Hypocreadium patellare Yamaguti, 1938 Atypical
|
publication ID |
https://doi.org/ 10.5281/zenodo.187863 |
|
publication LSID |
lsid:zoobank.org:pub:3C88C15C-FEE3-4578-8DDD-147B1385AA9D |
|
DOI |
https://doi.org/10.5281/zenodo.5633249 |
|
persistent identifier |
https://treatment.plazi.org/id/8B7087CD-FF8E-FF84-FF77-FEC4CD4D0E00 |
|
treatment provided by |
Plazi |
|
scientific name |
Hypocreadium patellare Yamaguti, 1938 Atypical |
| status |
|
Hypocreadium patellare Yamaguti, 1938 Atypical View in CoL form A
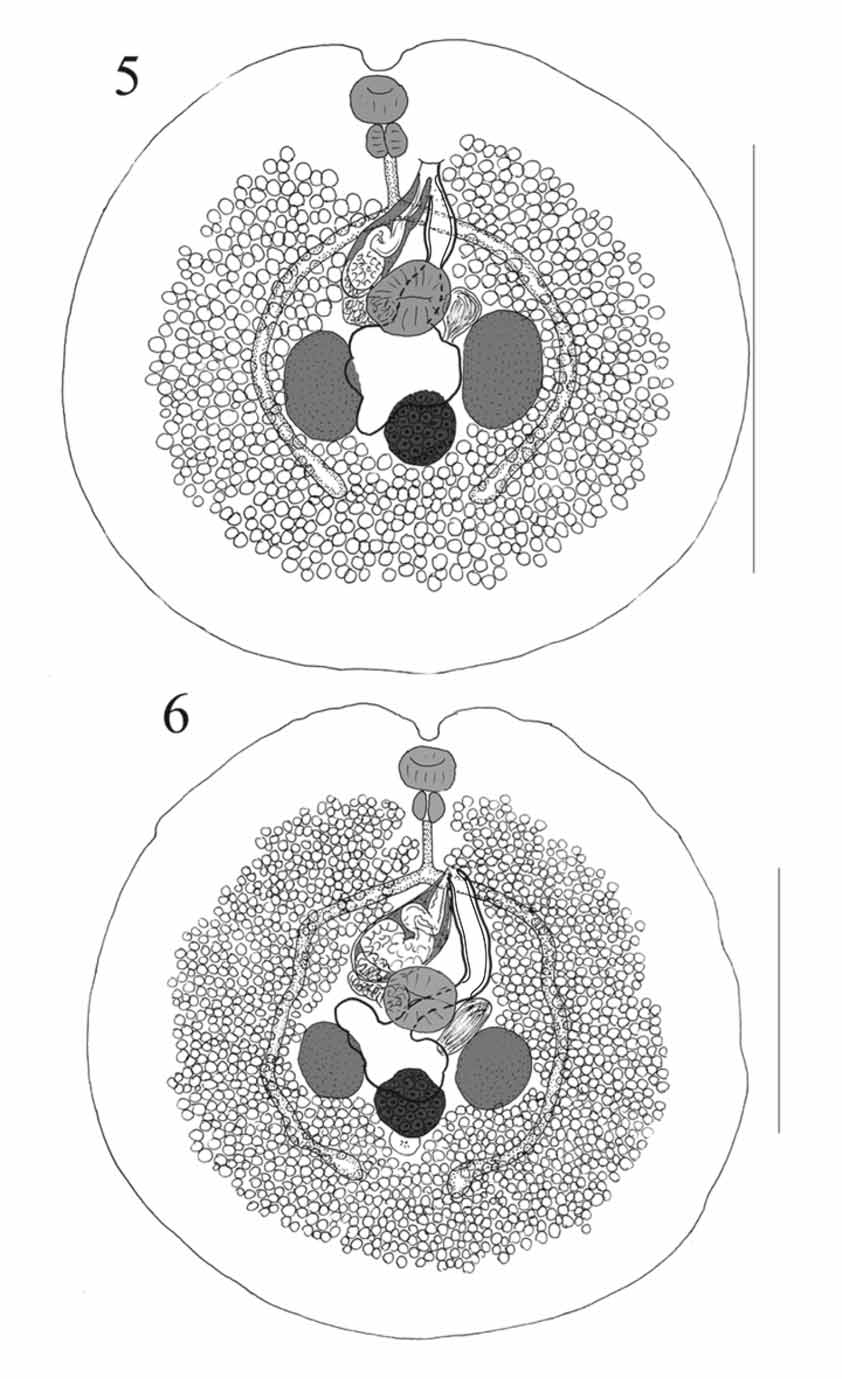
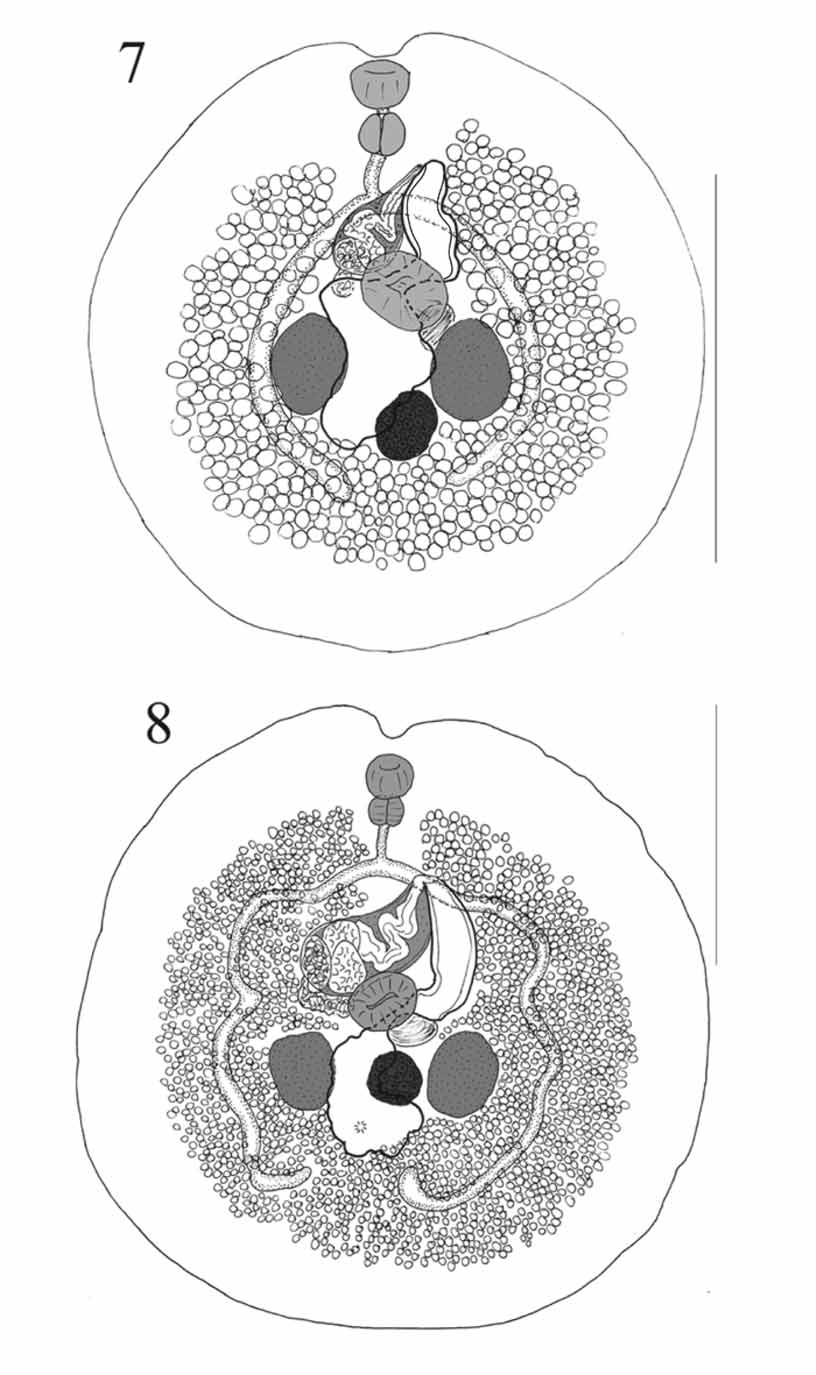
( Figures 5–7 View FIGURES 5 – 6 View FIGURES 7 – 8. 7 )
Hosts: Rhinecanthus aculeatus (Linnaeus, 1758) , Balistidae , black-bar triggerfish; Rhinecanthus verrucosus (Linnaeus, 1758) , Balistidae , blackbelly triggerfish.
Site: Intestine.
Localities: R. aculeatus and R. verrucosus , Palau (7°21’N, 134°31.47’E, Nov. 2001); R. aculeatus, Lizard Island (14°40’S, 145°28’E, August 2002).
Prevalences: Palau: R. aculeatus 1 of 2 (50%); R. verrucosus 1 of 1; Lizard Island: R. aculeatus 1 of 4 (25%):
Voucher specimens: R. aculeatus , Palau, QM G 230533 – 230534, BMNH 2009.2.12.24–25; Lizard Island, QM G 230535 – 230536, BMNH 2009.2.12.26; R. verrucosus , Palau, QM G 230537.
Description: Measurements in Tables 2 and 3. Body about as wide as long, virtually circular and with a distinct anterior notch. No tegumental spines seen. Pre-oral lobe short, distinct. Oral sucker oval, aperture subterminal. Ventral sucker slightly irregularly oval, just pre-equatorial. Prepharynx usually not evident. Pharynx broadly oval. Oesophagus distinct. Intestinal bifurcation in posterior forebody. Caeca narrow, arcuate around gonads, converge posteriorly, end blindly fairly near midline, in about mid-post-testicular region.
Testes 2, irregularly oval, symmetrical, in anterior hindbody, well separated. External seminal vesicle small, saccular, at level of ventral sucker. Cirrus-sac large, claviform, reaching from point dextro-lateral to ventral sucker diagonally across posterior forebody, reaches over left caecum; surrounded by scattered glandcells. Internal seminal vesicle oval. Pars prostatica bipartite, folded, lined with large anuclear cell-like bodies. Ejaculatory duct muscular, long, folded, lined with pavement [or carpet] of cobble-stone like small peduncles. Genital atrium large, often dilated with eggs. Genital pore sinistral at level of (i) R. aculeatus , Palau: anterior oesophagus (2 specimens), mid-oesophagus (1) and posterior oesophagus (1); (ii) R. verrucosus , Palau: anterior oesophagus (1); and (iii) R. aculeatus, Lizard Island: intestinal bifurcation (3).
Ovary more or less oval, intertesticular but extending post-testicular, closest to but usually separated from sinistral testis and separated from dextral testis. Seminal receptacle large, saccular, between sinistral testis and ventral sucker. Laurer’s canal opens dorsally to ovary, sinistral testis or between. Mehlis’ gland anterior to ovary. Uterus usually lies between ovary and dextral testis but does not pass into post-ovarian region (4 specimens), may pass slightly into post-ovarian region ( R. aculeatus , Palau, 2) or is restricted to pre-ovarian region ( R. aculeatus , Palau, 1), then anteriorly, narrowing dorsally to ventral sucker, then widens to form metraterm. Metraterm passes anteriorly, with thick muscular wall and thin sheath of gland-cells, often dilated with eggs. Vitellarium follicular, follicles numerous, surround gonads, well separated from body-margins, contiguous and reaching to midway between caecal termination and posterior extremity posteriorly, separated and reaching to about pharyngeal level anteriorly, lateral, ventral and median to caeca, but not dorsal.
Excretory pore at region of posterior part of ovary or just posterior. Vesicle division not seen, arms narrow, reach to level of oesophagus.
Remarks: This form keys to Hypocreadium patellare in Bray & Cribb (1996) based on the position of the genital pore. As discussed above, Machida & Kuramochi (1999) found that in material from the type host, the genital pore is at the ‘bifurcal level’, placing considerable doubt on the validity of this character. We feel it is better not to use this character as a differentiating feature, although we find it rather consistent. Ignoring this feature in the key brings into consideration H. balistes , which is reported from the same host-species from the Red Sea ( Nagaty, 1942). This form can, however, readily be distinguished from H. balistes by its anterior notch and its more circular body (width 100–108% of length vs 107–118%).
The distinction from Hypocreadium patellare is less clear as they both have a distinct anterior notch, so much so that we, at present, consider these forms conspecific with H. patellare . These specimens from Rhinecanthus spp. are generally smaller than the ‘typical’ specimens and more variable in regards the position of the genital pore and posterior extent of the uterus, although the variation found encompasses that of the typical form.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.