioning, however.
All species mentioned above occupy similar habitats, occurring between 200 to 2200 m, and are usually recorded encrusting the coral Lophelia or Madrepora (e.g. Harmelin & d’Hondt 1992; Zabala et al. 1993; Hayward & Ryland 1998). On Galicia Bank, C. obliquum n. sp. was found to be relatively frequent (88 colonies) and at almost all of the sampling stations, occurring between 779 and 1313 m depth. At five of these localities it coexisted with its congener C. exiguum . Copidozoum obliquum n. sp. was found encrusting corals and rocks in roughly equal numbers as well as shells of balanids in some instances.
One or more intramural buds were observed within the cystids of numerous zooids and avicularia of C. obliquum n. sp. Although the decisive factors inducing the formation of intramural buds are unkown, they are usually interpreted to follow upon death of a zooid caused by accidental damage or partial predation ( McKinney et al. 2003; Berning 2008), although partial starvation of the colony has to be considered as well (J.-G. Harmelin pers. comm. 2008). With their relatively unprotected and exposed frontal membrane, zooids of anascan cheilostomes are especially vulnerable to damage by mobile benthic organisms and saltational transport of sediment particles. Differential damage of avicularia and autozooids, however, suggests that the intramural buds were formed in response to partial predation. Whereas the skeletons of first-formed autozooids are usually intact, the cystids of the much smaller avicularia, in which intramural buds were formed, are often damaged ( Fig. 12, 13 View FIGURES 9 – 13 ). This pattern can hardly be explained by accidental damage or starvation but may be caused by the size of mouthparts used in feeding by the predator, such as the radula of nudibranch gastropods. While cystids of autozooids are large enough for the predator to feed on without damaging the skeletal walls, the radula may break the distinctly smaller avicularian cystids during feeding.
TABLE 4. Measurements (in mm) of Copidozoum obliquum n. sp.
| Mean | SD | Minimum | Maximum | N | |
|---|---|---|---|---|---|
| Autozooid length | 0.635 | 0.0929 | 0.447 | 0.784 | 19 |
| Autozooid width | 0.494 | 0.0624 | 0.383 | 0.575 | 19 |
| Opesia length | 0.520 | 0.0773 | 0.347 | 0.633 | 19 |
| Opesia width | 0.432 | 0.1140 | 0.313 | 0.691 | 19 |
| Ooecium length | 0.188 | 0.0104 | 0.177 | 0.202 | 5 |
| Ooecium width | 0.233 | 0.0130 | 0.218 | 0.247 | 5 |
| Avicularium length | 0.317 | 0.0163 | 0.301 | 0.347 | 7 |
| Avicularium width | 0.154 | 0.0197 | 0.119 | 0.172 | 7 |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
SubOrder |
Flustrina |
|
Family |
|
|
Genus |