Rhacophorus wui, Li & Liu & Chen & Wu & Murphy & Zhao & Wang & Zhang, 2012, Li & Liu & Chen & Wu & Murphy & Zhao & Wang & Zhang, 2012
|
publication ID |
https://doi.org/10.1111/j.1096-3642.2011.00790.x |
|
persistent identifier |
https://treatment.plazi.org/id/8A440809-FFDC-DD02-40BD-2394FD26F910 |
|
treatment provided by |
Marcus |
|
scientific name |
Rhacophorus wui |
| status |
sp. nov. |
RHACOPHORUS WUI SP. NOV.
( FIGS 1 View Figure 1 , 2 View Figure 2 , 3A, C, E; TABLE View Figure 3 3)
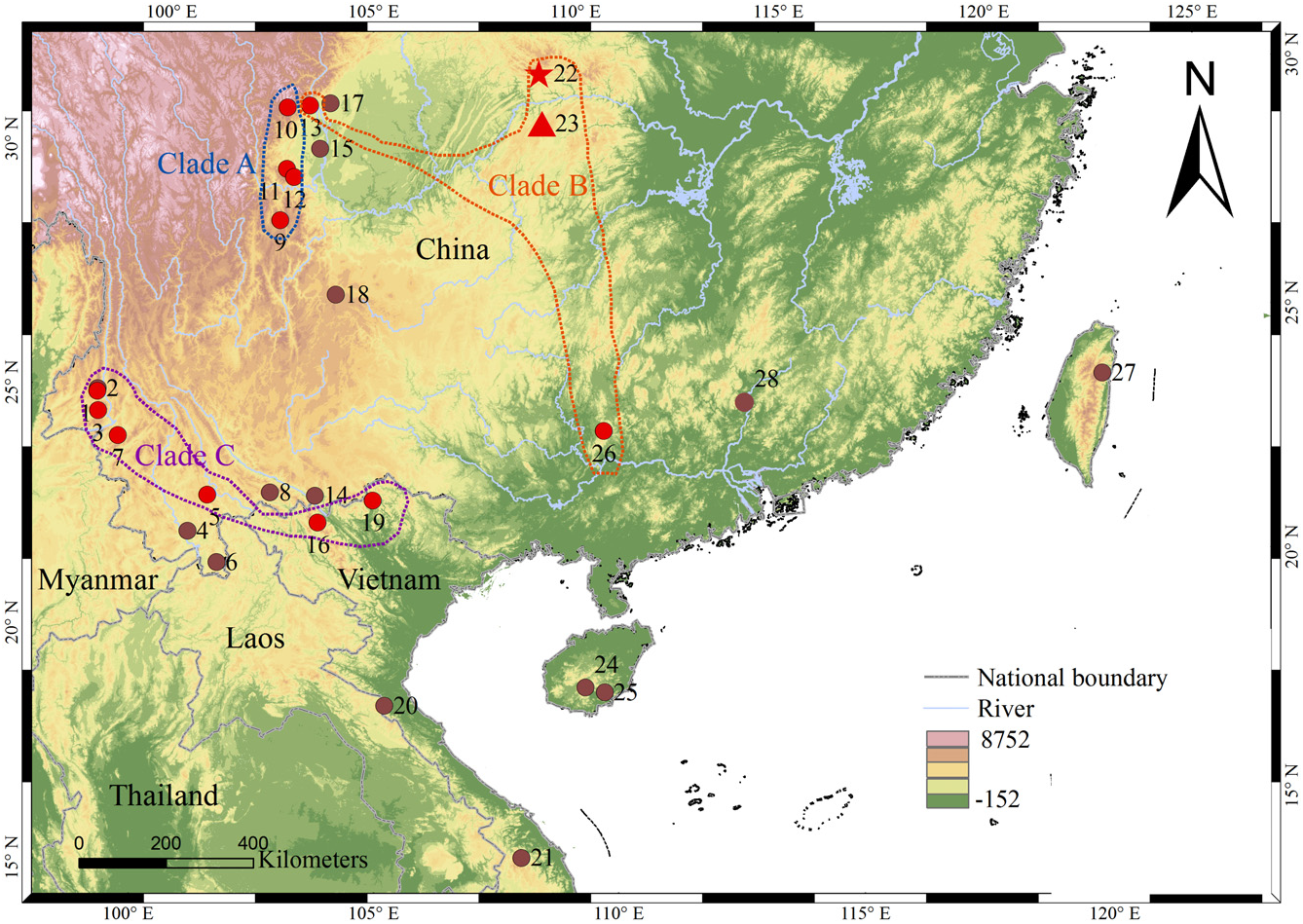
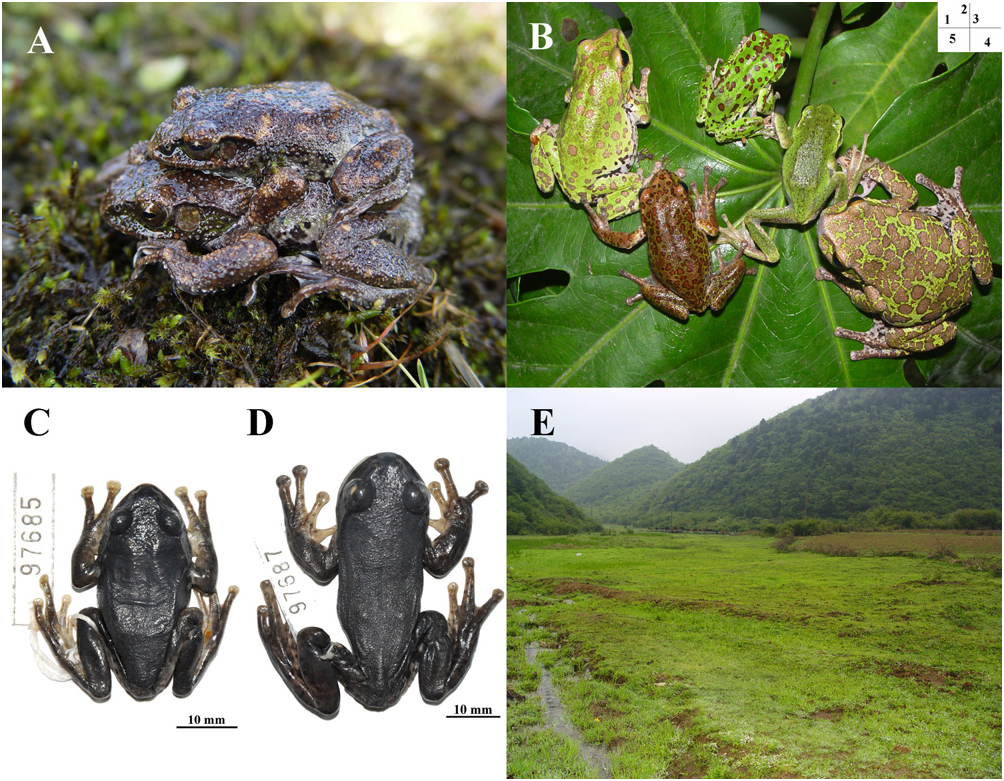
Holotype: CIB 97685 ( Fig. 3C View Figure 3 ; Table 3), an adult male, collected on 13 March 2006 by Jia-tang Li and Jian-li Xiong from Hanchi Village, Lichuan County, Hubei, China ( 30°31′30.94″ N, 109°05′39.05″ E, elevation 1913 m a.s.l.; Fig. 1 View Figure 1 ). GoogleMaps
Paratypes: CIB 97686, an adult female, and CIB 97689–97693 View Materials , five males, collected with the holotype .
Diagnosis: Rhacophorus wui sp. nov. can be distinguished from all other species of Rhacophorus by a combination of the following characters: (1) body size (adult males SVL 35.2–38.2 mm; adult females 48.6 mm); (2) both dorsal and ventral surfaces with irregularly distributed small tubercles, some with white or black spines on the tip; (3) tarsal fold obvious, with small white tubercles on the edge; (4) numerous light-brown spots on dorsum with dark yellowish brown edges; (5) tympanum distinct and round, third finger width larger than tympanum; (6) discs on fingers and toes enlarged and terminal phalanges Y-shaped.
Description of holotype: An adult male, SVL 36.6 mm; head width slightly greater than length (HW 13.9 mm; HL 12.9 mm); snout length (SL 6.0 mm), longer than diameter of eye (ED 4.6 mm), outline of snout in dorsal views pointed; ED much longer than DNE ( 2.9 mm); head flat, IND slightly longer than IOD (IND 4.4 mm; IOD 3.7 mm); cantus rostralis obvious and angular; nostrils closer to tip of snout than to eye, under the edge of canthus rostralis; loreal region is slightly concave; tympanum distinct, rounded, diameter (TD 2.5 mm) half of ED; vomerine processes, five vomerine teeth present; vocal sac single, externally expanded; tongue small sized, posterior one-eighth notched.
Forelimb 21.0 mm, hand length larger than half of FLL (HLT 12.7 mm); fingers with lateral fringes, relative length of finger is 3> 4> 2> 1 (FL 1 6.5 mm; FL 2 8.8 mm; FL 3 12.7 mm; FL 4 10.4 mm); discs expanded, with circummarginal groove at the end of phalanges; terminal phalanges Y-shaped; pad width (III) slightly larger than pad length (3FL 2.1 mm; 3FW 2.3 mm); hand webbing poorly developed, only at base; webbing formula for digits, following Myers & Duellman (1982), I trace–II2–3III2.5–2.25IV; subarticular tubercles present, distinct, convex, and rounded, a single subarticular tubercle on fingers I and II, two subarticular tubercles on finger III, three subarticular tubercles on finger IV; inner metacarpal tubercles small, oval, and distinct.
Hindlimbs relatively long; tibiotarsal articulation reaches to between tympanum and eye, when adpressed to the body; heels do not meet when folded at right angle to body; thigh length longer than tibia length (THL 17.2 mm; TIL 16.0 mm); toes with lateral dermal fringe, relative length of toes 4> 5> 3> 2> 1 (TL 1 6.0 mm; TL 2 8.5 mm; TL 3 12.5 mm; TL 4 17.2 mm; TL 5 13.5 mm); discs round, with ventral circummarginal groove, width of fourth toe disc relatively smaller than width of third finger disc; webbing formula for toes I2–2II1.5–2.5III2–2.5IV2.5–1.5V; base of outer two toes with row of small, white granules; inner metatarsal tubercles small, oval, and indistinct.
Skin on dorsum of body and limbs with strongly distributed, coarse tubercles, some with black or white spines on the tip, becoming feeble or weak on sides of body; belly grossly granulate; ventral surface of limbs areolate; tarsal fold obvious, with small tubercles on ridge; throat with somewhat loose skin.
Colour in life: Coloration varies from dark yellowish brown to light green on dorsal and lateral surfaces, with numerous light-brown spots that have dark yellowish brown edges ( Fig. 3A View Figure 3 ); individuals living on green grass versus mud light green versus darkyellowish brown, respectively. Upper surface of forelimb and hindlimb same as dorsum. Ventral surface creamy white with vague greyish brown blotches. Ventral sides of legs light reddish white, marbled with grey. Tips of finger light brown with scattered dark mottling; pattern retained when preserved. Inner side colour of thigh creamy white with vague brown spots.
Colour in preservative: Dorsal and lateral surfaces blackish brown ( Fig. 3C View Figure 3 ). Ventral surface light creamy yellow, with vague light greyish brown blotches. Posterior colour of thigh and arm dark brown with sparse reddish brown blotches. Inner side colour of tibia white with vague brown spots.
Variation: Measurements and ranges of the type series are given in Table 3. Female ( 48.6 mm SVL) larger than males ( 35.2–38.2 mm SVL). Snout bluntly pointed in the female and more sharply pointed in males. Males with internal subgular vocal sacs and two sublingual openings; nuptial pad on base of first finger in males during breeding season. Number of vomerine teeth varies: CIB 097693 View Materials with three teeth; CIB 097689 View Materials , CIB 097690 View Materials , and CIB 097692 View Materials with four teeth each; CIB 097685 View Materials and CIB 097691 View Materials with five teeth each; and six teeth in CIB 097686 View Materials . Webbing formula for fingers (with range) follows Myers & Duellman (1982: I trace–II(1.75–2.00)–3 III (2.25– 2.50)–(2.0–2.5)IV; toes, I(1.5–2)–2II(1–2)–2.5 III2 – 2.5 IV2.5–(1–2) V. Ventral surfaces either creamy white with vaguely brown blotches from throat to vent ( CIB 97695), light-brown blotches only on belly ( CIB 97697; CIB 97687), or covered with blotches ( CIB 97698; CIB 97696) .
Etymology: The name, Rhacophorus wui sp. nov., honours Guan-fu Wu, in recognition of his great contribution to Chinese herpetology.
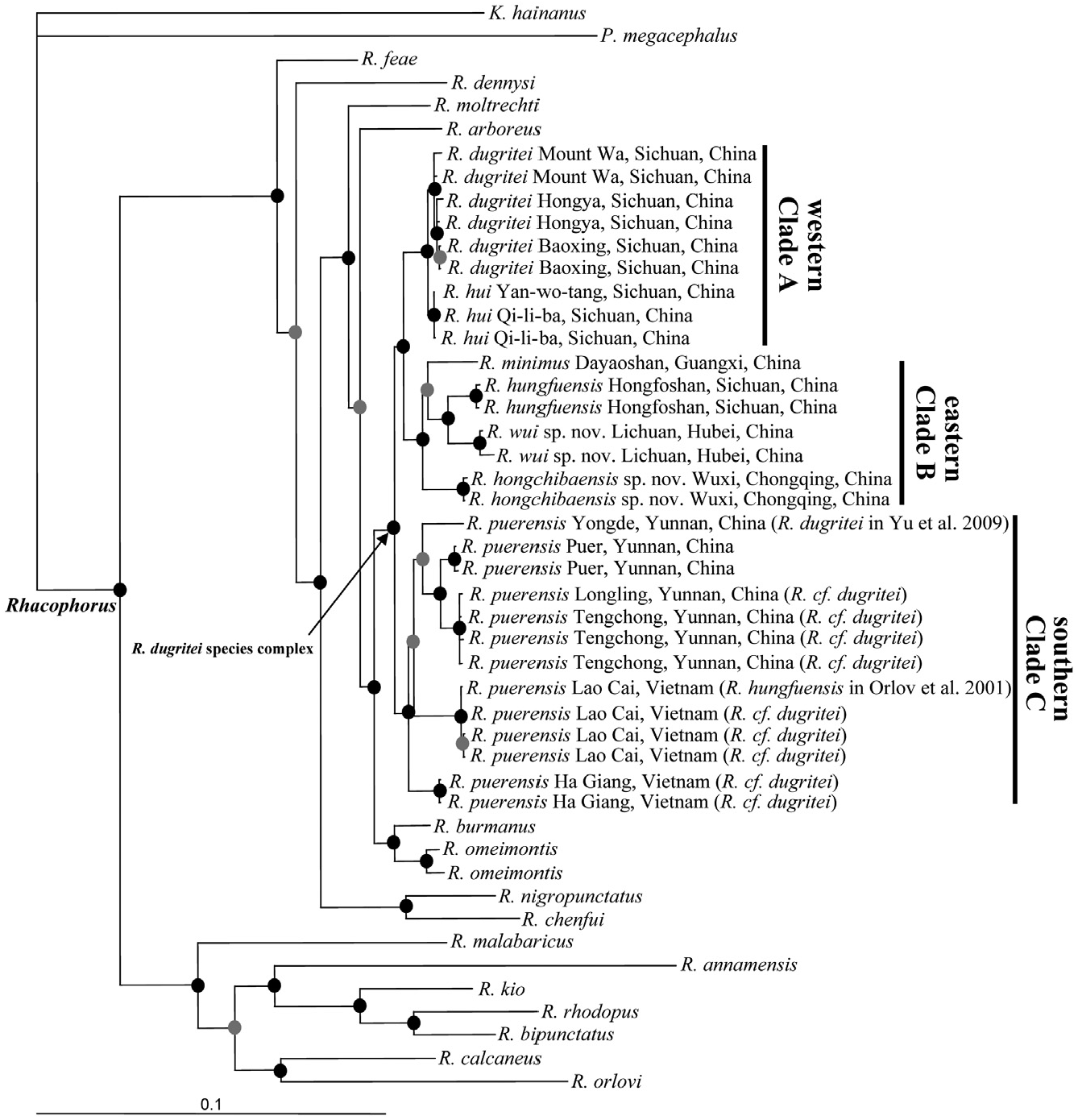
Comparisons: Rhacophorus wui sp. nov. is included within the genus because of expanded discs on the fingers and toes, terminal phalanges Y-shaped, intercalary element present, and webbing between fingers and toes ( Liem, 1970; Wilkinson & Drewes, 2000). Its placement is confirmed by our molecular phylogeny.
Similar morphologically to R. hongchibaensis sp. nov., R. wui sp. nov. can be distinguished from R. annamensis , R. bipunctatus , R. calcaneus , R. chuyangsinensis , R. kio , R. lateralis , R. malabaricus , R. nigropalmatus , R. orlovi , R. pardalis , R. reinwardtii , R. rhodopus , R. translineatus , and R. verrucopus by the lack of calcanar projections ( Wilkinson et al., 2005; Orlov et al., 2008). In lacking full webbing, this species differs from R. burmanus , R. dennysi , R. duboisi , R. dulitensis , R. feae , R. maximus , and R. omeimontis ( Liem, 1970; Jiang et al., 1987; Fei, 1999; Wilkinson & Drewes, 2000; Fei et al., 2009, 2010; Appendix 2).
Numerous light-brown spots on the dorsum clearly distinguish R. wui from the species with no spots on green dorsum, including R. arboreus , R. chengfui , R. dorsoviridis , R. hungfuensis , R. minimus , R. moltrechti , R. nigropunctatus , and R. schlegelii ( Liem, 1970; Jiang et al., 1987; Fei, 1999; Wilkinson et al., 2005; Fei et al., 2009, 2010; Appendix 2).
Rhacophorus wui sp. nov. differs from the similar Chinese species, R. dugritei , R. hui , R. hungfuensis , R. minimus , R. puerensis , and R. hongchibaensis sp. nov., by having a distinct tarsal fold and a dorsum with irregularly distributed tubercles that have white or black spines on the tip ( Fig. 3A View Figure 3 ; Appendix 2). Rhacophorus wui sp. nov. ( 35.2–38.2 mm) also differs from R. hongchibaensis sp. nov. ( 46.5–49.7 mm) in body size.
Distribution and ecology: The new species, known only from the type locality, occurs in an environment similar to that of R. hongchibaensis sp. nov. ( Fig. 3E View Figure 3 ). Both solitary males and amplexing pairs are found at night during the mating season, from March through June. Males perch on the vegetation near the ground or water. White foam nests with fertilized eggs are sited at the base of grassy vegetation near swampy pools. Males call with a loud ‘der-der-der’ both day and night, albeit more frequently at night.
| CIB |
Chengdu Institute of Biology |
| V |
Royal British Columbia Museum - Herbarium |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |