Myotis nimbaensis, Simmons & Flanders & Fils & Parker & Jamison D. Suter & Bamba & Douno & Mamady Kobele Keita & Morales & Frick, 2021
|
publication ID |
https://doi.org/ 10.5281/zenodo.4438059 |
|
DOI |
https://doi.org/10.5281/zenodo.4438061 |
|
persistent identifier |
https://treatment.plazi.org/id/867D87BA-1B55-ED02-BEB5-FE0517FD5AED |
|
treatment provided by |
Donat |
|
scientific name |
Myotis nimbaensis |
| status |
sp. nov. |
Myotis nimbaensis View in CoL , new species
Nimba Myotis
Figures 3–8 View FIGURE 3 View FIGURE 4 View FIGURE 5 View FIGURE 6 View FIGURE 7 View FIGURE 8
HOLOTYPE: AMNH 279589 , an adult male captured and released on 26 January 2018 at Kaiser Adit 3 and then collected on 2 February 2018 by Jon Flanders and Eric Moïse Bakwo Fils at Kaiser Adit 1 (fig. 2), Nimba Mountains , Guinea (N07.66499, W008.37223), field number BCIGU133 . The specimen was preserved whole in formalin and stored in ethanol, and the skull was subsequently extracted and cleaned. A 3 mm wing biopsy tissue sample was collected and stored in desiccant before the DNA was extracted at CIBIO-InBIO, University of Porto, Portugal. GoogleMaps
PARATYPE: AMNH 279590 , an adult female collected at the same time and place as the holotype, field number BCIGU132 . Like the holotype, the paratype was preserved in formalin and stored in ethanol after a 3 mm wing biopsy tissue sample was collected and stored in desiccant before the DNA was extracted. The skull was subsequently extracted and cleaned.
OTHER RECORDS: In addition to the captures of the holotype and paratype, we recorded echolocation calls from the male holotype on 26 January 2018. Once its distinctive call parameters were identified, we searched for its echolocation call signature in sound files recorded at the entrances of 10 mine adits between 2018–2019, including the type locality, within the Nimba Mountains’ Mining Concession in Guinea (fig. 1), using Song Meter SM4BAT acoustic detectors (Wildlife Acoustics). Echolocation calls closely matching those recorded from the release calls of the holotype were detected at the entrances of a total of five mine adits (fig. 1) with seasonal variation between sites and the highest activity rates recorded at the type locality. While the Nimba Mountains support an exceptionally diverse bat fauna, this is the first Myotis species observed using the adits and only the second Myotis species to be recorded across the whole mountain range ( Monadjem et al., 2016). Details of call frequency and structure, as well as comparisons with calls of congeneric species, are provided below in the section entitled Echolocation Calls.
DISTRIBUTION: Known only from the type locality and vicinity in the Guinean Nimba Mountains .
ETYMOLOGY: Myotis nimbaensis (“from Nimba ”) is named in recognition of the mountain range in which it was discovered. As an epithet referring to a place, nimbaensis is spelled the same way whether applied in combination with either a masculine or a feminine genus name. Woodman (1993) argued that Myotis should be considered feminine in gender, but Pritchard (1994) disagreed. Both of these authors overlooked a 1958 ruling by the International Commission on Zoological Nomenclature that fixed the gender of Myotis as masculine and placed the name as such on the Official List of Generic Names in Zoology ( International Commission on Zoological Nomenclature, 1958). So in this case, nimbaensis is masculine.
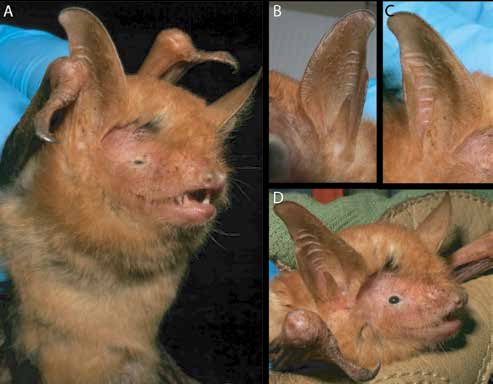
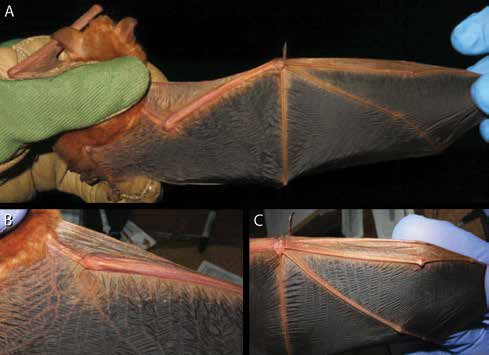
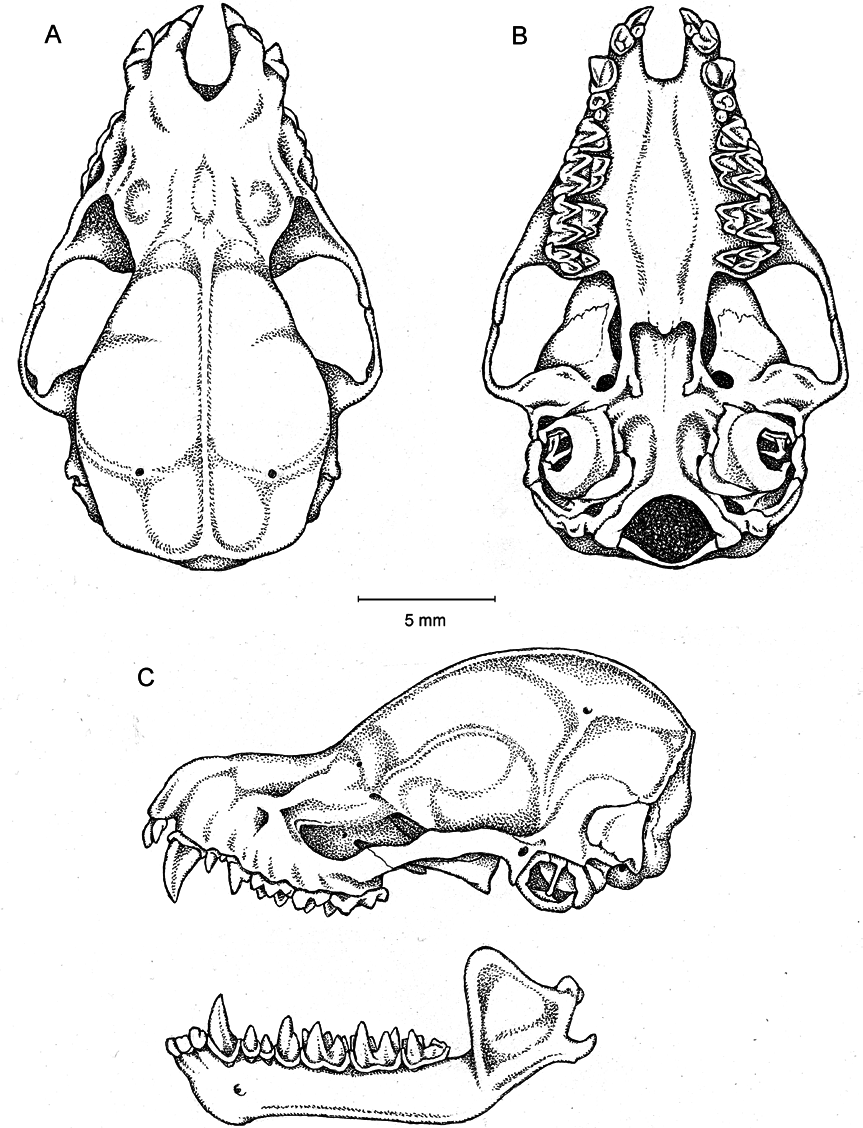
DIAGNOSIS AND DESCRIPTION: Myotis nimbaensis is diagnosed by a combination of the following characteristics: large size (FA 52.4–55.2 mm, mass 15.5–17.0 g; table 1) with females apparently somewhat larger than males; dorsal fur bright orange with strongly tricolored hairs (basal 1/3 of hair shaft black, middle 1/3 of shaft creamy white, distal 1/3 and tip bright orange to coppery red); ventral fur paler than dorsal fur, tricolored on belly (black base, buff-colored shaft that grades to orange at tip) grading to bicolored (lacking black base) on the sides near the wing membranes; ruff of brighter fur present around neck; pointed face with pale skin covered with orange fur, skin clearly visible through the fur around eye, mouth, and on rostrum; no black spots present on face; pinna with a rounded tip, relatively long and reaching beyond tip of nose when laid forward; pinna with strong distal emargination; skin of pinna pale orange brown becoming slightly darker near the tip, no rim of black around ear; tragus lanceolate, slightly less than half the length of the pinna; thumb brown; wing membranes strongly dichromatic black and orange, with plagiopatagium and dactylopatagium mostly black with narrow bands of orange along the metacarpals, phalanges, and across the membrane behind the forearm and upper arm; black pigmentation extends medially nearly to body and posteromedially to tibia; membrane between metacarpals I and II pale orange; propatagium pale orange; uropatagium orangish brown with orange fur on the proximal 1/3 of the dorsal surface and pale cream-colored fur on proximal 1/5 of ventral surface; no black spots on patagia; no conspicuous gland present in plagiopatagium behind humerus; pale ventral fur extends onto proximal plagiopatagium in narrow strip along body, does not extend past knee; relatively small foot with length approximately 2/5 of tibia length; foot and toes brown, with sparse long brown hairs on the dorsal surface of each toe; wing membrane essentially naked except close to the body, where it has a sparse covering of cream colored hairs on the ventral surface; wing membrane attaches to foot at base of first toe; calcar long, more than twice the length of the hind foot, runs approximately 2/3 of the length of the uropatagium border; skull large for Myotis (GLS = 19.48–19.73 mm; CIL = 18.92–18.93 mm; CBL = 18.31–18.81 mm) with globular braincase, well-defined sloping forehead, and elongated supraorbital region all contributing to the appearance of a clearly defined and elongate rostrum; rostrum with shallow medial depression in nasal region; sagittal crest moderately well developed in both sexes; lambdoid crest weakly developed in both sexes; dental formula I2/3, C1/1, P 3/3, M3/3 = 38; I1 and I2 each with a well-developed posterior cusp; upper canine robust, crown height approximately 2 × that of P4, basal area (as seen in occlusal view) roughly equal to that of P4; anterior two premolars (P2 and P3) much smaller than last premolar (P4); P3 with less than half the basal area of P2 and shifted lingually to be partly excluded from the toothrow although still visible in lateral view; P4 large, sharply pointed, taller than M1; lower incisors each with 4 main cusps; i3 with distal accessory cuspules; p3 relatively large, approximately three quarters the basal area of p2, and fully in line in toothrow, not shifted lingually; lower molars myotodont.
COMPARISONS: External and craniodental measurements for Myotis nimbaensis and other congeneric African species with which it might be confused are provided in table 1. In addition to M. nimbaensis , three other large Myotis (subgenus Chrysopteron ) species with orange dorsal fur occur in Africa: M. tricolor , M. welwitschii , and M. morrisi . Descriptions and measurements of these species can be found in Hill and Morris (1971), Hill et al. (1988), Taylor (2000), and Monadjem et al. (2010); the latter work also includes color photographs and echolocation call spectrograms.
Myotis nimbaensis can be easily distinguished from M. morrisi on the basis of size, with M. morrisi being smaller in all external and craniodental dimensions (table 1). The ventral pelage of M. morrisi is also different—rather than being tricolored as in M. nimbaensis , M. morrisi has unicolored dull cream white ventral fur that is tinged a faint brown on the flanks and chin. The skull and dentition of M. morrisi is very similar to that of M. nimbaensis although the braincase of M. morrisi is somewhat more globular and lacks the sagittal crest seen in M. nimbaensis .
g BMNH 22.12.17.76.
Myotis nimbaensis is similar in size and general coloration to the widespread species M. welwitschii , but these taxa can be easily distinguished because M. nimbaensis completely lacks the prominent black spots seen on the face and patagia of M. welwitschii (figs. 3–6). Color of the pinnae is also different—bright orange to coppery red with a black edge in M. welwitschii compared to pale orange brown with no black edge in M. nimbaensis . The thumb in M. welwitschii is bright orange whereas it is brown in M. nimbaensis . Both species are strongly dichromatic with black wing membranes and orange along the digits and forearm, but pigmentation of the plagiopatagium near the body and hind legs is different in the two species. Myotis welwitschii has a broad band of orange along the side of the body that extends anteriorly past the elbow to run along the forearm and posteriorly to the ankle, so black pigmentation is limited to the more distal and posterior portions of the plagiopatagium. In contrast, the orange is much less extensive and the black is more extensive in M. nimbaensis ; the black pigmentation approaches the body wall, extending anteriorly into the plagiopatagium behind the humerus and posteriorly to the tibia in M. nimbaensis , so there is no broad band of orange along the body in that species. The uropatagium in M. welwitschii is also bright orange whereas it is darker and more brownish in M. nimbaensis . There are additional differences in features of the dorsal and ventral pelage—the dorsal fur in M. welwitschii is bicolored (black at base and otherwise coppery red) rather than being tricolored as in M. nimbaensis , and M. welwitschii has unicolored ventral pelage (whitish tinged with coppery red) rather than the distinctive tricolored ventral fur in seen in M. nimbaensis .
Craniodental measurements (table 1) suggest overall similarity of M. nimbaensis with M. welwitschii ; small differences in a few dimensions are not interpretable given the small sample sizes. Both species have lambdoidal crests that are weakly developed but clearly visible, and moderately well-developed sagittal crests in both sexes. The dentition of M. welwitschii includes two very small anterior upper premolars that are both included in the toothrow; P3 is smaller than P2, but P3 is not displaced lingually as it is in M. nimbaensis .
Myotis tricolor is a widespread species that shows considerable morphometric variation across its range ( Koopman, 1989, 1994; Taylor, 2000; Monadjem et al., 2010). Myotis nimbaensis is roughly equivalent in overall size (e.g., forearm length and body mass) to the largest individuals of M. tricolor reported in the literature, although none of the specimens of M. tricolor that we measured were as large as M. nimbaensis . The only known specimen of M. tricolor from West Africa—a male from Liberia referred to that species by Koopman (1989) —is much smaller than M. nimbaensis , having a forearm length of 46.0 mm compared to 52.4 mm for the male paratype of M. nimbaensis . The pelage of M. tricolor is similar to that of M. nimbaensis , and M. tricolor similarly lacks black spots on the face or tail membrane. However, these species can be distinguished easily based on coloration of the wing membranes: M. tricolor has wings that are dark brown rather than the distinctive dichromatic orange and black wings seen in M. nimbaensis . The pinnae of M. tricolor are dark brown compared with the pale orange brown seen in M. nimbaensis , and the emargination is more proximally located (nearly at the midpoint of the lateral edge of the pinna) in M. tricolor compared to the clearly distal location of the emargination in M. nimbaensis . The uropatagium of M. tricolor is also darker brown (rather than orange brown as in M. nimbaensis ) and has a dense covering of coppery red fur proximately (more sparse in M. nimbaensis ). In terms of craniodental features, M. nimbaensis has a greater condylobasal length (18.9 mm for both sexes) than seen in M. tricolor (15.9–18.5 mm including data from table 1 and Monadjem et al., 2010). Although our comparative sample of M. tricolor was not large, we found that most other craniodental measurements similarly appear to distinguish M. nimbaensis and M. tricolor , with M. nimbaensis the larger of the two species.
Sagittal crest development in Myotis tricolor is variable, with females typically lacking any sagittal crest development. In contrast, both male and female M. nimbaensis have well-developed sagittal crests (though again, our sample size is small). Upper premolars in M. tricolor are somewhat variable, but P3 is always less than half the basal area of P2 and is always shifted lingually as in M. nimbaensis . However, in many individuals the reduction in P3 is greater than in M. nimbaensis , and this tooth is completely excluded from the toothrow (only partly excluded in M. nimbaensis ). The p3 of M. tricolor is one half to two thirds the basal area of p2, and p3 is sometimes displaced lingually from the toothrow, compared to a slightly larger p3 that is at least three quarters the size of p2 and which is not displaced in M. nimbaensis .
Another orange Myotis species from Africa is M. bocagii , which occurs across much of tropical Africa and is broadly sympatric with M. tricolor and M. welwitschii (Monadjem and Jacobs, 2017a; Patterson, 2019). Myotis bocagii can be easily distinguished from M. nimbaensis based on its dark brown wings and considerably smaller size in all dimensions (e.g., FA = 36.0– 40.5 mm; mass = 6.0–9.0 g.; CBL = 13.0–15.0 mm; CIL = 14.0– 15.2 mm; Koopman, 1994; Monadjem et al., 2010).
Myotis (subgenus Chrysopteron ) species with yellow or orange dorsal fur and dichromatic orange and black wings are not limited to Africa, but also occur in Asia. At least six Asian species ( M. bartelsi , M. formosus , M. hermani , M. rufoniger , M. rufopictus , and M. weberi ) exhibit color patterns with dichromatic wing pigmentation superficially similar to that of M. nimbaensis ( Csorba et al., 2014) . Csorba et al. (2014) gave diagnoses and measurements of these species that provide a basis for distinguishing them from M. nimbaensis . These authors recognized two pelage and skin color patterns among the Asian species: a “ rufoniger type ” and a “ formosus type.” Species exhibiting “ rufoniger type ” morphology include M. bartelsi , M. hermani , M. rufoniger , and M. weberi . These species can be distinguished from M. nimbaensis based on darker dorsal pelage that has four bands of color (individual dorsal hairs black basally, pale yellow distally, then darkening to deep red before terminating in a black tip; Csorba et al., 2014) rather than the bright orange tricolored dorsal fur of M. nimbaensis . Species with “ rufoniger type ” coloration are also considerably darker ventrally than M. nimbaensis , having hairs with a black base, followed by either a pale yellowish section that progressively darkens distally to deep red, or are otherwise entirely deep red ( Csorba et al., 2014). The ear is also edged with black in rufoniger - type species ( Csorba et al., 2014), a trait lacking in M. nimbaensis (or any other African species of Chrysopteron ). The thumb and underside of hind foot are entirely black in species with “ rufoniger type ” coloration ( Csorba et al., 2014) but not in M. nimbaensis , which has an orange-brown thumb and a brown foot. Myotis rufoniger has flight membranes with a broad band of red that extends from the ankle to the forearm along the side of the body, and black pigmentation is limited to the more distal and posterior portions of the plagiopatagium ( Bhak et al., 2017). In contrast, the black pigment is more extensive in M. nimbaensis , extending much closer to the body including reaching the tibia and into the membrane behind the humerus, and the pale portions of the wings are more orange than red.
Members of the Myotis rufoniger group vary somewhat in size, but they are all large bats roughly comparable in size to M. nimbaensis . Measured forearm lengths of M. rufoniger (FA = 45.0–56.0; Csorba et al., 2014) overlap with those of M. nimbaensis , but cranial dimensions of M. rufoniger (e.g., GLS = 16.98–19.24 mm; ZB = 10.04–12.24 mm) do not overlap, with M. nimbaensis being the slightly larger species. Dental morphology is also somewhat different; M. rufoniger has a P3 that is roughly two thirds the size of P2 and which is usually in line with the other check teeth (rarely displaced lingually; Csorba et al., 2014), compared with M. nimbaensis , in which P3 is less than half the size of P2 and is lingually displaced. Considerable overlap in most dimensions exists between M. nimbaensis and M. weberi (FA = 49.7–53.5 mm; GLS = 19.15–19.72 mm; ZB = 12.37–12.67 mm; Csorba et al., 2014). However, M. weberi as described by Csorba et al. (2014) can be distinguished by having a relatively short upper canine that is only about 1.5 × the height of P4 (C height is ~2 × height of P 4 in M. nimbaensis ) and a p3 that is no more than half the basal area of p2 (p3 is at least three quarters the basal area of p 2 in M. nimbaensis ).
In contrast to Myotis rufoniger and M. weberi , both M. bartelsi (FA = 53.4 mm; GLS = 20.42 mm; ZB = 13.41 mm) and M. hermani (FA = 56.0–60.0 mm; GLS = 20.10–21.77 mm; ZB = 13.4–14.10 mm) are somewhat larger than M. nimbaensis in most dimensions ( Csorba et al., 2014). As described by Csorba et al. (2014), M. bartelsi can be distinguished by having strong lambdoid crests (weakly developed in M. nimbaensis ) and a P3 that is fully out of line with the rest of the toothrow and not visible in lateral view (partially out of line and visible in lateral view in M. nimbaensis ). The forehead is also less clearly developed and strongly sloped in M. bartelsi compared to M. nimbaensis , and the rostrum appears somewhat shorter in M. bartelsi in lateral view. Myotis hermani as described by Csorba et al. (2014) can be distinguished by its overall very robust skull and exceptionally well-developed sagittal and lambdoid crests (more weakly developed in M. nimbaensis ), very large upper canine with a basal area exceeding that of P4 (basal areas of C and P4 subequal in M. nimbaensis ), minute P3 less than one quarter the size of P2 (P3 only slightly less than one half the size of P 2 in M. nimbaensis ), P3 fully out of line with the rest of the toothrow and not visible in lateral view (only partially out of line and visible in lateral view in M. nimbaensis ), p3 with half the basal area of p2 (p3 is at least three quarters the basal area of p 2 in M. nimbaensis ), and p3 partly lingually displaced out of the toothrow (p3 fully in toothrow in M. nimbaensis ).
Asian Myotis (subgenus Chrysopteron ) species exhibiting “ formosus type ” external morphology are M. formosus and M. rufopictus ( Csorba et al., 2014) . These species can be distinguished from M. nimbaensis based on pelage color and banding. The dorsal fur of formosus - type species has individual hairs that have a very narrow brown base and are pale yellow distally for 80–100% of their length, or grade into a brown tip, with banding sometimes not evident as the color changes gradually ( Csorba et al., 2014). Overall, the general aspect of the dorsal fur in M. formosus and M. rufopictus is pale yellow-brown ( Csorba et al., 2014). This pattern is very different from the distinctly banded tricolored orange fur of M. nimbaensis . Ventral fur of M. formosus and M. rufopictus is either bicolored light yellow with a narrow brown base, or unicolored light yellow ( Csorba et al., 2014), again quite different from tricolored buff to orange ventral fur of M. nimbaensis . The ear is faintly edged with black in the formosus - type species ( Csorba et al., 2014) but not in M. nimbaensis . As in M. rufoniger , the dark pigment in the wing membranes of M. formosus is limited to more distal and posterior portions of the plagiopatagium and does not extend to the tibia and membranes next to the body as it does in M. nimbaensis .
Measurements and craniodental features also distinguish Myotis nimbaensis from M. formosus and M. rufopictus . In terms of measurements, M. nimbaensis is only trivially different or falls within the range of variation in measurements reported for M. formosus (FA = 45.5–53.0 mm; GLS = 17.91–19.45 mm; ZB = 11.76–12.94 mm; Csorba et al., 2014). However, M. formosus as described by Csorba et al. (2014) can be distinguished based on having a sagittal crest that is missing or only very weakly developed (moderately well developed in M. nimbaensis ), P3 that is not visible in lateral view of the skull (visible in M. nimbaensis ), and a p3 with half the basal area of p2 (p3 is at least three quarters the basal area of p 2 in M. nimbaensis ). In addition, the skull of M. formosus lacks an expanded supraorbital region and appears to have a shorter rostrum than M. nimbaensis when seen in lateral view. Myotis rufopictus (FA = 51.0– 52.5 mm; GLS = 18.2 mm; ZB = 11.17; Csorba et al., 2014) appears slightly smaller than M. nimbaensis , but both species are known from very few specimens, so the value of this observation is questionable. As described by Csorba et al. (2014), the skull profile of M. rufopictus ascends almost evenly with no frontal depression (i.e., no clearly defined break between rostrum and sloping forehead as seen in M. nimbaensis ). Like Myotis formosus , M. rufopictus additionally lacks an expanded supraorbital region and it appears to have a shorter rostrum than M. nimbaensis when seen in lateral view. Morphology of P 3 in M. rufopictus is unknown, but p3 is a very small tooth less than one quarter the basal area of p2 and displaced lingually halfway out of the line of toothrow (p3 at least three quarters the area p2 and not displaced in M. nimbaensis ).
ECHOLOCATION CALLS: Based on the echolocation calls recorded on the release of the holotype (n = 22), Myotis nimbaensis emits a broad bandwidth (84.1 ± 13.1 kHz; FMAX: 104 kHz, FMIN: 20 kHz) steep frequency-modulated pulse with a peak frequency of 42.5 kHz (± 4.2 kHz), an average call duration of 3.5 ms (± 0.3 ms), and an interpulse interval of 113 ms (± 5.3 ms; fig. 9). In comparison, echolocation calls of Myotis welwitschii have a lower peak frequency (34 kHz), shorter bandwidth (28.3 kHz) and shorter duration (2.4 ms; Schoeman and Jacobs, 2008: Monadjem et al., 2010: Collen, 2012). While there may be geographic variation in call parameters between East African and Southern African Myotis tricolor populations, calls recorded from Southern Africa have a slightly higher peak frequency (47.8±3.1 kHz) but are shorter in bandwidth (46±23.9 kHz) and duration (3.3±0.6 ms; Schoeman and Jacobs, 2008: Monadjem et al., 2010). Echolocation calls of Myotis morrisi are unknown. Myotis bocagii , the other known Myotis species found on the Nimba Mountains, have calls with a similar peak frequency (41–43 kHz) but are shorter in bandwidth (36 kHz) and duration (2–2.8 kHz) (Schoeman and Waddington, 2011; Collen, 2012; Monadjem et al., 2017a).
MOLECULAR ANALYSES: Molecular analyses of mitochondrial cytochrome b sequences from selected specimens ( appendix 2 View APPENDIX 2 ) support recognition of Myotis nimbaensis as a distinct species (table 2, figs. 10 and 11). Our analyses recovered well-supported clades consistent with results of Csorba et al. (2014) and Patterson et al. (2019), and in this context Myotis nimbaensis was found to represent a distinct lineage that nests within the subgenus Chrysopteron as sister to the M. tricolor complex (the clade consisting of M. tricolor 1, 2, and 3 of Patterson et al., 2019). Monophyly of Myotis nimbaensis , the M. tricolor complex, and a clade consisting of these two lineages was highly supported (>95% bootstrap). Myotis nimbaensis is 5.18% different than M. tricolor 1, 5.43% from M. tricolor 2, and 5.08% than M. tricolor 3 (table 2).
NATURAL HISTORY: The type locality habitat of Myotis nimbaensis occurs at the ecotone of high-altitude grassland, montane savanna, and gallery forest habitats of the Nimba Mountains around 1400 m elevation (fig. 1). As far as is known, the preferred (or exclusive) roosting habitat for this species is subterranean features. The two individuals that we captured were emerging from day roosting in an abandoned mine adit (fig. 1), and echolocation calls similar to the release calls of Myotis nimbaensis recorded at other nearby adits, apparently confirming association of M. nimbaensis with these underground structures. Myotis nimbaensis coroosts with other subterranean roosting bats and was detected at mine adits that house colonies of several hundred Rhinolophus guineensis and Hipposideros lamottei , the latter a bat species endemic to the Nimba Mountains ( Monadjem et al., 2013).
Foraging habitat for Myotis nimbaensis is unknown, but the six mine adits where M. nimbaensis echolocation calls were recorded occur in similar ecotone habitats at the interface of high-elevation grassland, montane savanna, and gallery forests along headwaters of the Zié, Gouan, and Zougué rivers in the northern Guinean Nimba Mountains.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
SubFamily |
Myotinae |
|
Genus |
|
|
SubGenus |
Chrysopteron |