Gangesia, WOODLAND, 1924 GANGESIINAE MOLA, 1929
|
publication ID |
https://doi.org/10.1093/zoolinnean/zlac098 |
|
publication LSID |
lsid:zoobank.org:pub:142F33C8-72A5-4BDAAEAE-B2F0DBAB5413 |
|
DOI |
https://doi.org/10.5281/zenodo.7972998 |
|
persistent identifier |
https://treatment.plazi.org/id/862DC000-FFBB-FFFB-0DD7-FC0EFA89F8E4 |
|
treatment provided by |
Plazi |
|
scientific name |
Gangesia GANGESIINAE MOLA, 1929 |
| status |
|
GENUS GANGESIA WOODLAND, 1924 View in CoL View at ENA
Diagnosis: Proteocephalidae : Gangesiinae . Scolex with four uniloculate suckers, sucker generally with several rows of hooklets on anterior rim, rarely without hooklets. Rostellum-like organ eversible, spherical in shape, longer than, or as long as, suckers, usually armed with one, two or several circles of hooks, rarely without hooks; hooks may differ in shape and size between rows. Retractor muscles prominent, forming a wide band around rostellum-like organ. Testes medullary, in single field. Cirrus-sac pre-equatorial. Genital pore irregularly alternating. Ovary medullary, bilobed. Vagina posterior or anterior to cirrus-sac; near genital atrium vaginal sphincter present. Vitelline follicles medullary, some follicles paramuscular (between muscle fibres of inner longitudinal musculature to cortex); lateral band of vitelline follicles occupies almost total length of proglottids. Uterus medullary, with development of type 1 of de Chambrier et al. (2004). Ventral osmoregulatory canals median (internal) to vitelline follicles. Parasites of catfishes ( Siluriformes ) in the Indomalayan and Palaearctic regions.
Type-species: Gangesia bengalensis (Southwell, 1913) View in CoL .
Note: Even though the subfamily Gangesiinae View in CoL View at ENA has not been revealed to be monophyletic, a morphology-based key to all genera previously placed in this subfamily is provided to facilitate identification of its species, as the previous key of Ash et al. (2012) primarily focused on the presence or absence of rostellar hooks and did not include Pangasiocestus .
KEY TO THE GENERA OF THE SUBFAMILY GANGESIINAE MOLA, 1929 View in CoL
1a. Common genital atrium present ..................................................................................................................... 2
1b. Common genital atrium absent (i.e. male and female genital pores open separately). In Siluridae View in CoL . Iraq, Russia................................................................................................................ Postgangesia Akhmerov, 1969 View in CoL
2a. Type 2 uterine development (sensu de Chambrier et al., 2004); vagina always anterior to cirrus-sac .......................................................................................................................................................... 3
2b. Type 1 uterine development (sensu to de Chambrier et al., 2004); vagina anterior or posterior to cirrus-sac .......................................................................................................................................................... 4
3a. Apical depression present on the apex of the scolex; scolex contains longitudinal muscle bundles; lateral band of vitelline follicles formed by several rows; testes similar in size. In Bagridae View in CoL ( Rita rita View in CoL ). India........................................................................................................ Ritacestus de Chambrier et al., 2011 View in CoL
3b. Scolex without apical depression and longitudinal muscle bundles; lateral band of vitelline follicles formed by a single row; testes of different sizes. In Pangasiidae View in CoL ( Pangasius larnaudii View in CoL ). Cambodia............................................................................. Pangasiocestus Scholz & de Chambrier, 2012 View in CoL
4a.Lateral band of vitelline follicles occupies almost entire length of proglottids; proglottids anapolytic ....... 5
4b. Lateral band of vitelline follicles restricted to region between genital pore and anterior margin of ovary; proglottids apolytic. In Schilbeidae View in CoL ( Clupisoma garua View in CoL ). India.................................. Vermaia Nybelin, 1942 View in CoL
5a. Testes in single field; rostellum-like organ spherical..................................................................................... 6
5b. Testes in two fields; rostellum-like organ disc-like or flattened. In Malapteruridae View in CoL ( Malapterurus electricus View in CoL ). Africa .................................................................................................. Electrotaenia Nybelin, 1942 View in CoL
6a. Ventral osmoregulatory canals median (internal) to vitelline follicles; rostellum-like organ longer than, or as long as, suckers; strong retractor muscles form a wide band. In Siluridae View in CoL , Bagridae View in CoL and Schilbeidae View in CoL . Southern and eastern Asia ..................................................................................... Gangesia Woodland, 1924 View in CoL
6b. Ventral osmoregulatory canal lateral (external) to vitelline follicles; rostellum-like organ distinctly smaller than suckers; retractor muscles present only on lateral sides of rostellum-like organ. In Siluridae View in CoL ( Silurus glanis View in CoL and S. soldatoƲi View in CoL ). Europe, China? ............................................................. Silurotaenia Nybelin, 1942 View in CoL
SPECIES COMPOSITION OF GANGESIA
Ash et al. (2015) synonymized numerous poorly described, insufficiently differentiated species of Gangesia from the Indomalayan region. Since then, Jasrotia & Kaur (2017) described G. punjabensis Jasrotia & Kaur, 2017 from Wallago attu in India based on alleged molecular support (GenBank accession number KY018599). However, the sequence KY018599 of Jasrotia & Kaur (2017) includes obvious errors, because it includes the primer regions (containing errors) used by the authors for PCR amplification and sequencing. This error questioned the quality of the sequence. Moreover, if we exclude the primer regions from KY018599, there are only two single cytosine insertions left, both situated close to the 5ʹ and 3ʹ ends of the sequence, i.e. positions of likely lower sequencing quality. Otherwise, the KY018599 lsr DNA fragment is identical with the remaining four representatives of Gangesia bengalensis (from an identical host species) in the GenBank database. In its morphology, G. punjabensis shares all taxonomically important characters with G. bengalensis , including rostellar hook number (47–54), number of rows of rostellar hooks (two), number of rows of hooklets on sucker (five to eight), relative size of the cirrus-sac (about one-quarter of width of proglottids) (see: Ash et al., 2012: figs 1A–E, 2 and Jasrotia & Kaur, 2017: figs 1, 2A–B, 3). Therefore, we consider G. punjabensis a new synonym of G. bengalensis .
Later on, Jasrotia et al. (2019) described another new species of Gangesia , namely Gangesia harikeensis Jasrotia et al., 2019 , based on two specimens collected from the same fish host, Wallago attu Bloch & Schneider, 1801 , in Punjab, India with molecular data (GenBank accession no. MK127923). As in the case of G. punjabensis , their molecular analysis is questionable; the node at which the species is placed has no support value and the analysis also placed it as a sister-taxon to the clade consisting of all other Gangesia species. We have also found discrepancies between the data provided in the text and those taken from illustrations ( Jasrotia et al., 2019: figs 1, 2), such as measurements of the width of the rostellar-like organ, sucker width and length of the blade of rostellar hooks. Although the specimens of G. harikeensis were strongly contracted and deformed (see Jasrotia et al., 2019: fig. 2), the important taxonomic characters, such as the length of the scolex, presence of double rows of rostellar hooks, number of testes, relative size of cirrus-sac and the host species, make it indistinguishable from G. bengalensis . Therefore, G. harikeensis is also synonymized with G. bengalensis .
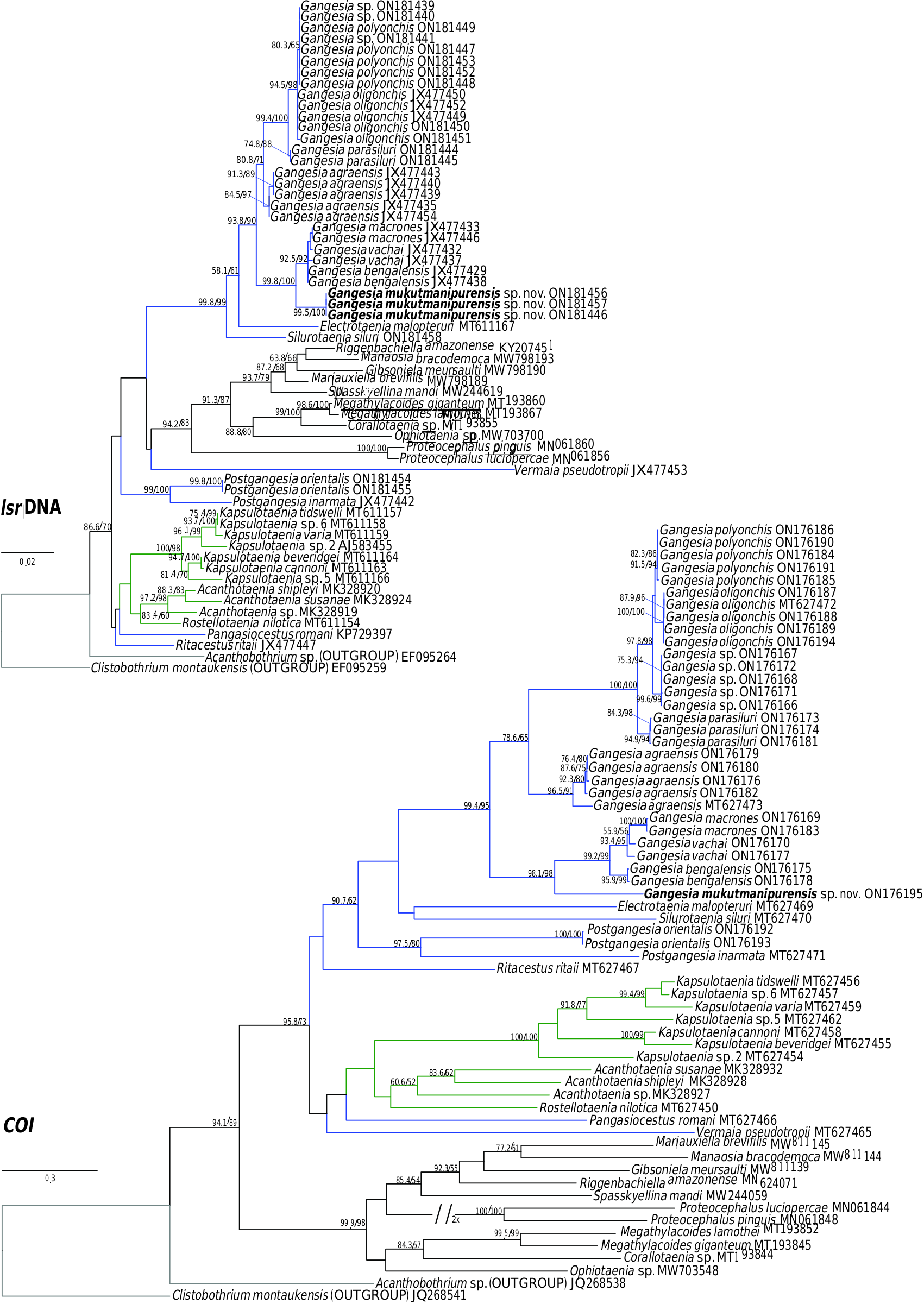
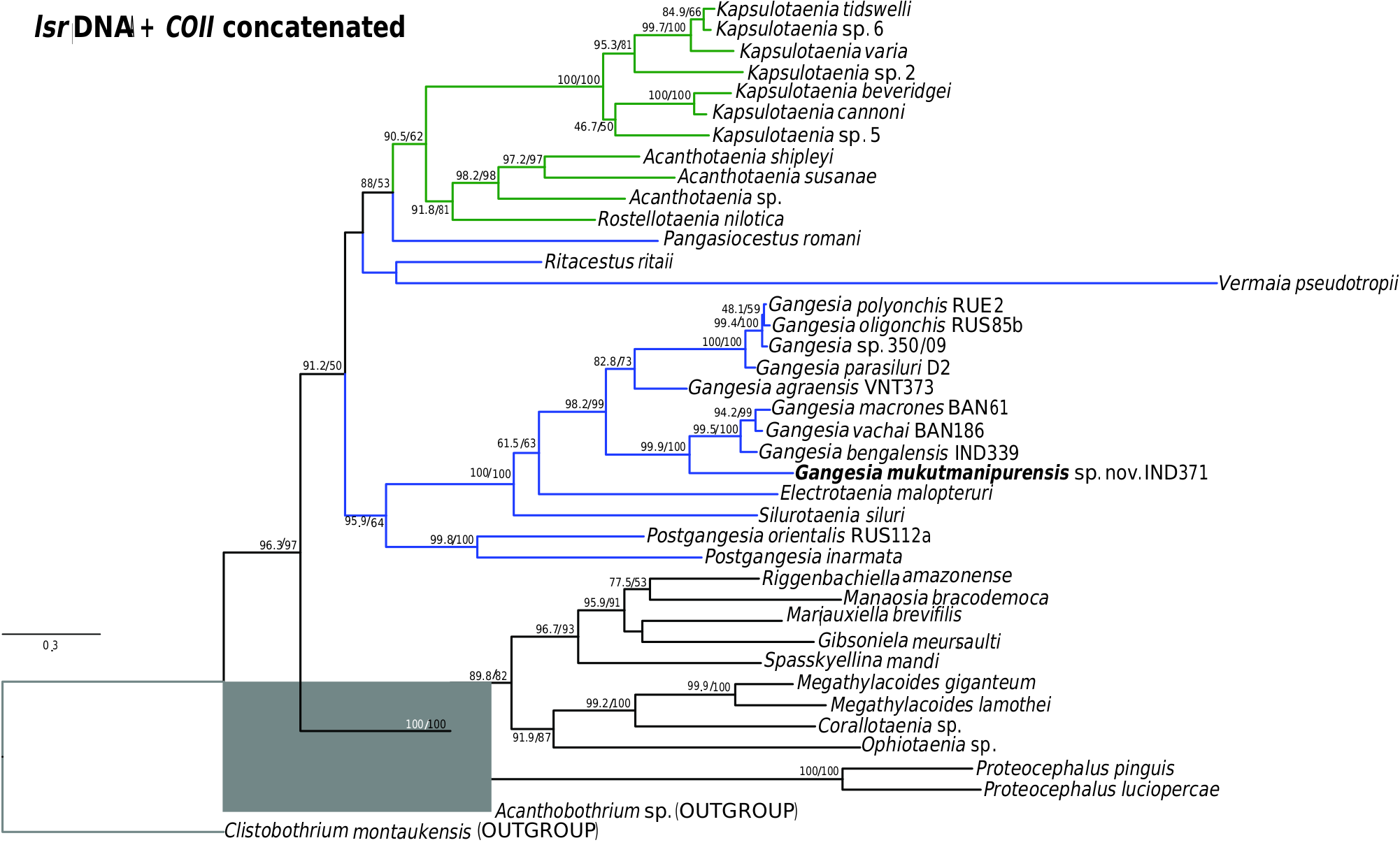
Specimens of Gangesia sp. collected by M. Oros and T. Scholz from Silurus cf. soldatoƲi Nikolskii & Soin in China ( Ash et al., 2015) in 2009 and 2015, provided to the present authors (MHNG-PLAT 67056), form a distinct group based on COI and concatenated data ( Figs 1 View Figure 1 , 2 View Figure 2 ; Supporting Information, Fig. S1 View Figure 1 ) and probably represent a distinct and undescribed species of Gangesia . Unfortunately, these specimens do not have mature and gravid proglottids, which are necessary for formal description of a new species.
As stated previously, the new species G. mukutmanipurensis is unique among species of Gangesia in lacking hooks on the rostellum-like organ and hooklets on the anterior rim of the suckers. This peculiarity of its scolex morphology is arguably a case of secondary loss of structures. These reversals appear to be rare among cestodes but have been documented in at least two genera of cyclophyllidean cestodes, namely Taenia Linnaeus, 1758 and Hymenolepis Weinland, 1858 , where rostellar hooks are absent in the adults of some species ( Šlais, 1973). The presence of different rostellar types (armed rostellum, rudimentary unarmed rostellum) or the lack of a rostellum in multispecies lineages of Hymenolepididae confirm that rostellar morphology is not a reliable indicator of relationships or taxonomy ( Haukisalmi et al., 2010; Neov et al., 2019). Neov et al. (2019) showed that partial or entire reduction of the rostellar apparatus is an apomorphic condition that arose separately in all lineages of hymenolepidids. Similarly, multigene analyses of the genus Taenia have demonstrated that the absence of rostellar hooks in T. saginata Goeze, 1782 and T. asiatica Eom & Rim, 1993 is a case of secondary loss ( Knapp et al., 2011; Nakao et al., 2013). Host associations in different biogeographical regions may play a crucial role for such evolutionary processes ( Hoberg, 2006).
Life-history strategies of parasites are often specific to a particular phase/stage of their life cycle ( Kuris & Lafferty, 2000; Poulin, 2011). Interaction of larval helminths with their intermediate hosts can be markedly different from that of adult worms and their definitive hosts ( Poulin, 2011). Little is known about the life cycle of species of Gangesia , except for two experimental studies of Demshin (1985) and Shimazu (1999) that confirmed planktonic copepods and prey fish as the first and second intermediate (or paratenic) hosts, respectively. Procercoids are formed in copepods, which are ingested by prey fish and grow to plerocercoids, which are infective to the final (definitive) hosts in which the worms mature. In Gangesia parasiluri , hooklets appear on the rostellum-like organ in procercoids and are also present in plerocercoids, but then disappear and are replaced by newly formed hooks in adults ( Shimazu, 1999).
It is generally accepted that the scolex, being the attachment organ, is often adapted to the particular morphology and physiology of the host intestine, and that ‘mucosal topography’ is a ‘critical resource’ for enteric helminths ( Hayunga, 1991). It is also presumed that highly host-specific helminths are especially adapted to the architecture of their preferred intestinal sites ( Hayunga, 1991). Evidence from certain Tetraphyllidea-like cestodes – the group from which proteocephalids may have evolved – suggests that compatibility between scolex morphology and attachment site, along with gut physiology, may determine host specificity ( Williams, 1960, 1966, 1968a, b; Randhawa & Burt, 2008). In addition, features of the strobila, specifically the shape of the proglottids, may also closely match the intestinal architecture ( Joy et al., 2009). The evolutionary loss of hooks from the scolex of the new species cannot be readily explained at this time, but it is reasonable to propose that the uniqueness of the scolex in this new taxon may be an adaptive evolutionary change related to the internal gut morphology and physiology of its fish host, Ompok bimaculatus .
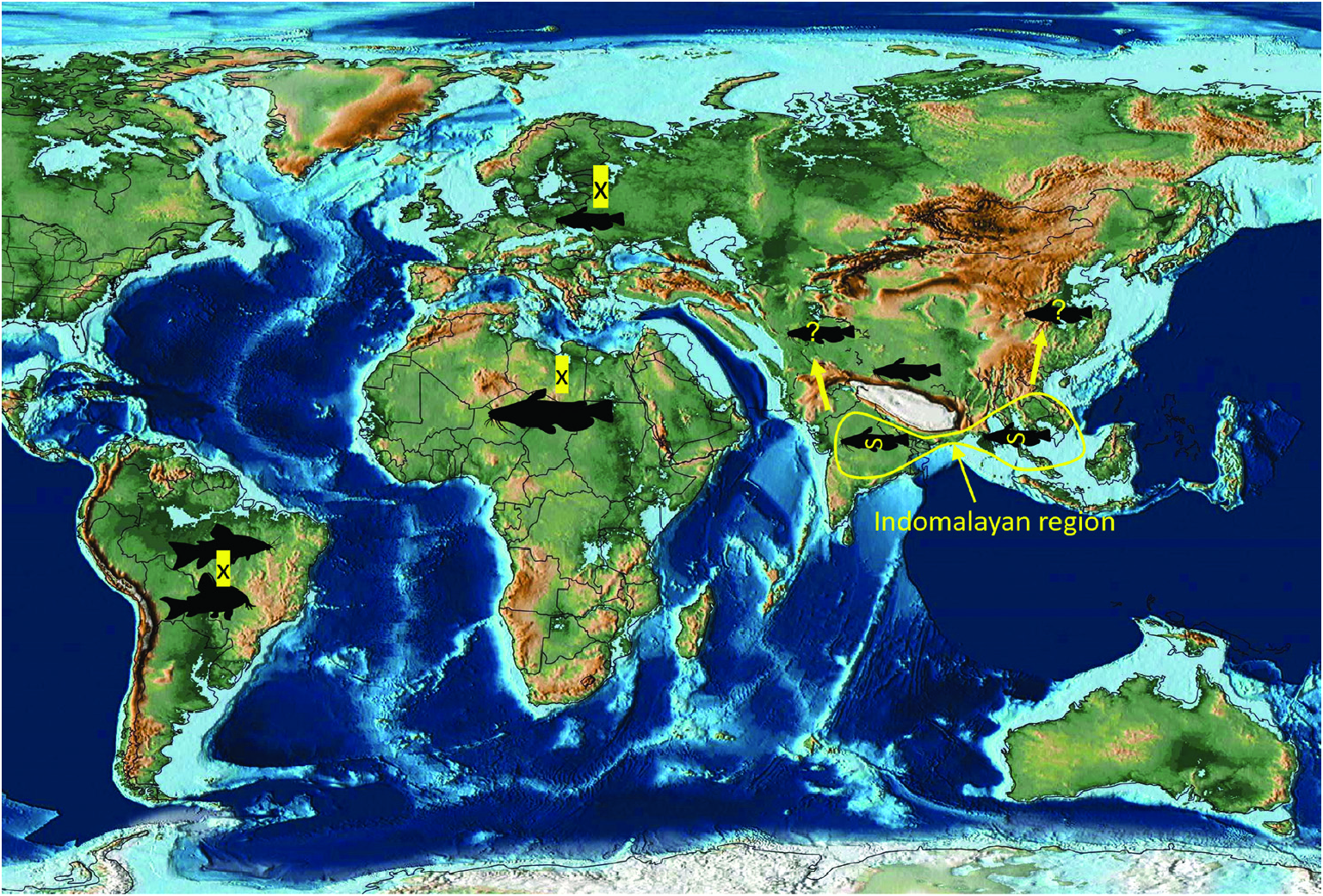
ORIGINS AND HISTORICAL BIOGEOGRAPHY OF EARLY BRANCHING PROTEOCEPHALID LINEAGES (‘ GANGESIINAE’ SENSU LATO) IN THEIR CATFISH HOSTS
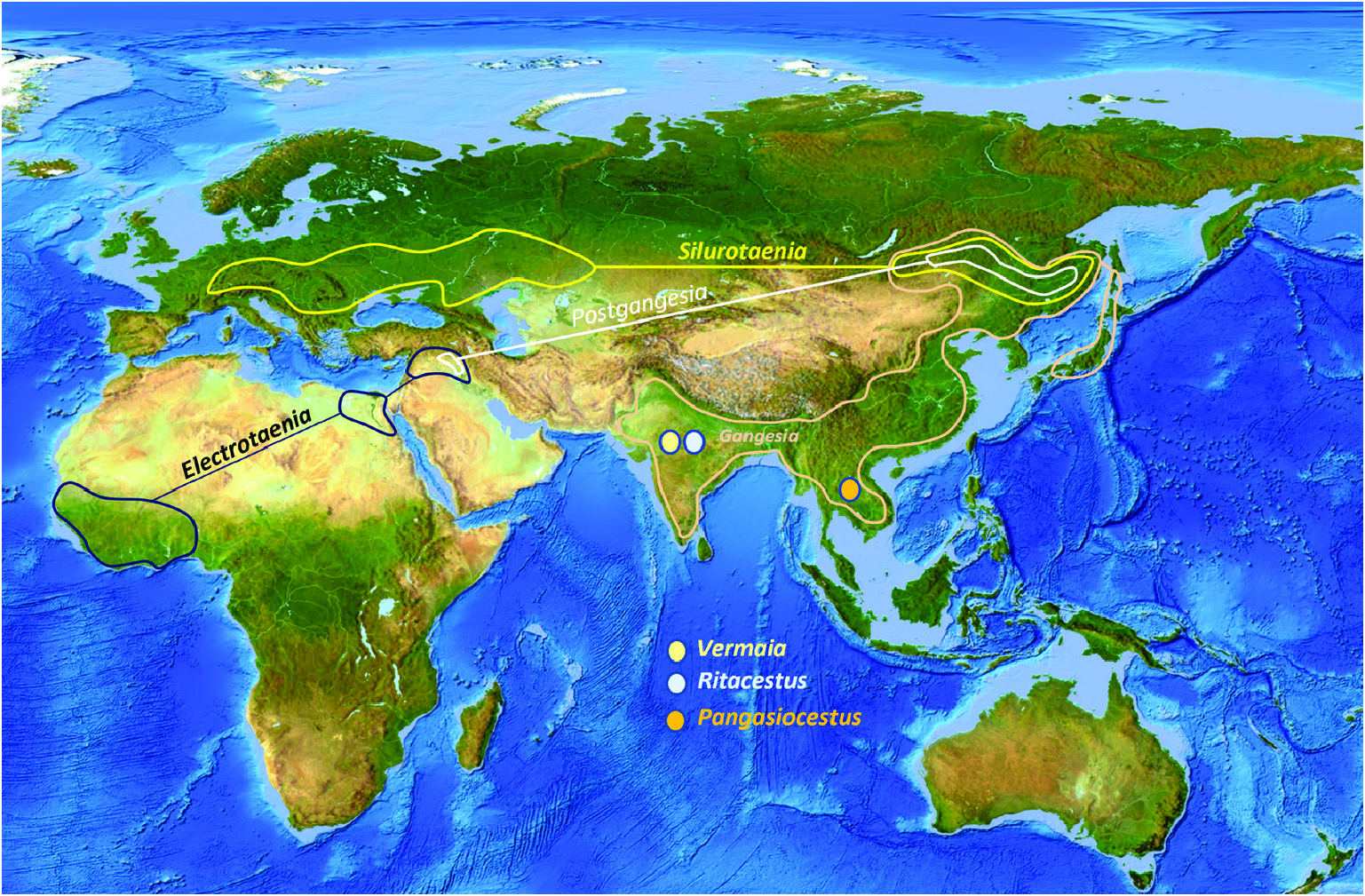
Previous studies ( Ash et al., 2012, 2015) have clarified the taxonomy, host associations and contemporary distribution of several early branching lineages of proteocephalid tapeworms conventionally placed in Gangesiinae , a group of tapeworms occupying the freshwaters of the Indomalayan and contiguous Palaearctic biogeographical regions ( Fig. 7 View Figure 7 ). The hosts of species of Gangesia include mainly catfishes of the families Siluridae and Bagridae , with one species in a schilbeid catfish ( Eutropichthys Ʋacha Hamilton, 1822 : Schilbeidae ). The sisterlineages of Gangesia are Silurotaenia , a parasite of a silurid catfish in the Palaearctic region, and Electrotaenia , a parasite of Malapterurus electricus (Gmelin, 1789) (Malapteruridae) in North Africa ( Fig. 7 View Figure 7 ). Representatives of the Acanthotaeniinae , parasites of varanid lizards and snakes, together with several lineages of the Gangesiinae s.l., form the basal groups of proteocephalids. Three monotypic genera are currently recognized in Gangesiinae , all narrowly specific for their siluroid hosts in the Indomalayan region, and whose phylogenetic relationships to the remaining basal groups remain unclear. Ritacestus ritaii (Verma, 1926) occurs in India as a parasite of Rita rita (Hamilton, 1822) , a catfish conventionally placed in Bagridae , but found to be deeply separated from the bagrid clade ( Kappas et al., 2016). Vermaia pseudotropii (Verma, 1928) is a parasite of the schilbeid catfish, Clupisoma garua (Hamilton, 1822) in India. Pangasiocestu s is associated with the pangasiid catfish, Pangasius larnaudii Bocourt, 1866 , in Cambodia. In addition, species of Postgangesia are found in Silurus spp. in the Palaearctic region. The phylogeny, host associations and contemporary distribution provide the basis for analysing the historical biogeography of these tapeworm lineages in their catfish hosts, although we are currently handicapped by the poor resolution of relationships among the early diverging clades ( Pangasiocestus , Ritacestus , Postgangesia and Vermaia ).
Despite the poor resolution of the phylogenetic relationships among groups occupying the base of the proteocephalid tree, their widespread and largely exclusive association with catfishes ( Actinopterygii: Siluriformes ) suggest that a discussion of the historical biogeography of catfishes, particularly the siluroids, which host all of the known basal lineages in question (conventional Gangesiinae ), may provide insight into the origins of these host–parasite associations.
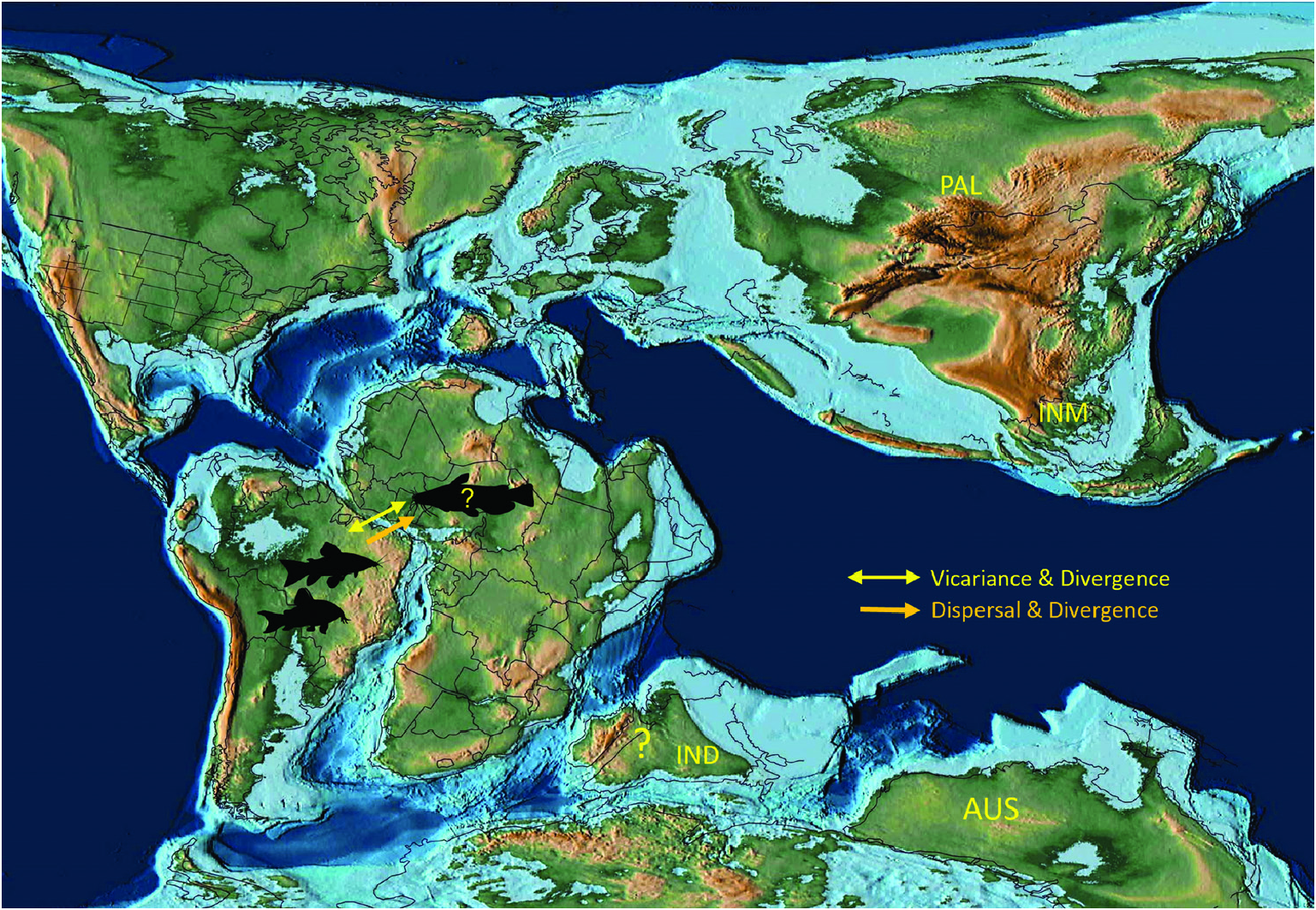
Using phylogenetic and biogeographical analyses based on five nuclear genes and two separate timecalibration methods based on the fossil evidence, Chen et al. (2013) generated chronograms that placed siluriform origins, respectively, at 97 Mya or 117 Mya and the origin of siluroids at 79 Mya or 86 Mya. The chronogram from a molecular clock analysis by Kappas et al. (2016) places the origin of the catfishes (order Siluriformes ) in the early Cretaceous (133 Mya) and the divergence of the well-supported Siluroidei at 97 Mya. Both studies support the hypothesis of Gondwanan siluriform origins approximately when Africa and South America were completing their final separation ( Fig. 8 View Figure 8 ). The analysis of Dai et al. (2018) has the siluriforms originating at 86–74 Mya, close in age to the oldest fossil records of catfishes in South America. More importantly for our study, these calibrated reconstructions suggest that siluroids originated at least 43–24 Mya after the final separation of Gondwana and Laurasia (~140 Mya) and shortly after, or approximately with, the separation of Africa and South America (~110–100 Mya; Fig. 8 View Figure 8 ). This also means that Siluroidei diverged after India separated from both Africa and Australia and began its long journey in isolation ( Smith et al., 1994; Scotese & Wright, 2018), before finally docking on to the Eurasian landmass in the Eocene, between 50 and 45 Mya ( Scotese, 2016; Scotese & Wright, 2018). Meanwhile Siluroidei diversified into the ‘Big Asia’ and ‘Big Africa’ clades in Africa and Laurasia during the Late Cretaceous ( Kappas et al., 2016), with a remarkable radiation in the Cenozoic ( Diogo, 2004; Kappas et al., 2016). The presence of Late Cretaceous (Maastrichtian) freshwater siluriform fossils in India ( Cione & Prasad, 2002) – also the oldest catfish fossil in Asia – indicates that India did have freshwater catfishes during its long isolation, but the limited and fragmentary nature of the fossils do not allow a robust analysis of its affinities with other siluriforms.
When considered together, the phylogeny and host associations of gangesiine tapeworms and the evolutionary history of siluroids suggest one or more possible scenarios for the origins of their associations. The deepest node in the phylogeny of Proteocephalidae is a polytomy comprising several branches of Gangesiinae (s.l.) along with proteocephalids ( Acanthotaeniinae ) in varanid lizards and snakes. The oldest possible host association in Gangesiinae would have been that of Ritacestus ritaii with Rita rita , a catfish belonging to an early branching (~97 Myr) lineage of siluroids (but see discussion in the next section). The other associations are probably much younger. The host of Vermaia , Clupisoma garua , is part of the large Afro-Asian catfish family Schilbeidae , estimated to be 50–55 Myr old. Even allowing for the wider distribution of Pangasiocestus in Pangasiidae Bleeker, 1858 , this association is also not older than ~40–45 Myr, the age of Pangasiidae (and of P. larnaudii , the host of the monotypic Pangasiocestus romani Scholz & de Chambrier, 2012 ). Postgangesia spp. are parasites of Silurus catfishes, a relatively recently evolved lineage (~23 Myr, Kappas et al., 2016). The remaining gangesiines ( Electrotaenia + Silurotaenia + Gangesi a) form a clade that is also part of this unresolved basal polytomy. Malapterurus electricus , the host of Electrotaenia , is an old lineage of catfishes (divergence at ~60 Mya). Therefore, several lineages of hosts were already present in Eurasia when India finally merged with it ( Fig. 9 View Figure 9 ).
The evolution of the remaining gangesiines, i.e. species of Gangesia , involves radiation in catfish hosts, including several silurids ( Wallago attu , Ompok bimaculatus and Silurus asotus ) and bagrids ( Tachysurus fulƲidraco , Sperata seenghala and Mystus spp. ). Bagridae evolved ~62 Mya, with Tachysurus fulƲidraco (syn. Pelteobagrus fulƲidraco ) originating around the same time (~22 Mya) as Silurus . The age of other bagrid hosts of Gangesia , such as S. seenghala (the host of G. macrones in India), remains unknown but, given the position of G. macrones on the tree, we can infer that it too was part of a Neogene radiation of these tapeworms. The origin of the hookless Gangesia mukutmanipurensis in the silurid Ompok bimaculatus is also firmly embedded in the radiation of Gangesia in catfishes of the Indomalayan region during this geological period. Whether Gangesiinae evolved early in the evolutionary history of siluroid catfishes will depend on the position of lineages that are associated with evolutionarily older siluroid lineages, such as Rita , Malapterurus and Pangasius , relative to those in younger catfish clades, such as Silurus .
AN ‘ OUT OF INDOMALAYA’ OR AN ‘ INTO INDOMALAYA’ HYPOTHESIS FOR GANGESIINAE ?
The current polytomy of several gangesiine lineages at the base of the phylogenetic tree limits our reconstructions of the historical biogeography (assuming that this is not a ‘hard’ polytomy) of these tapeworms. Nevertheless, several of these deep lineages of Gangesiinae ( Pangasiocestus and Ritacestus ) are associated with the Indomalayan region, and the diversification of Gangesiinae (e.g. Gangesia ) also involves this region. The siluroid Pangasius , host of Pangasiocestus , is endemic to South and South-East Asia (the Indomalayan region). Rita rita , a species of an early branching siluroid lineage (and host of Ritacestus ), is widely distributed across the Indian subcontinent and Burma. All other species of Rita are also restricted to South Asia. Despite its old evolutionary age, Rita probably did not evolve by drift vicariance when India separated from Africa because it is not consistent with the geological history of India. By the time India became contiguous with Eurasia, the predominant siluroid host groups had already originated and diversified in Africa and Eurasia, and access from the east and west would have allowed siluroids from these regions to extensively colonize the freshwaters of India. It is likely that Rita rita formed an early part of that colonization, although its nearest relatives among catfishes remain unknown. Rita has been conventionally placed in Bagridae , but the mitogenome analysis refutes that classification.
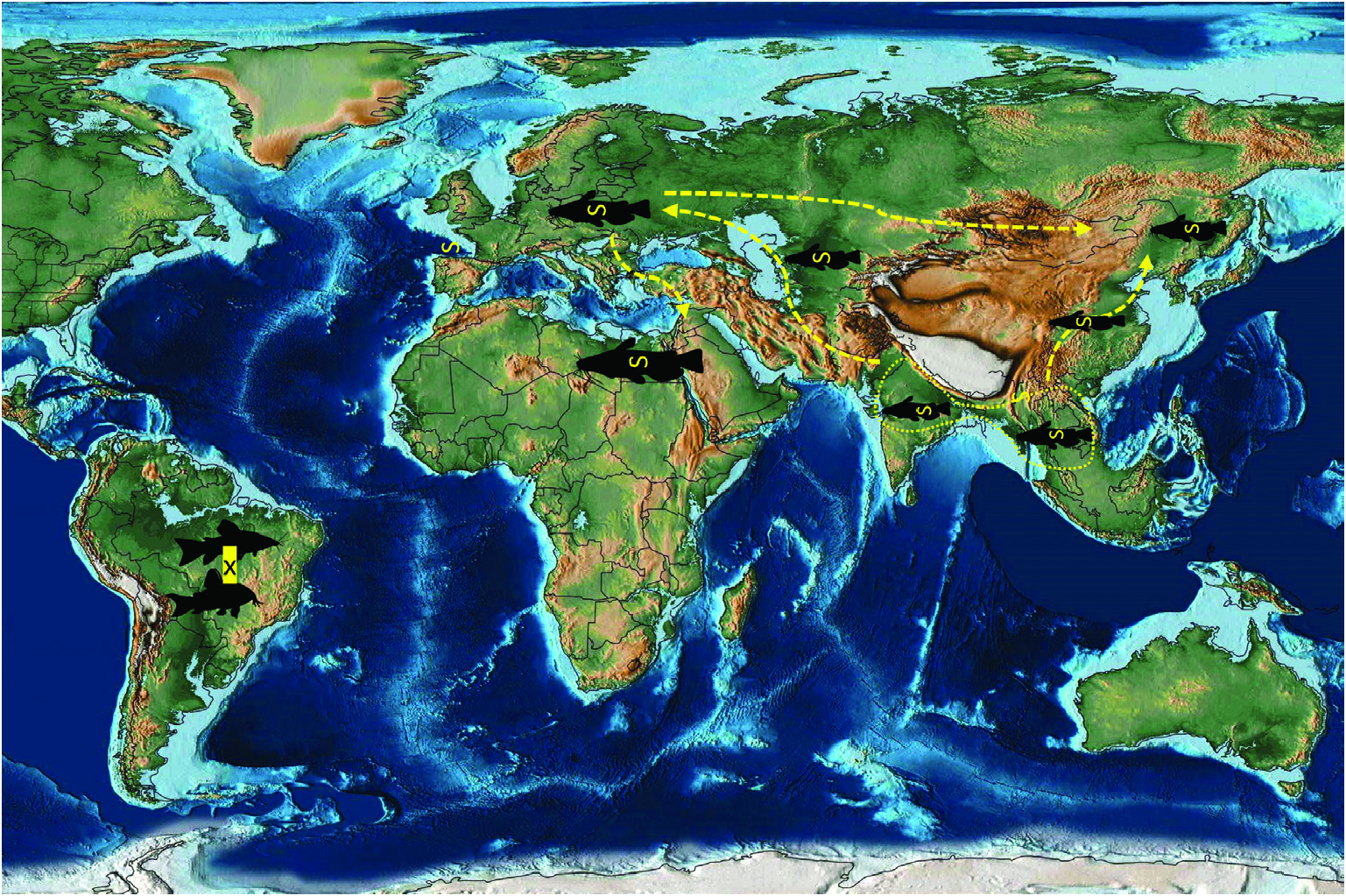
An integrative approach to the question of gangesiine origins leads us to consider an ‘Out of Indomalaya’ dispersalist hypothesis. Given the age of the remaining host groups of Gangesiinae , i.e. between 62 Mya ( Bagridae ) and 24 Mya ( Siluridae ), one may hypothesize that Gangesiinae (s.l., i.e. including Pangasiocestus , Ritacestus and Vermaia ) originated in the Indomalayan region during the Palaeogene of the Cenozoic ( Fig. 9 View Figure 9 ), thereafter dispersing to Europe and subsequently Africa ( Fig. 10 View Figure 10 ), with range expansion and areas of diversification in South and South-East Asia ( G. macrones , G. bengalensis, G. Ʋachai and G. mukutmanipurensis ) and in north-east Asia ( G. oligonchis , G. parasiluri and G. polyonchis ). That Gangesiinae are not a group with deep Gondwanan origins is also supported by the absence of these tapeworms in sub-Saharan Africa, despite schilbeids and bagrids being present there, and in South America. The earliest branching catfish groups, Diplomystidae and Loricariidae of South America, are not hosts of gangesiines or any other early branching proteocephalids. Despite the remarkable diversity of proteocephalids in South American catfishes ( de Chambrier et al., 2015, 2017), the foregoing discussion suggests that the Indomalayan region is the original homeland of these early diverging groups of proteocephalids.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
SubFamily |
Gangesiinae |