Glenognatha Simon, 1887
|
publication ID |
https://doi.org/10.11646/zootaxa.4069.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:52FC658C-78C7-49FC-9961-8AC43CA03101 |
|
DOI |
https://doi.org/10.5281/zenodo.5666920 |
|
persistent identifier |
https://treatment.plazi.org/id/8E0E8F67-7D1D-FF9A-ACBA-0DA0FAA8D7AA |
|
treatment provided by |
Plazi |
|
scientific name |
Glenognatha Simon, 1887 |
| status |
|
Glenognatha Simon, 1887 View in CoL View at ENA
Glenognatha Simon, 1887: 193 View in CoL , type species by monotypy Glenognatha emertoni Simon, 1887 View in CoL . Mimognatha, Banks, 1929: 90 , type species by monotypy Mimognatha foxi Banks, 1929 . Synonymized by Levi, 1980: 64. Hivaoa, Berland, 1935: 50 , type species by original designation Hivaoa argenteoguttata Berland, 1935 . Synonymized by Levi,
1980: 64.
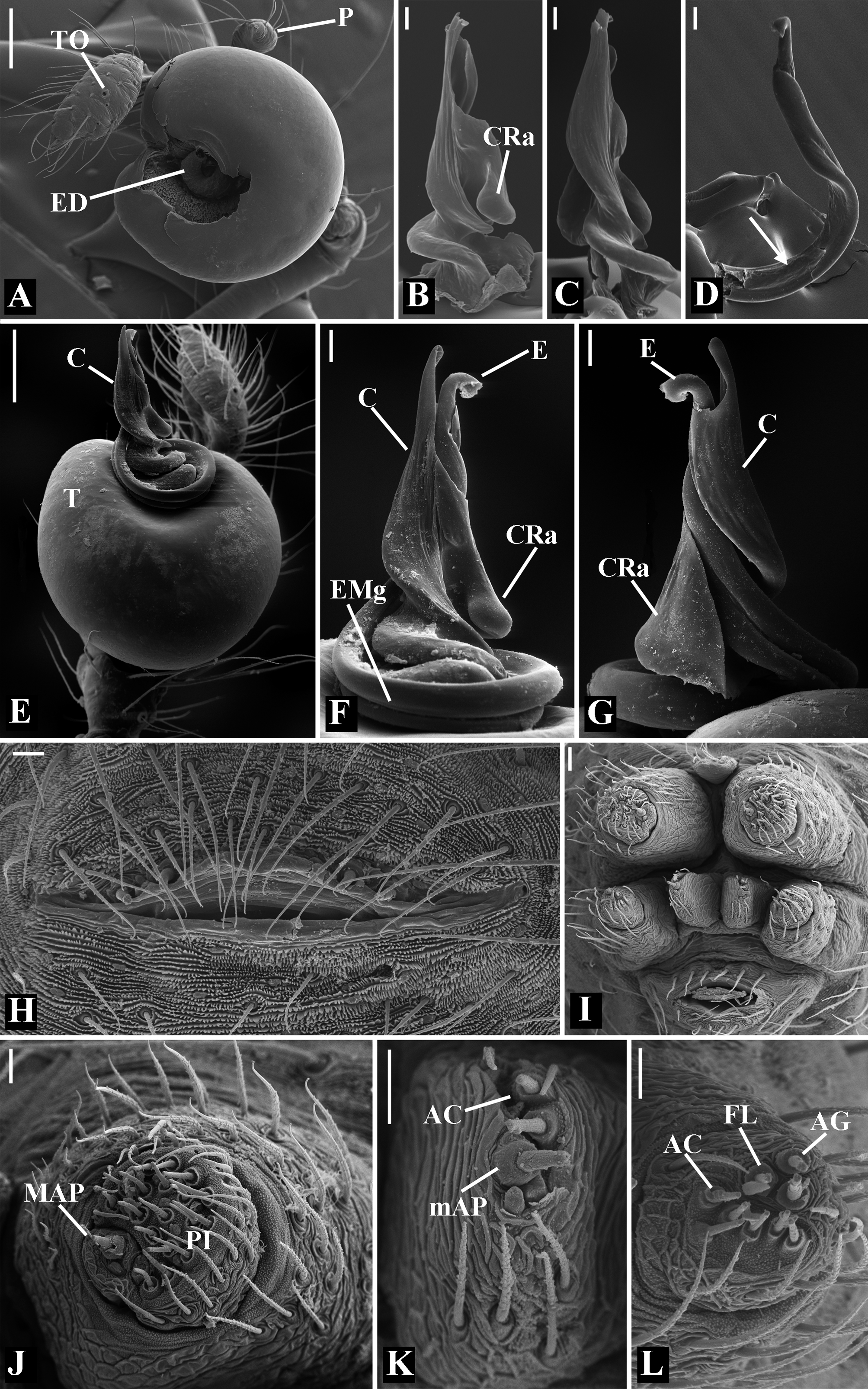
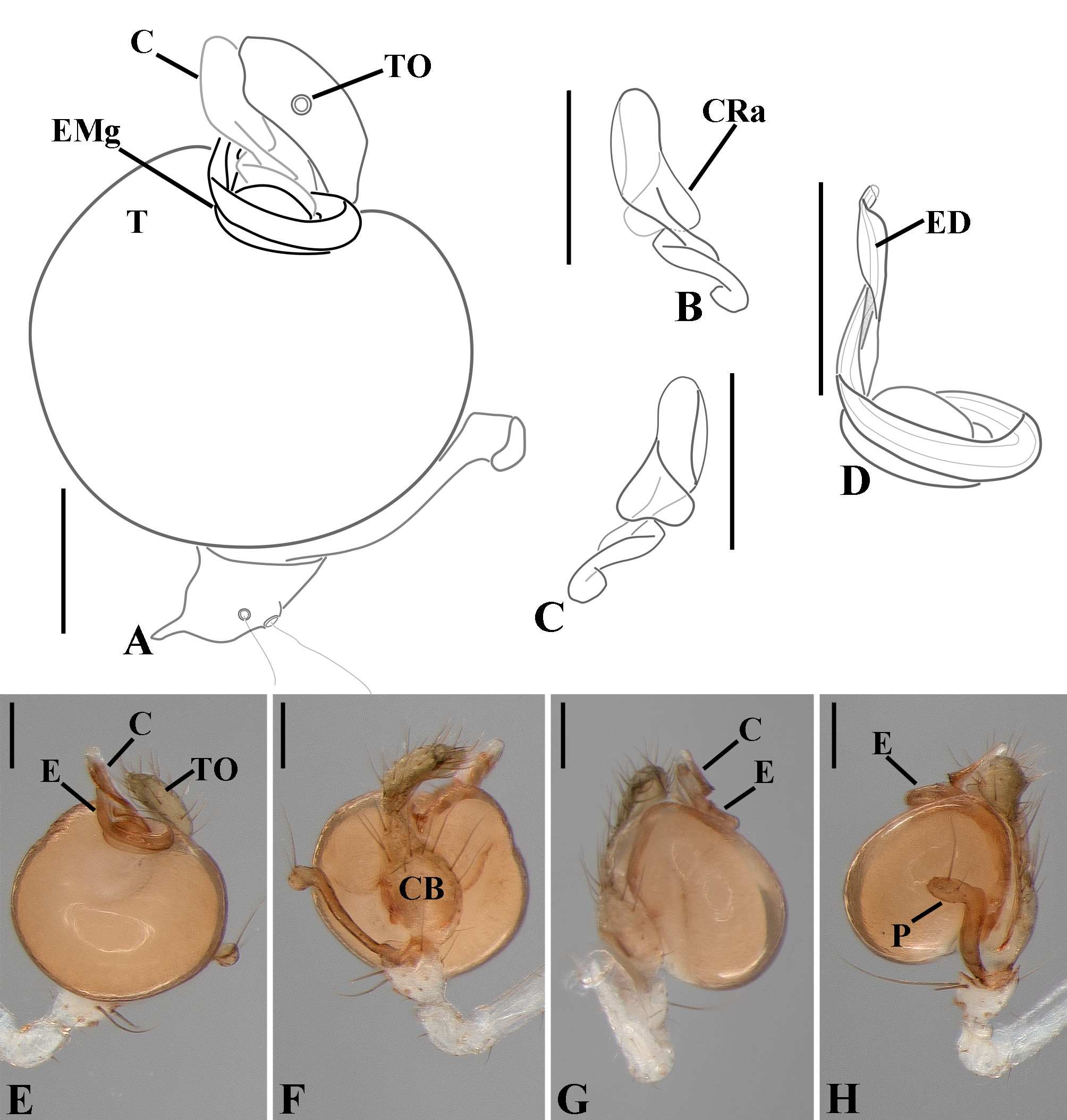
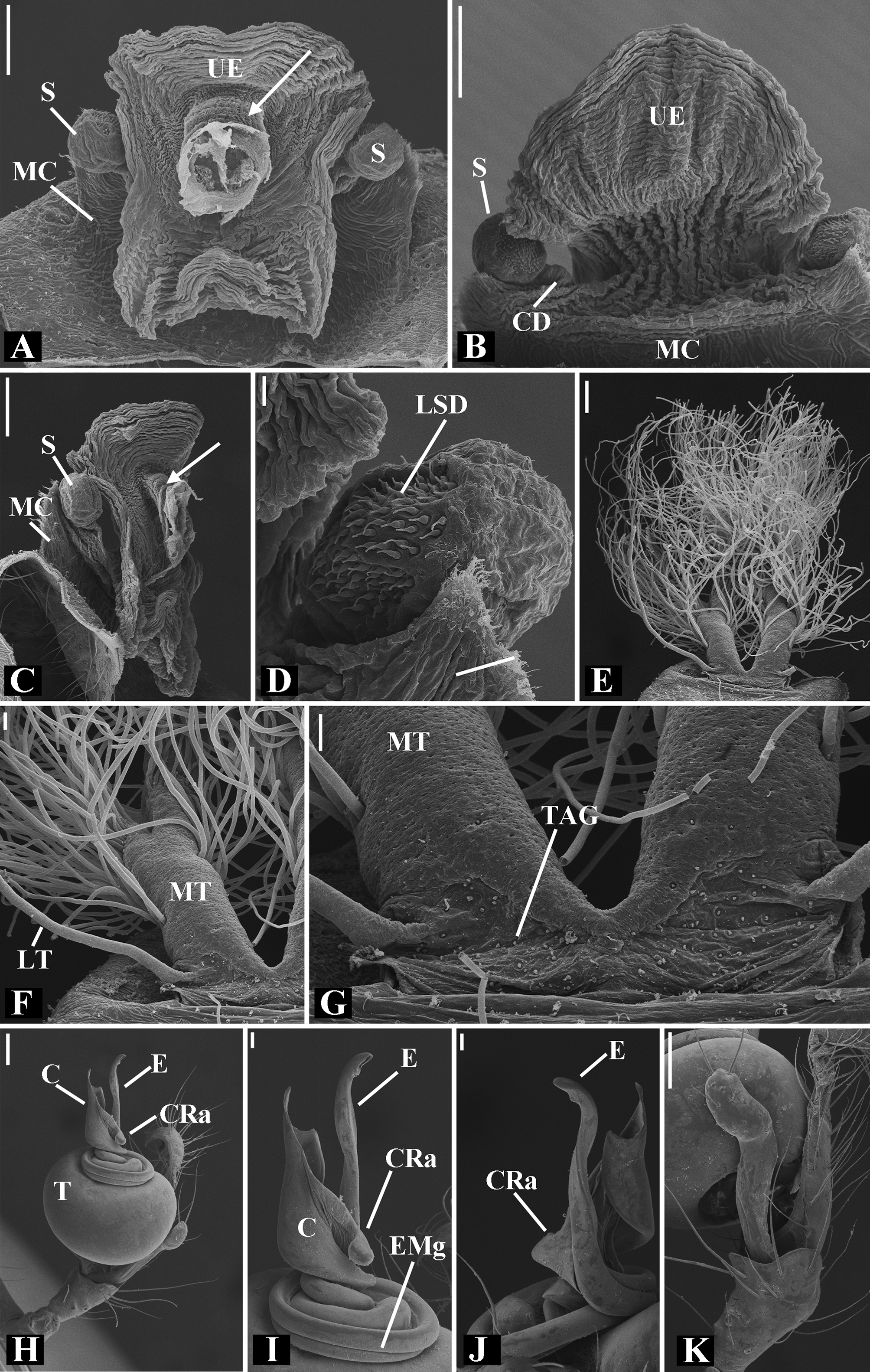
Diagnosis: Males of Glenognatha resemble those of Pachygnatha by the presence of a retrolateral apophysis on the conductor ( Fig. 74G View FIGURE 74 ). It can be distinguished from the latter genus by the paracymbium with the distal portion oriented in opposite direction to the cymbium as seen in retrolateral view ( Figs. 11H View FIGURE 11 , 16H View FIGURE 16 , 23H View FIGURE 23 , 27H View FIGURE 27 , 28G View FIGURE 28 , 40H View FIGURE 40 , 47H View FIGURE 47 , 49B View FIGURE 49 , 70J View FIGURE 70 , 91H View FIGURE 91 , 99H View FIGURE 99 , 128H View FIGURE 128 ), the folded retrolateral apophysis on the conductor lamina ( Figs. 18B View FIGURE 18 , 23F, L View FIGURE 23 , 28F View FIGURE 28 , 54C View FIGURE 54 , 79C View FIGURE 79 , 84E View FIGURE 84 , 92G View FIGURE 92 ) and the embolus with a medial groove in its basal portion ( Figs. 18B View FIGURE 18 , 23O View FIGURE 23 , 43D View FIGURE 43 , 47H View FIGURE 47 , 49F–G View FIGURE 49 , 54 A View FIGURE 54 , 58H View FIGURE 58 , 125I View FIGURE 125 ). Females can be diagnosed by an anteriorly displaced tracheal spiracle ( Figs. 9G View FIGURE 9 , 14G View FIGURE 14 , 30 A View FIGURE 30 , 31G View FIGURE 31 , 50G View FIGURE 50 , 60C View FIGURE 60 , 80G View FIGURE 80 , 126C View FIGURE 126 ) and a tracheal system composed of two median trunks divided into numerous distal and lateral tracheoles flanked by two unbranched lateral tracheae ( Figs. 12C–F View FIGURE 12 , 18E–G View FIGURE 18 , 24 A View FIGURE 24 , 41D–F View FIGURE 41 , 48G–J View FIGURE 48 , 49H–J View FIGURE 49 , 74I –J View FIGURE 74 , 92J–L View FIGURE 92 , 103F–H View FIGURE 103 , 121D–G View FIGURE 121 ). As currently delimited, the genus Dyschiriognatha also shares these diagnostic features. Nevertheless, its synonymy with Glenognatha cannot be objectively proposed (see ‘Discussion’ section).
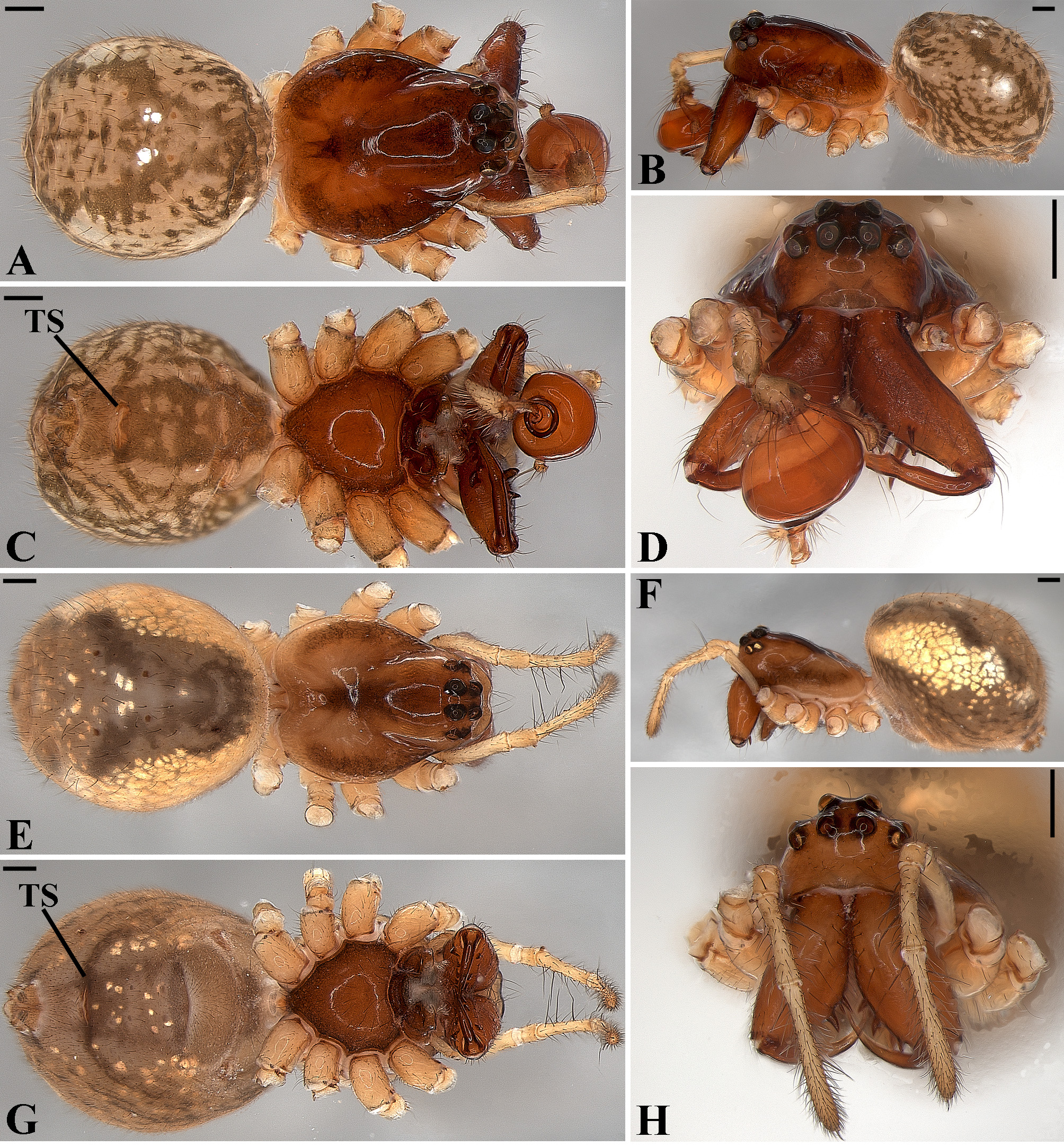
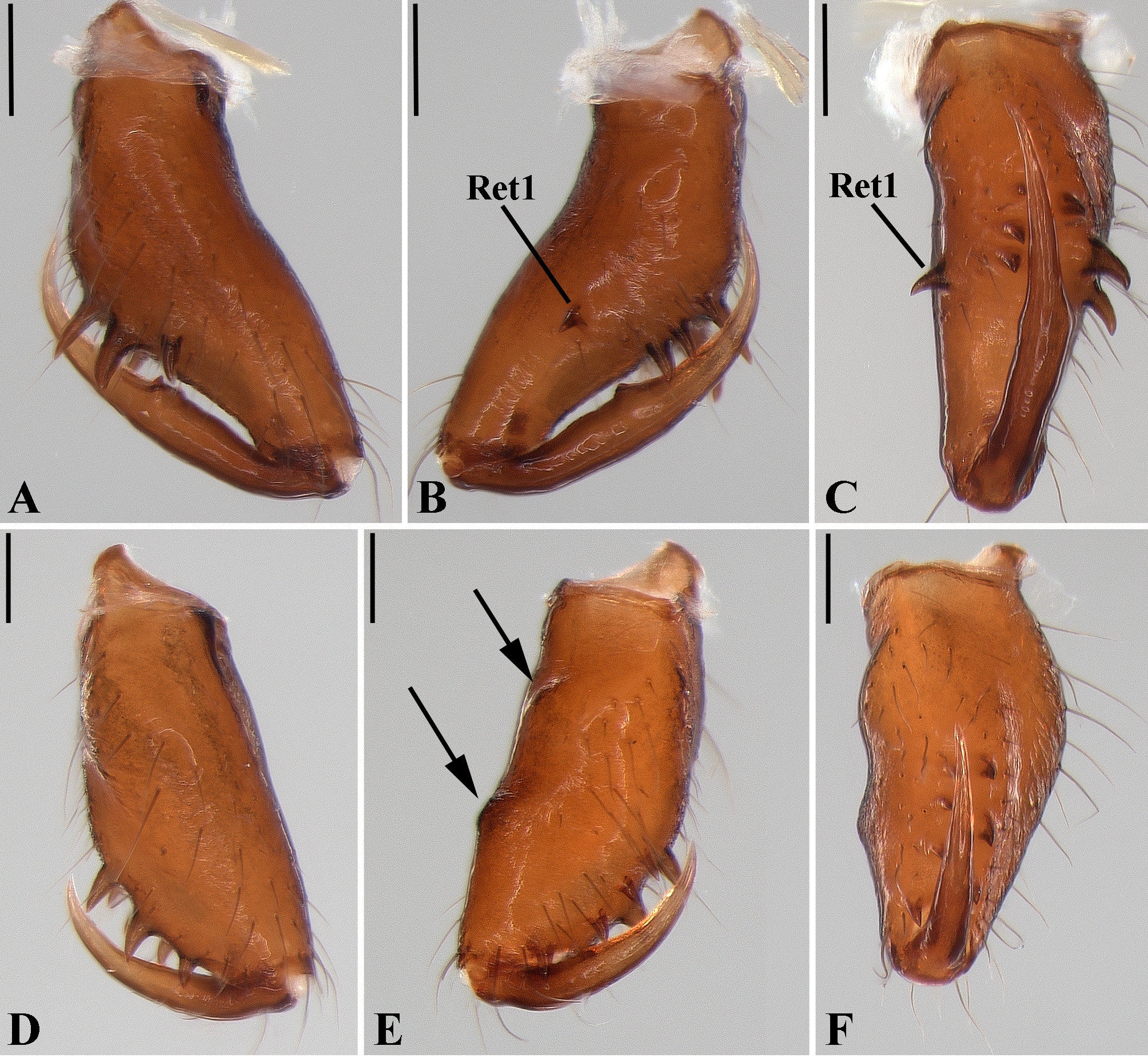
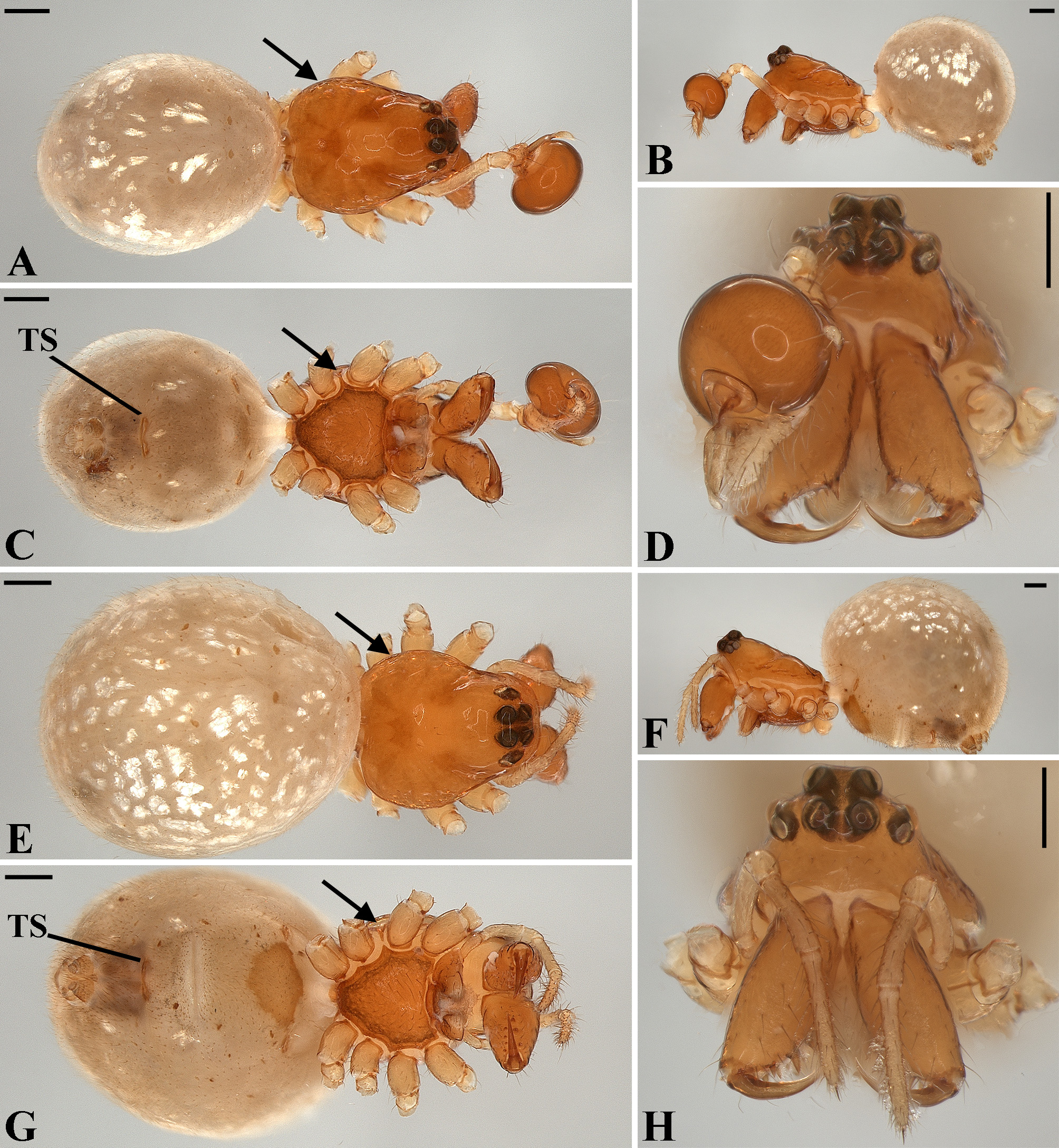
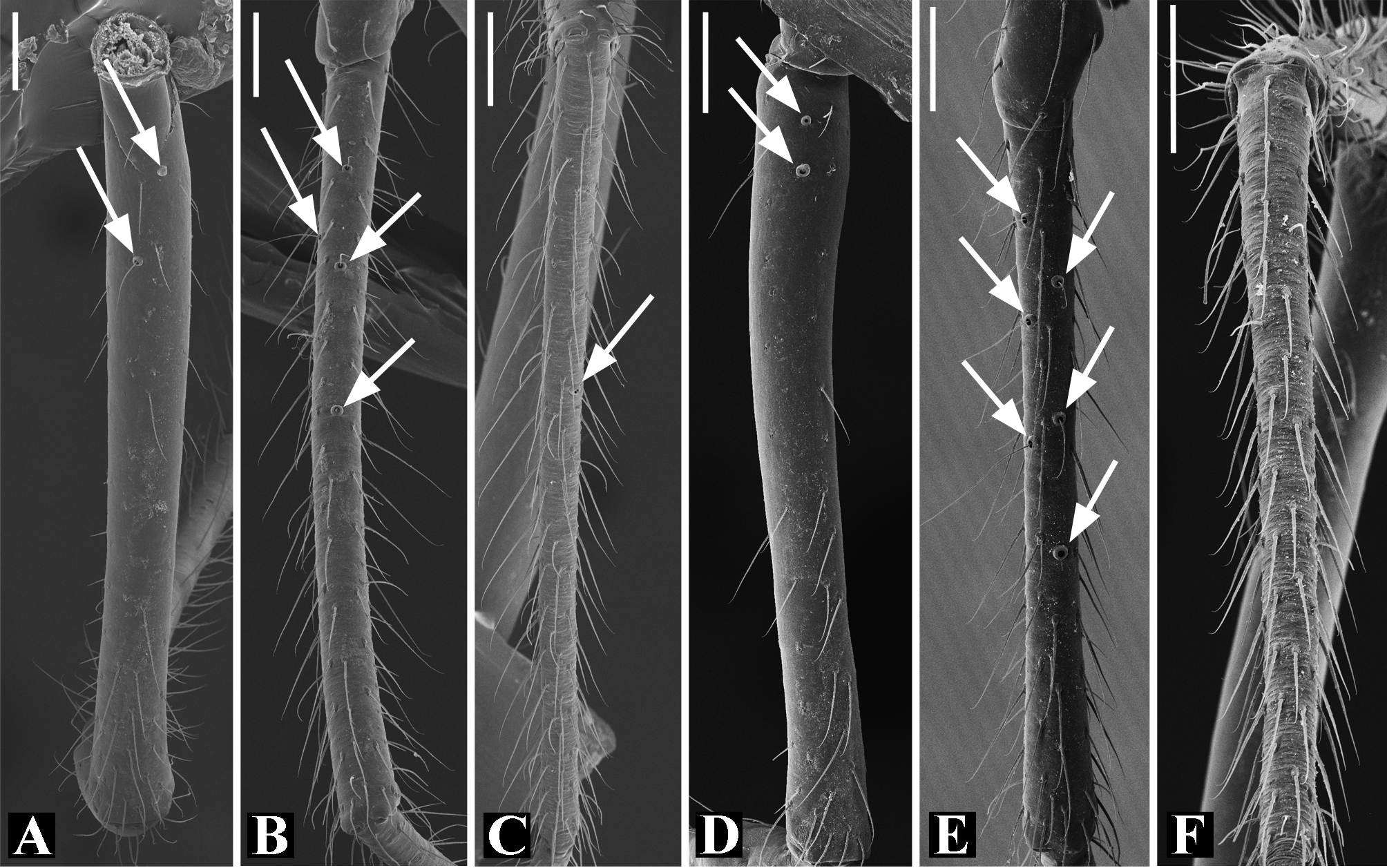
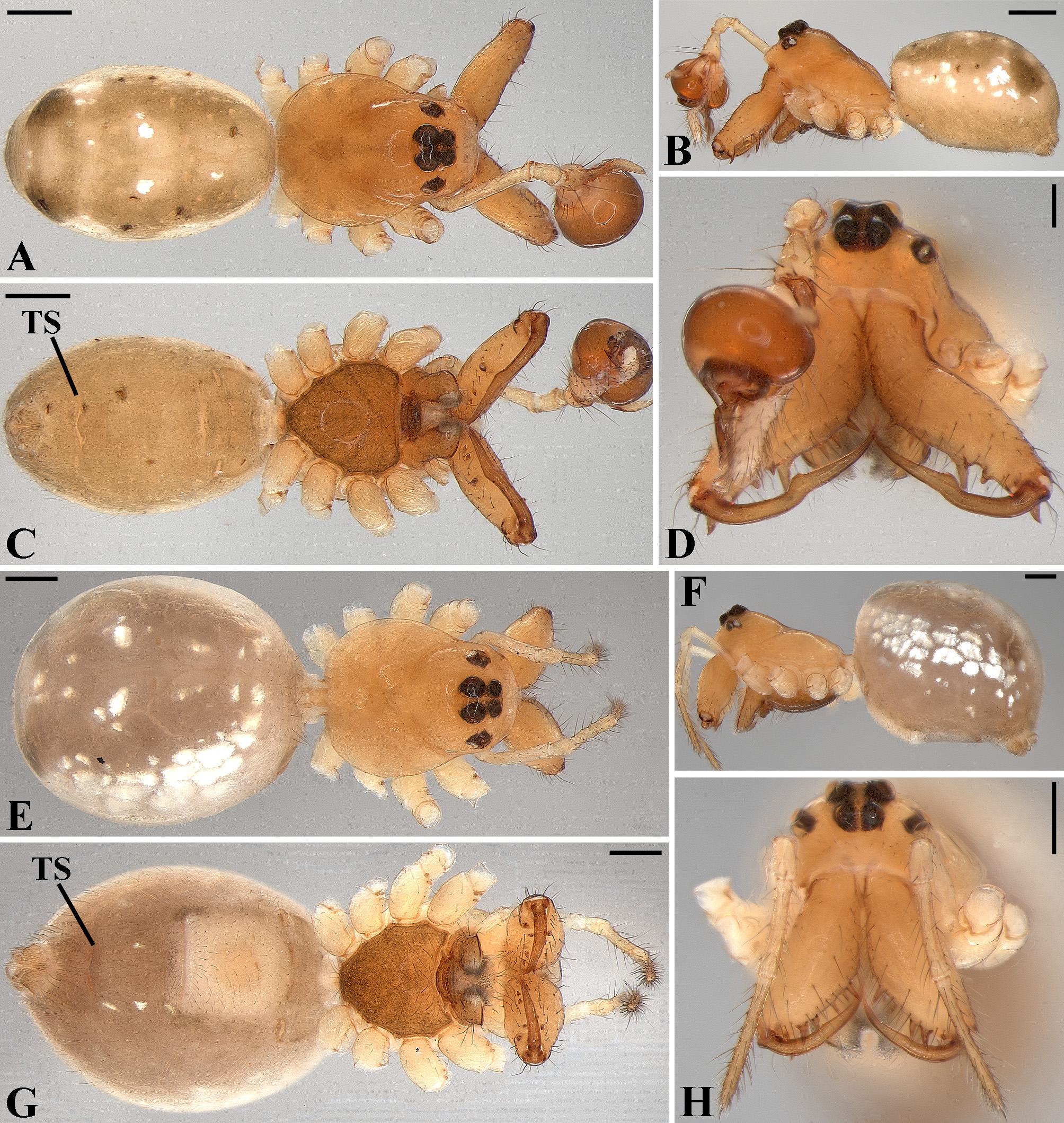
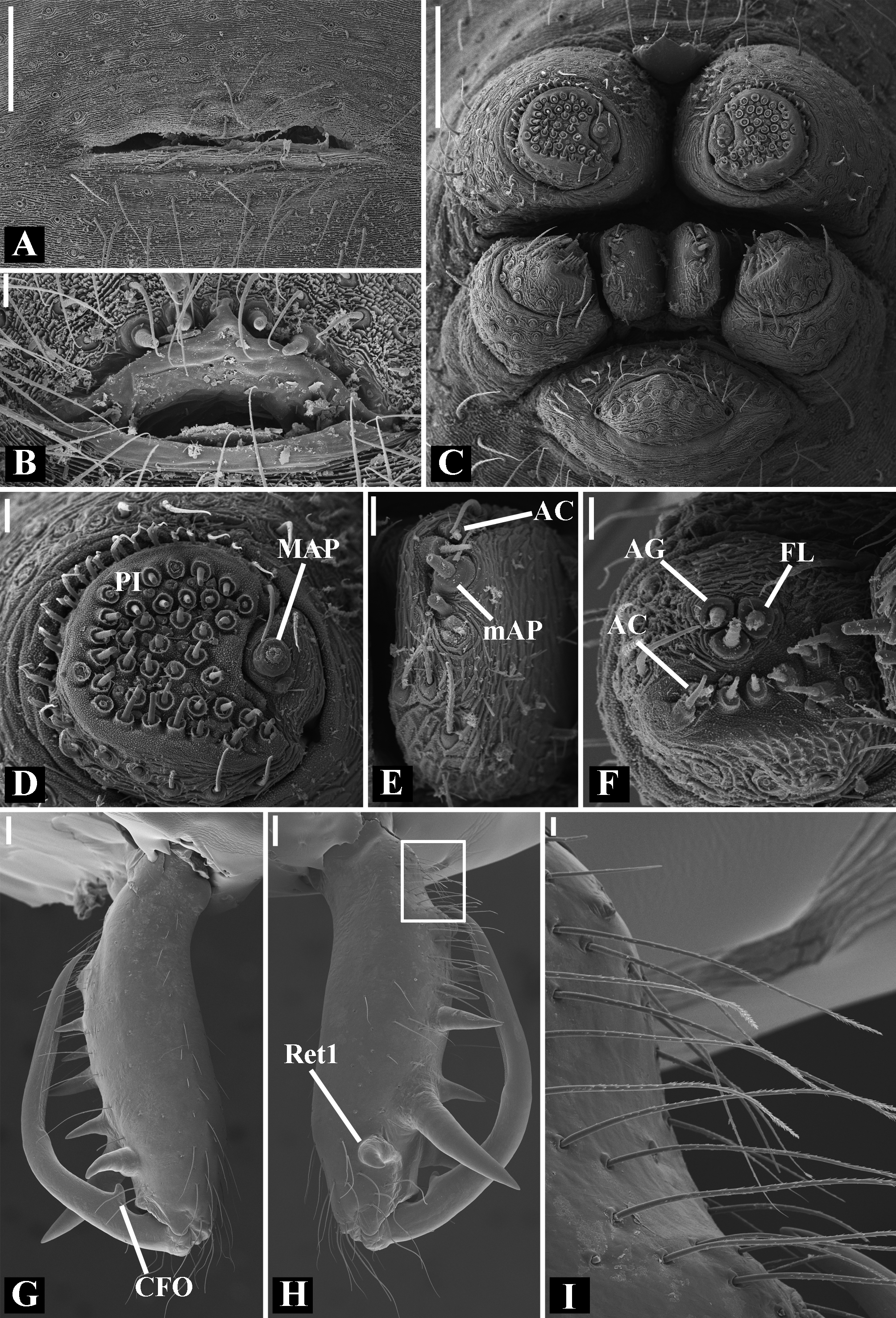
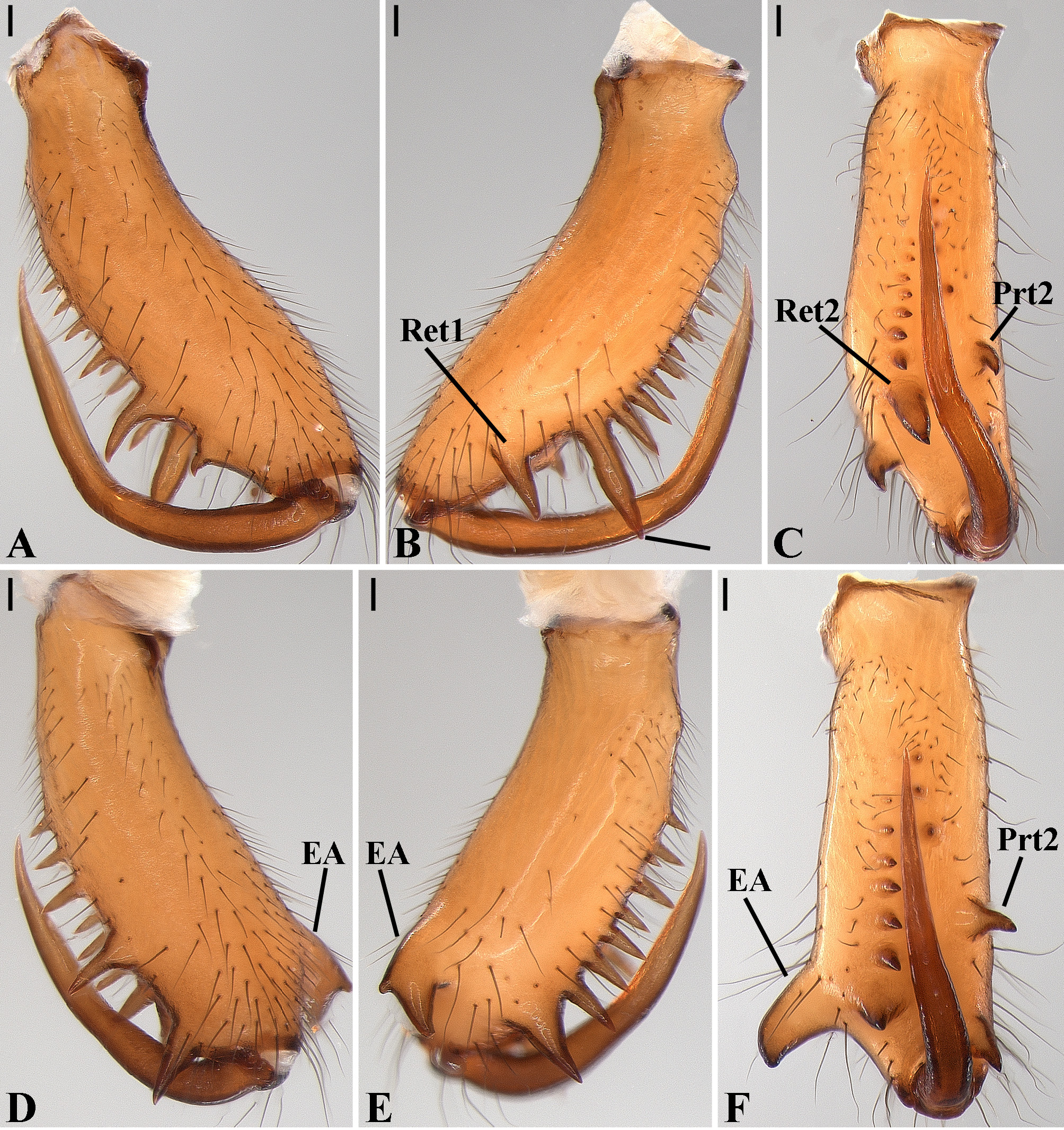
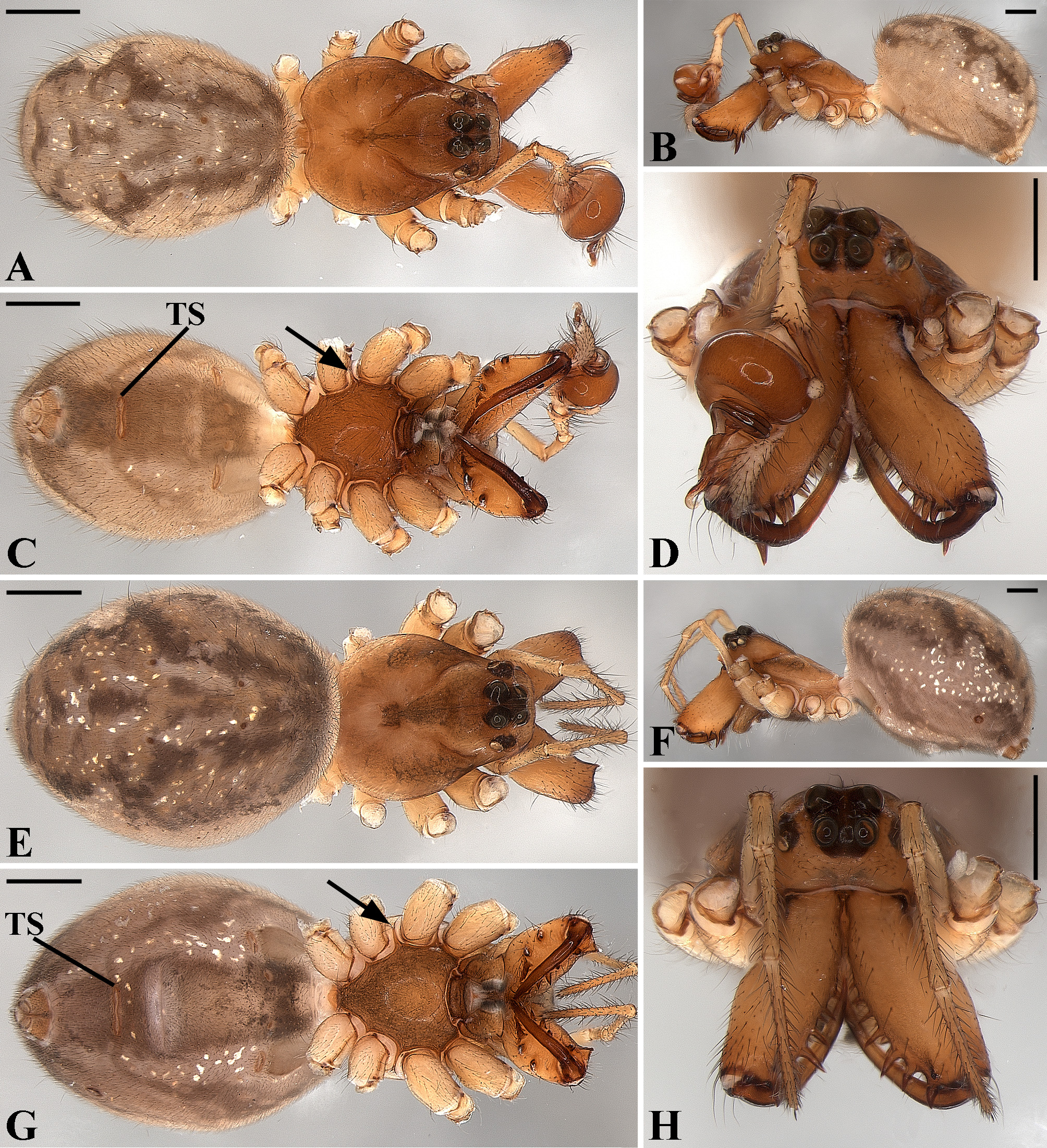
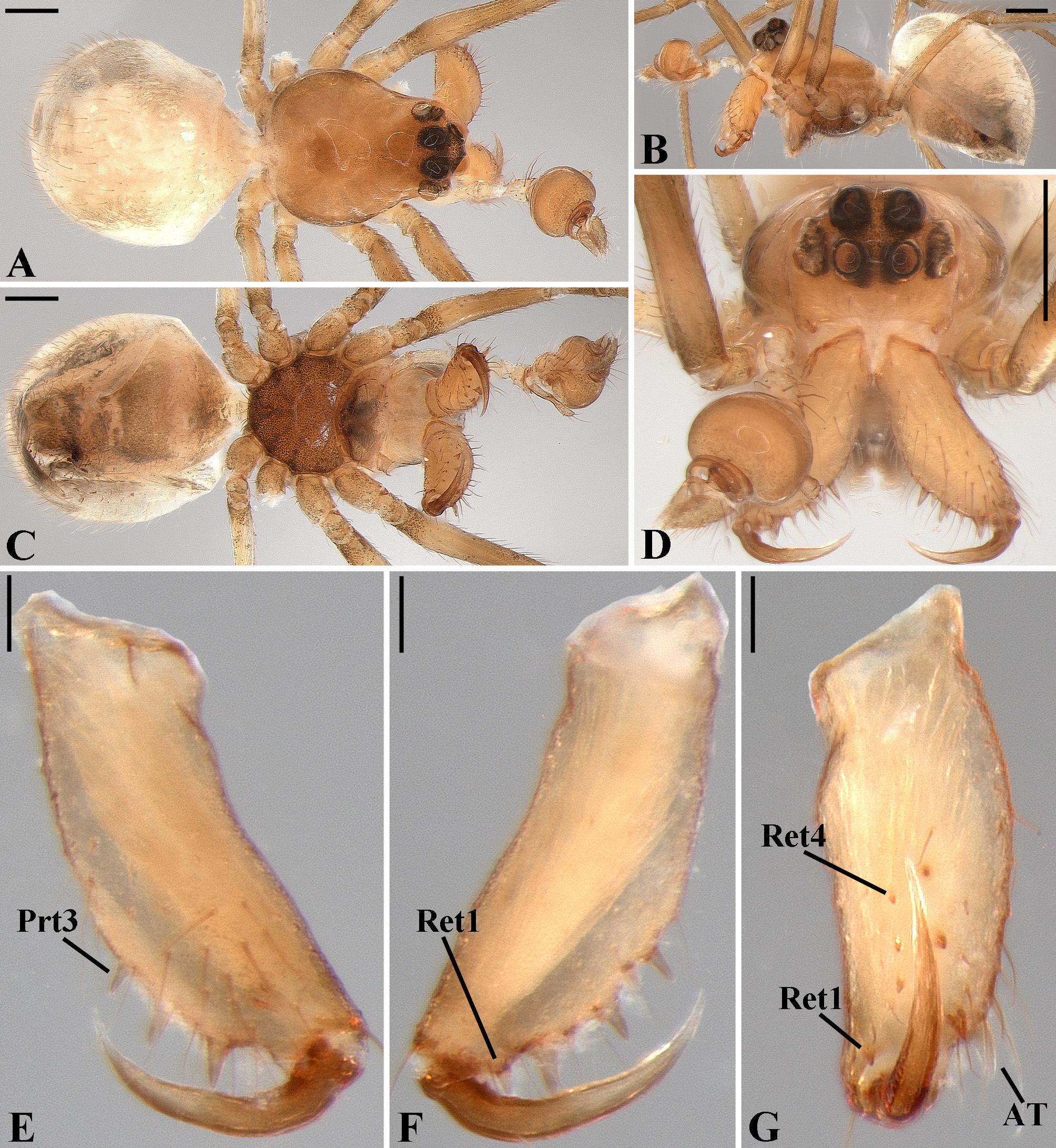
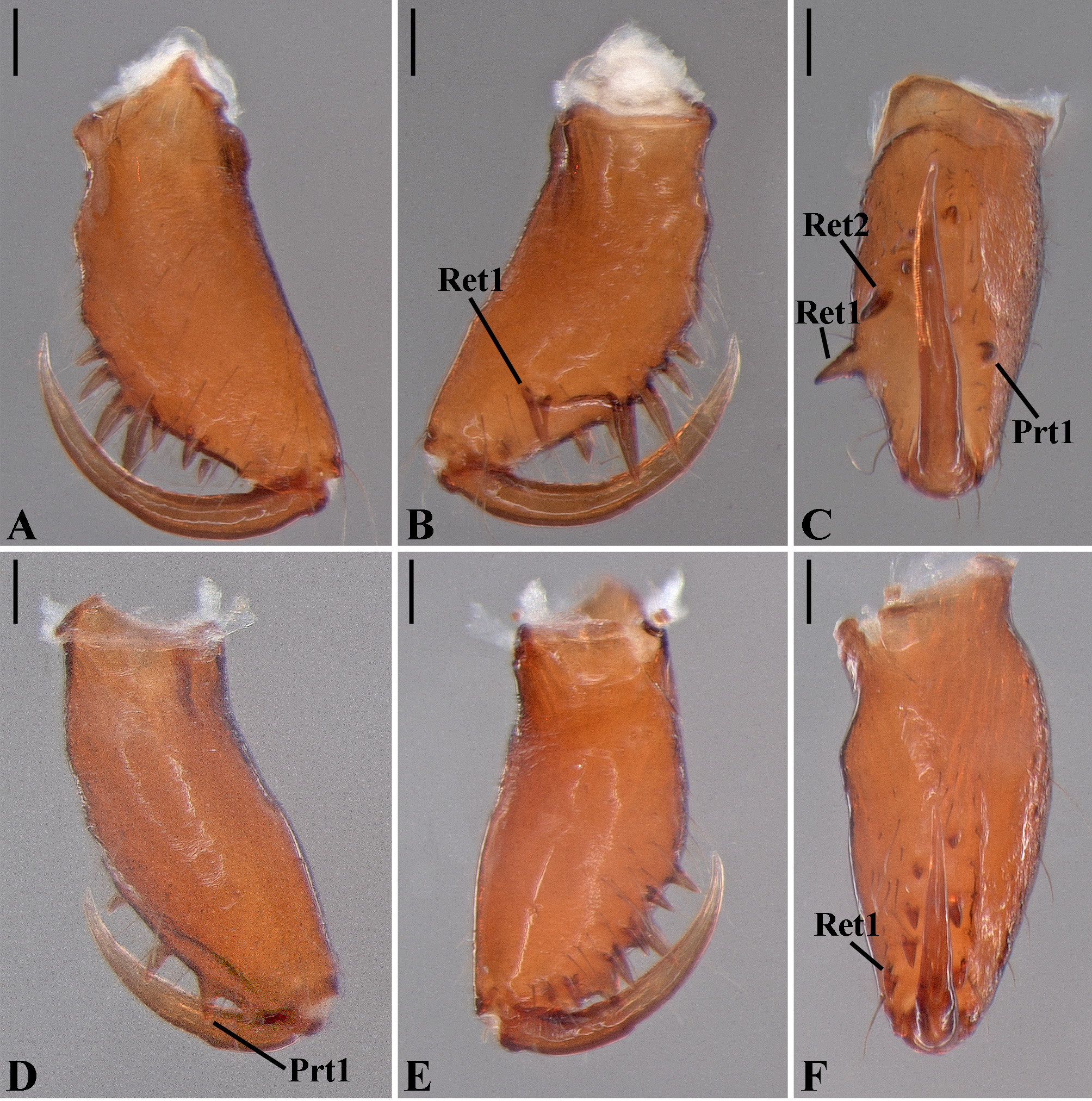
Description: Total length 1.25–4.60 males, 1.40–4.80 females. Carapace longer than wide, 0.62–2.4 long in males and 0.61–2.05 long in females; pale yellow to dark brown ( Figs. 14 A View FIGURE 14 , 19 A View FIGURE 19 , 38 A View FIGURE 38 , 45 A View FIGURE 45 , 75 A View FIGURE 75 ). Sternum as wide as long and prolonged between coxae in some species ( Figs. 25C View FIGURE 25 , 31C View FIGURE 31 , 50C View FIGURE 50 , 56C View FIGURE 56 , 60C View FIGURE 60 ). Labium trapezoidal, wider than long, and rebordered. Eyes subequal in size, lateral eyes slightly smaller. Clypeus height 1.10—3.21. Chelicerae large, sexually dimorphic, with 3–5 promarginal teeth and 3–10 retromarginal teeth ( Figs. 20 View FIGURE 20 , 46 View FIGURE 46 , 51 View FIGURE 51 , 57 View FIGURE 57 , 81 View FIGURE 81 , 86, 101, 115, 127). Cheliceral teeth numbers vary widely particularly on the retromargin, with some specimens presenting asymmetric teeth counts. Cheliceral boss well developed. Cheliceral fangs long and in some species with a well-developed retrolateral outgrowth ( Figs. 39 A View FIGURE 39 , 46 A View FIGURE 46 , 51 A View FIGURE 51 , 68B View FIGURE 68 , 90E View FIGURE 90 ). Legs without spines (except in G. hirsutissima ), slightly longer in males than in females. Femur I length varies from 0.75 to 3.45 in males and from 0.67 to 3.4 in females. Leg formula I>II>IV>III. Femora dorsal surface with one to six trichobothria ( Figs. 30G View FIGURE 30 , 44 A View FIGURE 44 ). Glenognatha foxi , G. heleios , G. iviei and G. spherella lack trichobothria on femur III and IV. Tibia dorsal surface with four to ten trichobothria ( Figs. 30H View FIGURE 30 , 44E View FIGURE 44 ). Metatarsi I, II and III, first half, with one trichobothrium longer than the diameter of the article (two in G. hirsutissima ). Metatarsus IV without trichobothria except in G. florezi (one trichobothrium), G. camisea (one), G. gouldi (one), G. emertoni (one) and G. hirsutissima (two).
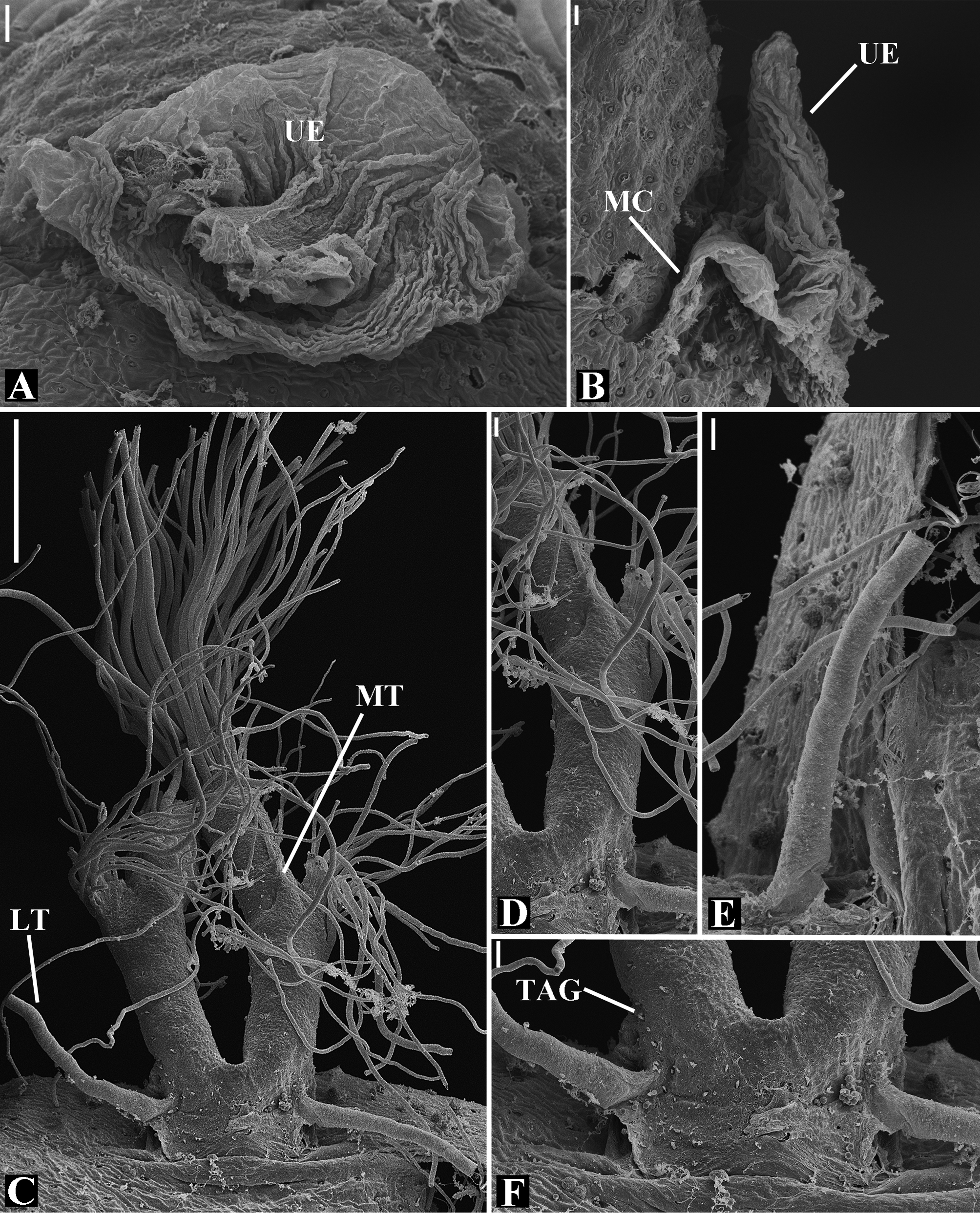
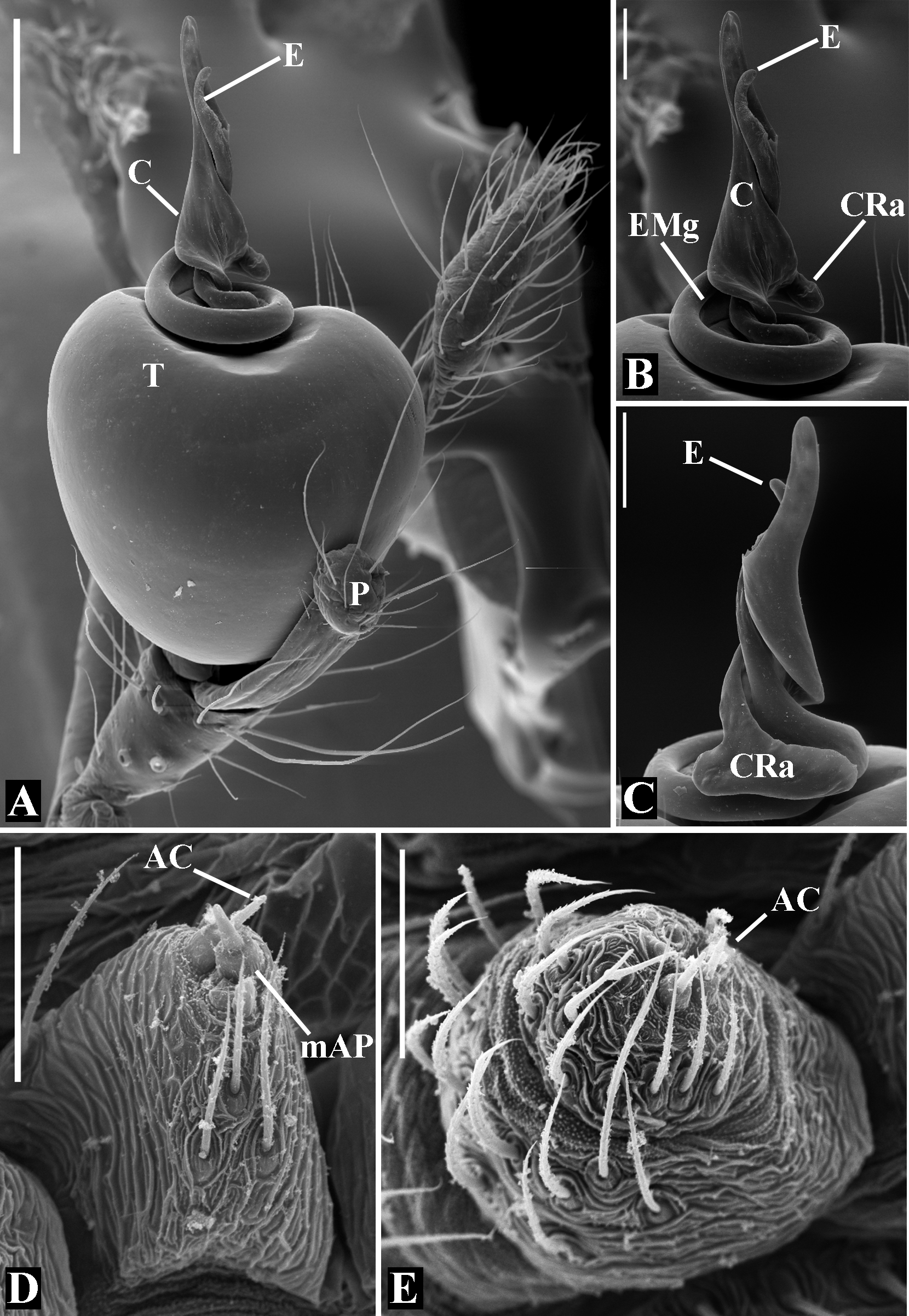
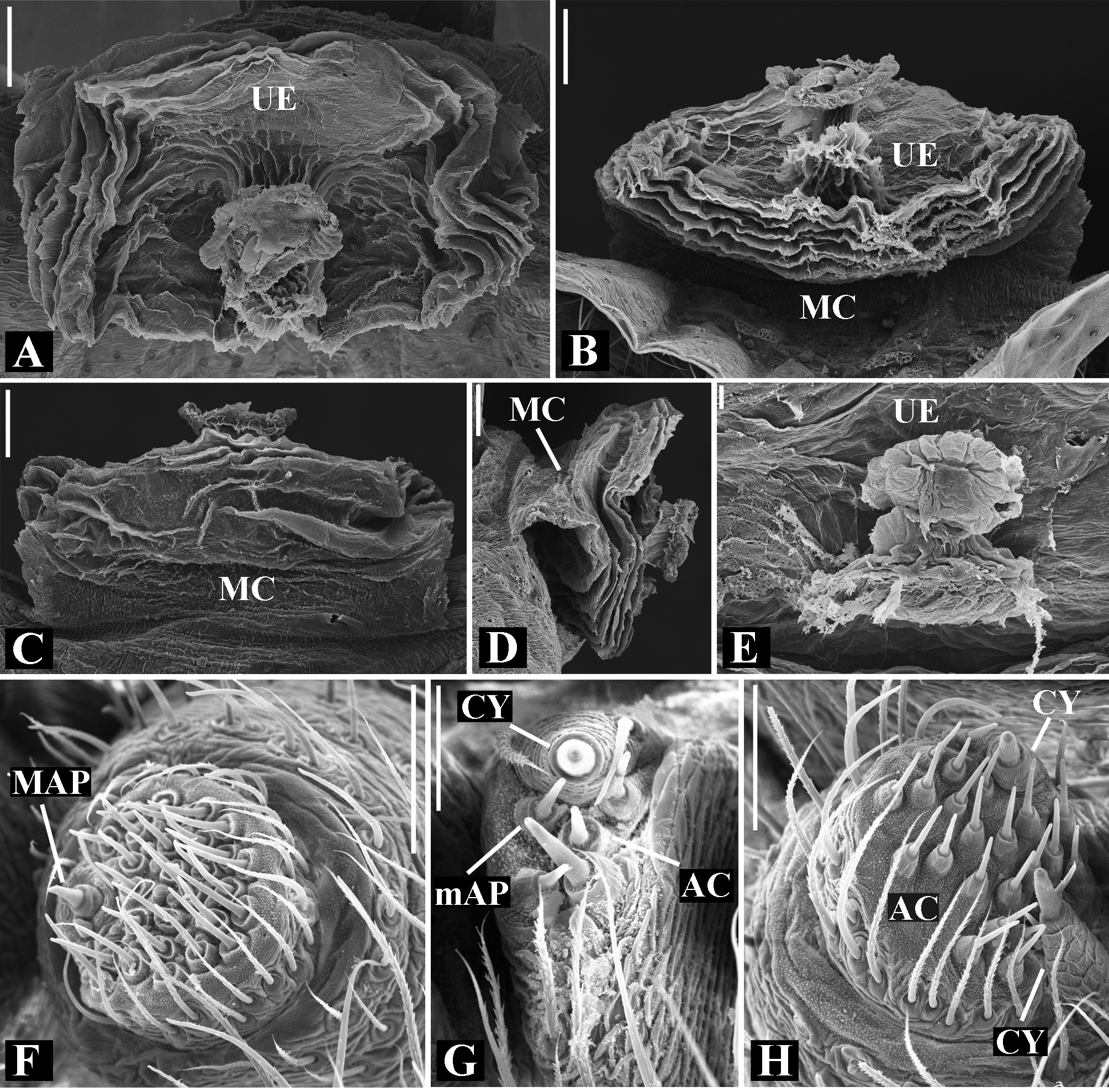
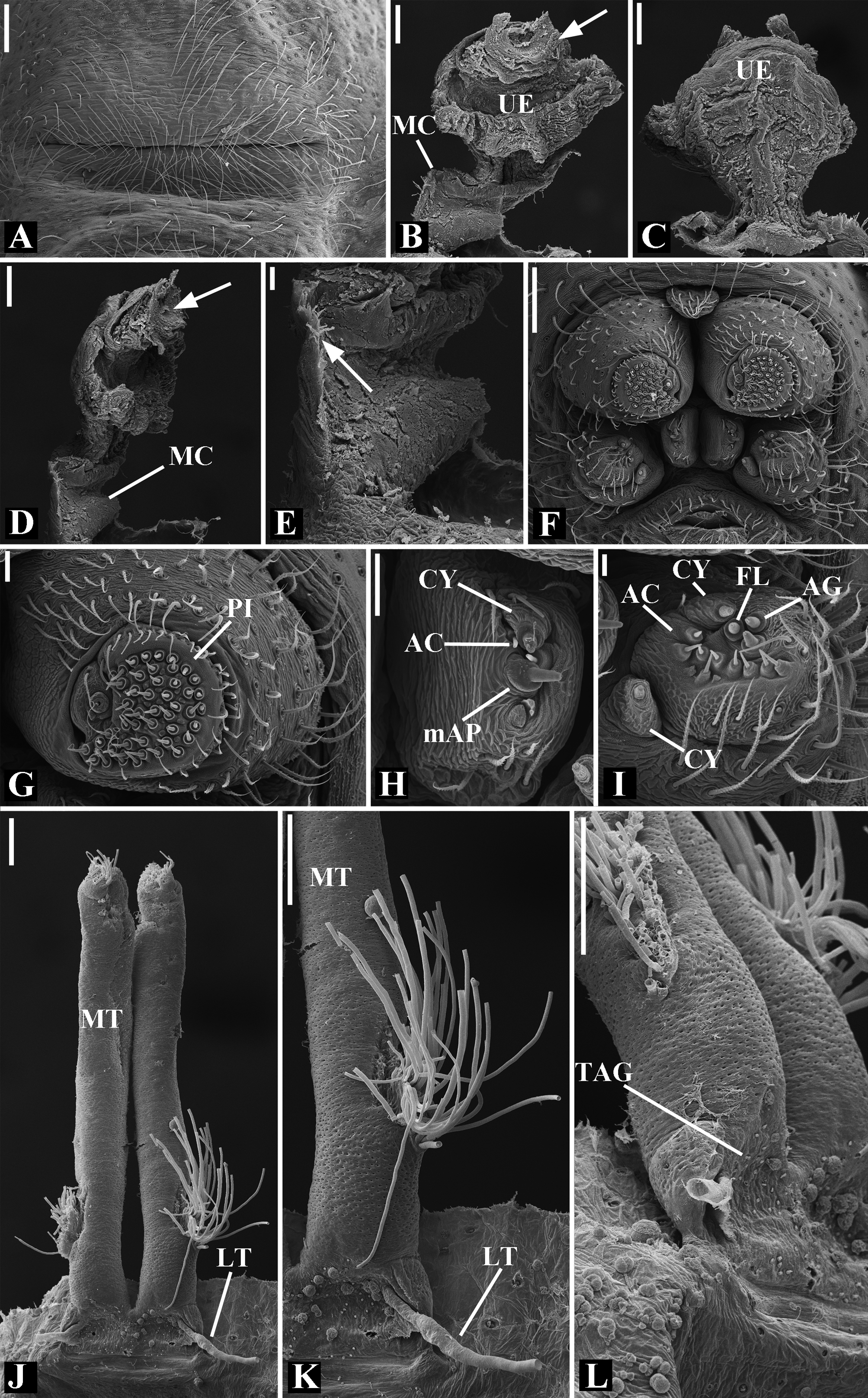
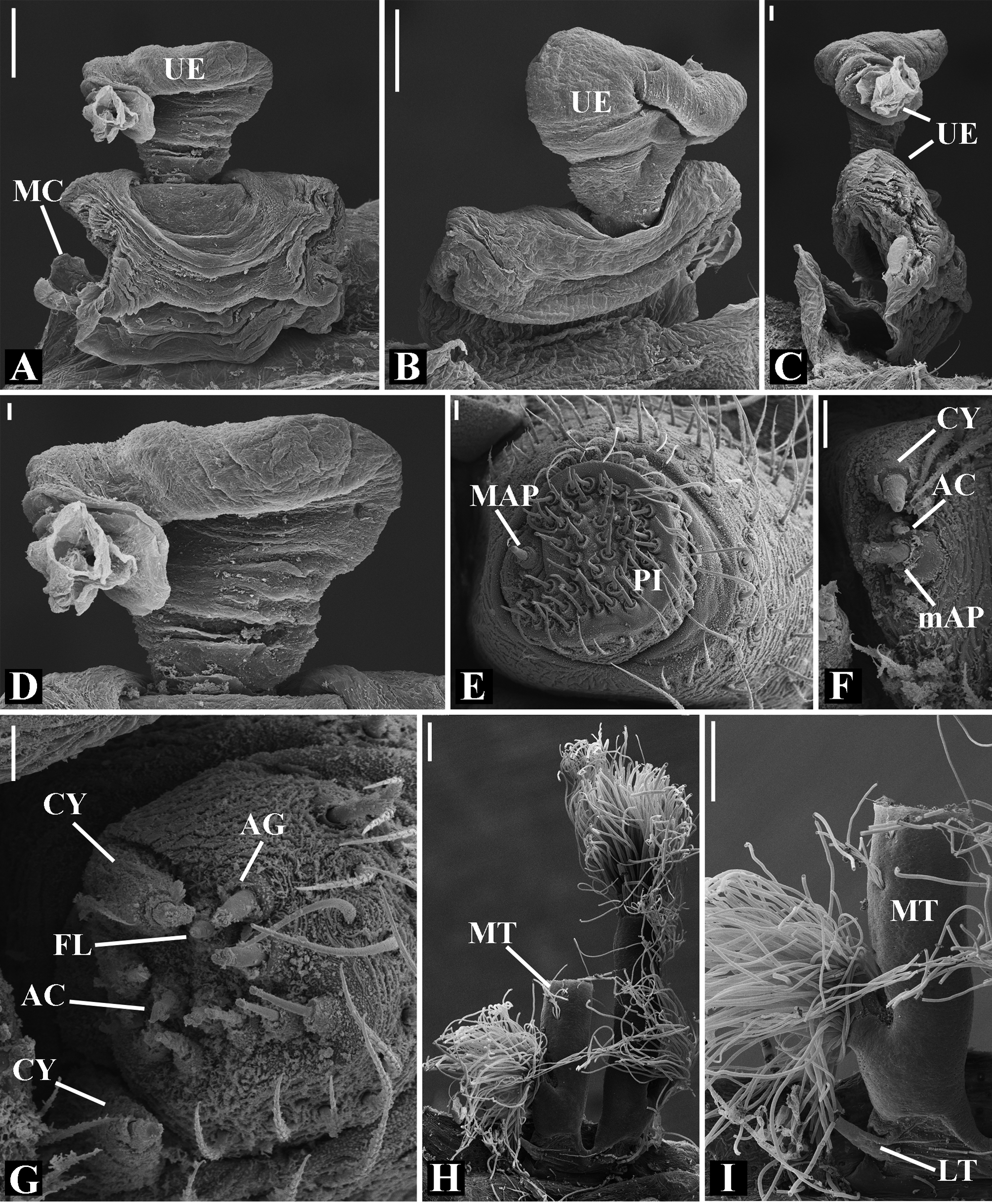
Abdomen oval to spherical. Dorsal pattern usually with disperse silver guanine patches combined with irregular dark longitudinal bands. Lateral surface also covered with a similar pattern of guanine deposits and dark bands. Ventral surface usually with a pale black median band and irregular silver guanine patches. Posterior tracheal spiracle wider than long and anteriorly displaced. Tracheal spiracle glands occupying both sides of the atrium and the basal region of the median trunks ( Figs. 12F View FIGURE 12 , 24D View FIGURE 24 , 41F View FIGURE 41 , 48J View FIGURE 48 , 83E View FIGURE 83 , 92L View FIGURE 92 , 103H View FIGURE 103 , 129I View FIGURE 129 ). ALS with more than 12 piriform spigots ( Figs. 17F View FIGURE 17 , 24F View FIGURE 24 , 30D View FIGURE 30 , 55D View FIGURE 55 , 63E View FIGURE 63 , 64F View FIGURE 64 , 103I View FIGURE 103 , 121H View FIGURE 121 ). PMS with one to five aciniform spigot between the cylindrical and the minor ampullate spigot ( Figs. 13D View FIGURE 13 , 17G View FIGURE 17 , 24G View FIGURE 24 , 30E View FIGURE 30 , 55E View FIGURE 55 , 103J View FIGURE 103 , 121I View FIGURE 121 ), nubbin posterior to the mAP. PLS with 4 to 25 AC spigots on both sexes ( Figs. 13E View FIGURE 13 , 17H View FIGURE 17 , 24H View FIGURE 24 , 30F View FIGURE 30 , 49L View FIGURE 49 , 63G View FIGURE 63 , 103K View FIGURE 103 , 121J View FIGURE 121 ). Females AG–FL triplet anterior to the AC spigots and almost parallel with the anterior CY spigot ( Figs. 24H View FIGURE 24 , 53I View FIGURE 53 , 63G View FIGURE 63 , 103K View FIGURE 103 , 121J View FIGURE 121 , 129L View FIGURE 129 ), except in G. florezi that lacks the triplet ( Fig. 17H View FIGURE 17 ). PLS triplet also present in adult males ( Figs. 30F View FIGURE 30 , 49L View FIGURE 49 , 55F View FIGURE 55 , 64H View FIGURE 64 , 104L View FIGURE 104 ), except in G. boraceia , G. lacteovittata and G. florezi ( Figs. 13E View FIGURE 13 , 24K View FIGURE 24 ). Epiandrous fusules usually four, arranged in a transverse line ( Figs. 30C View FIGURE 30 , 43B View FIGURE 43 , 49M View FIGURE 49 , 55B View FIGURE 55 , 64D View FIGURE 64 , 79D View FIGURE 79 , 104H View FIGURE 104 ), although some species have up to eleven fusules such as in G. florezi ( Fig. 18H View FIGURE 18 ).
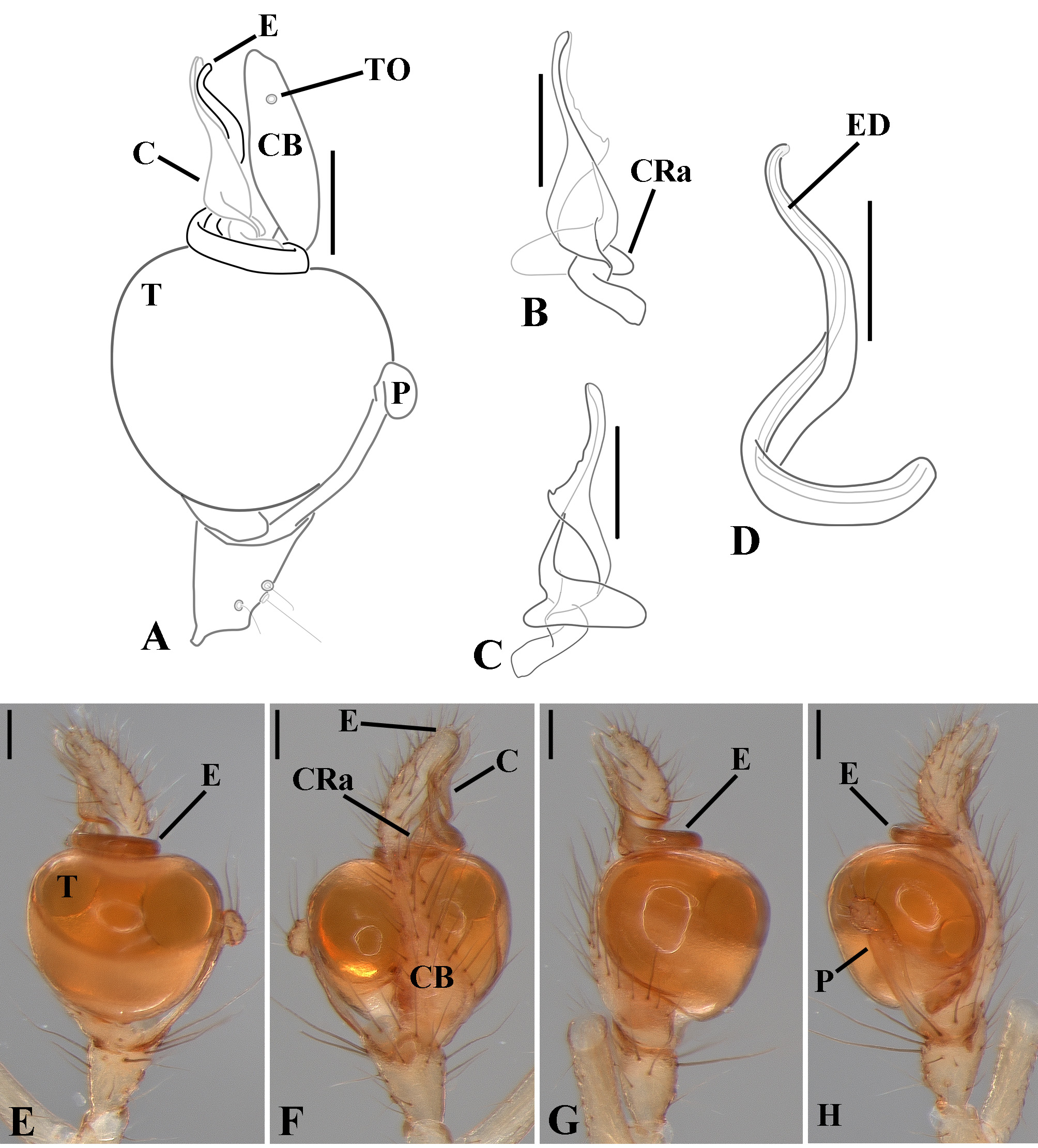
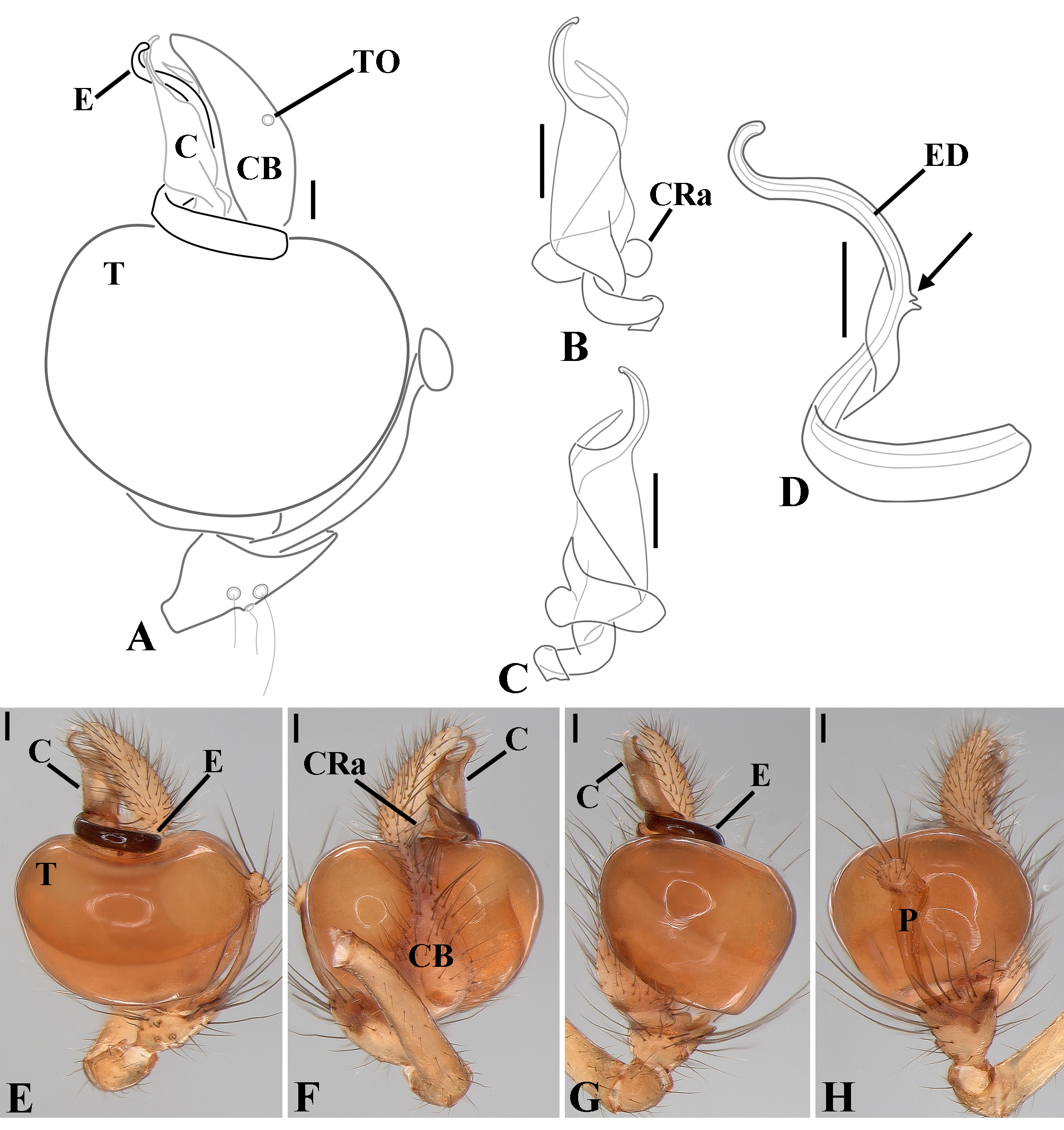
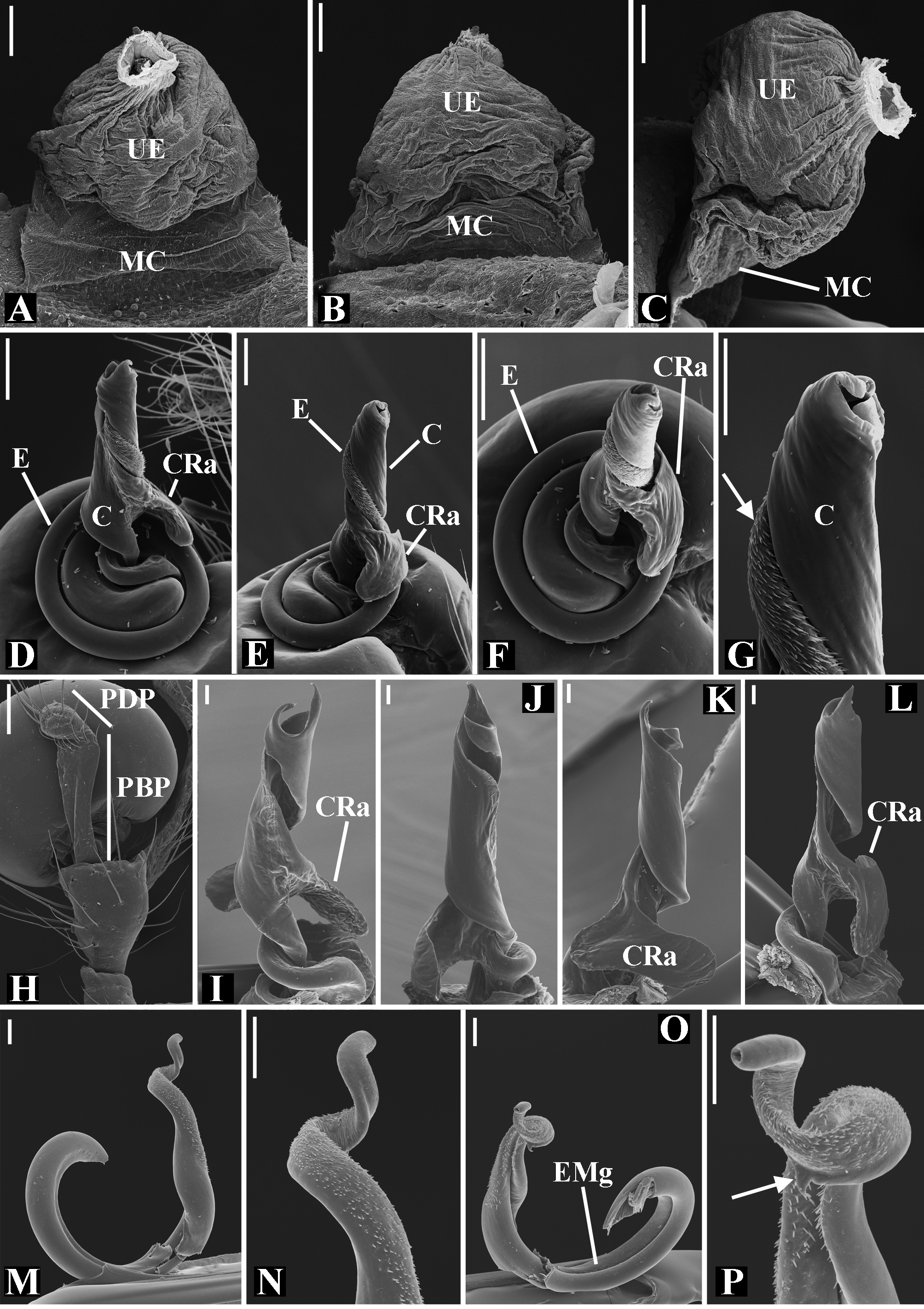
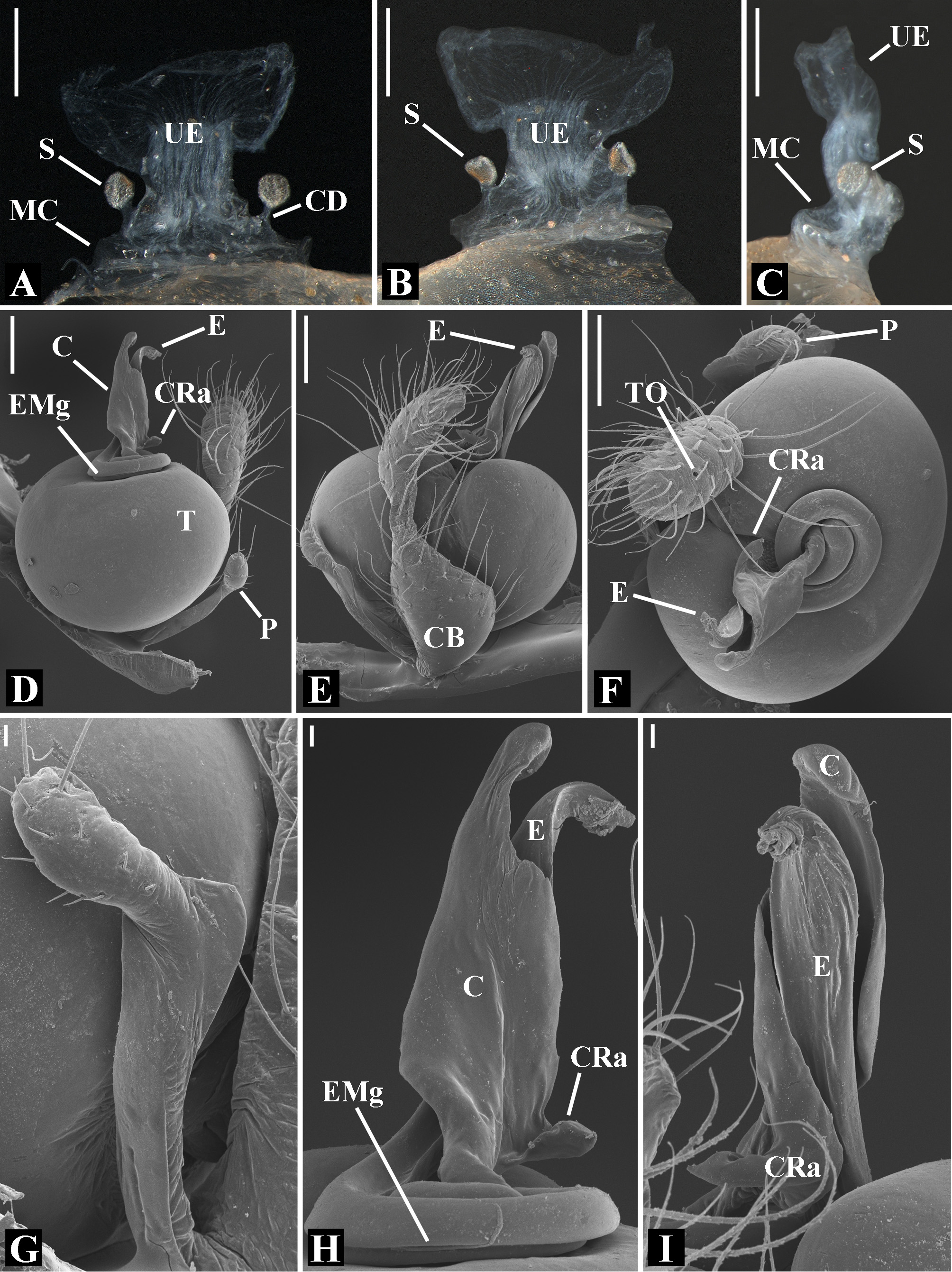
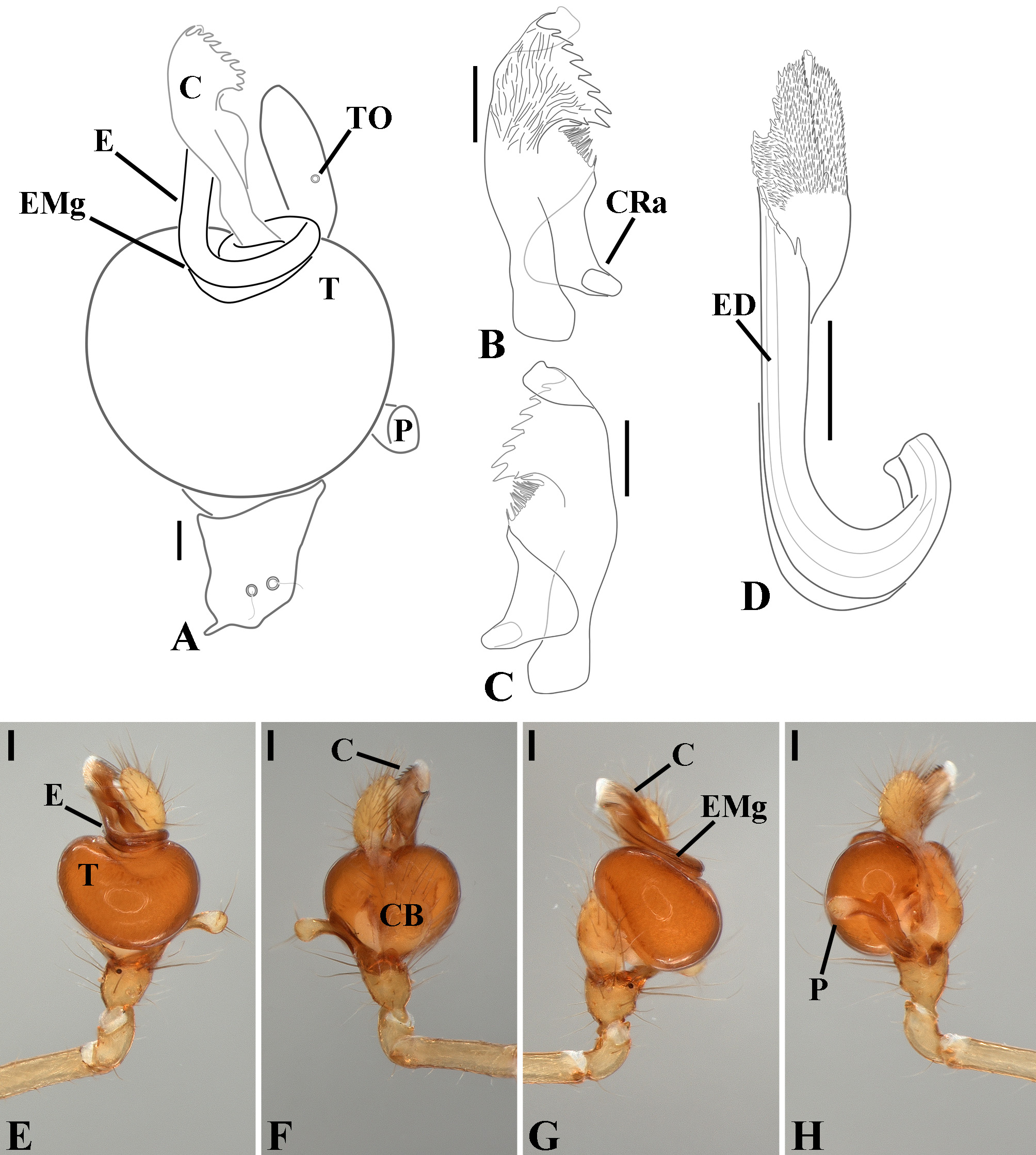
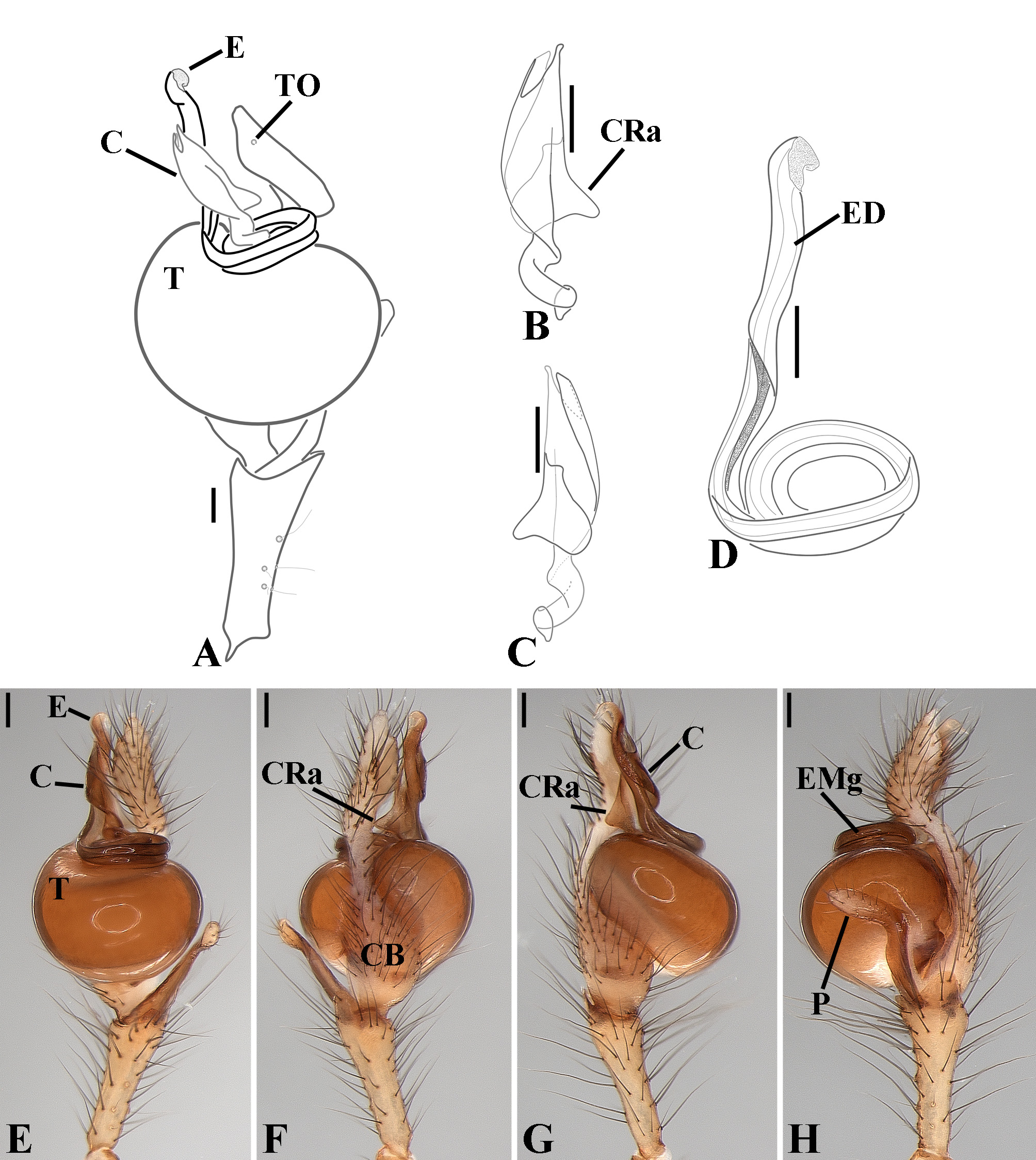
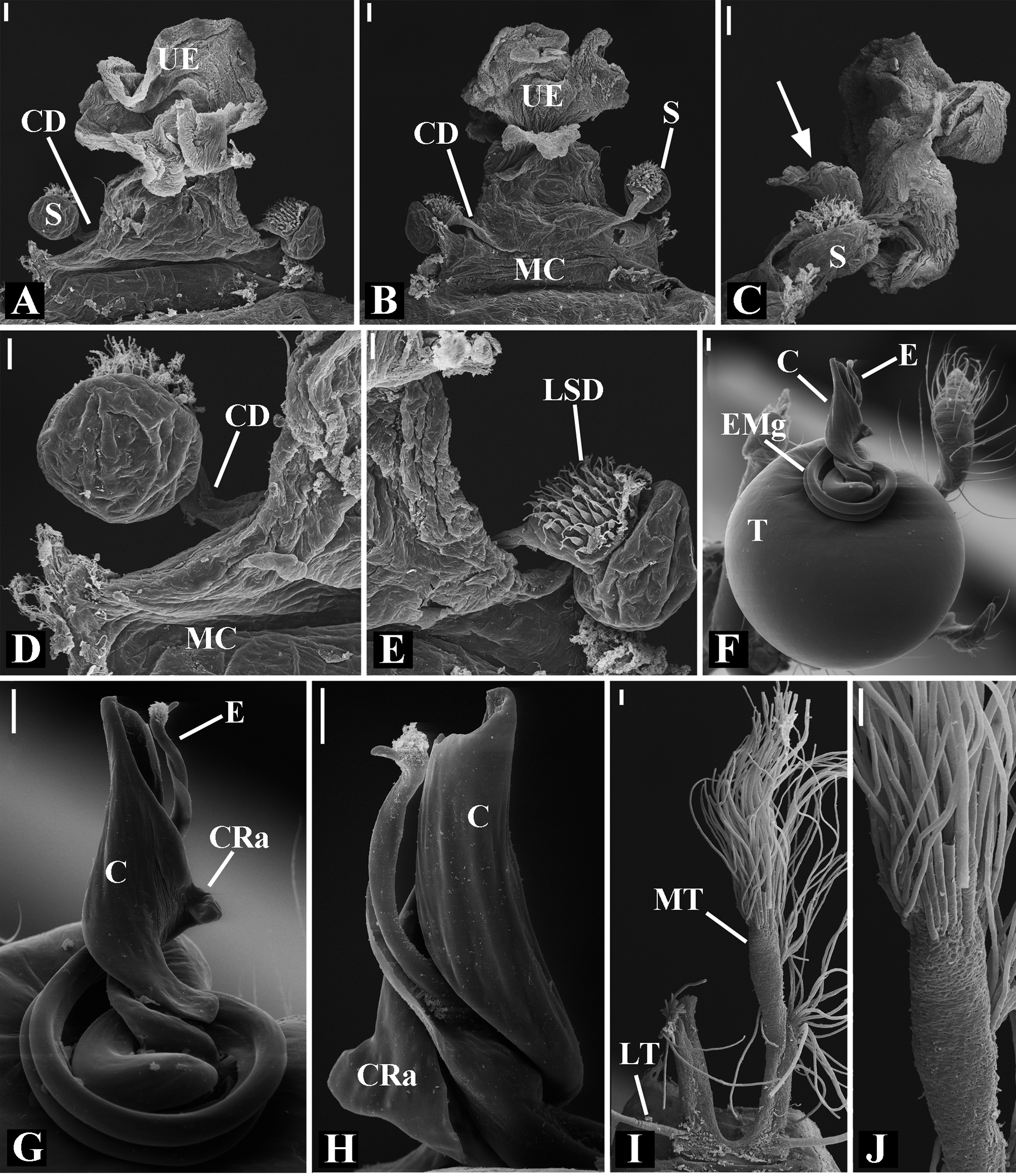
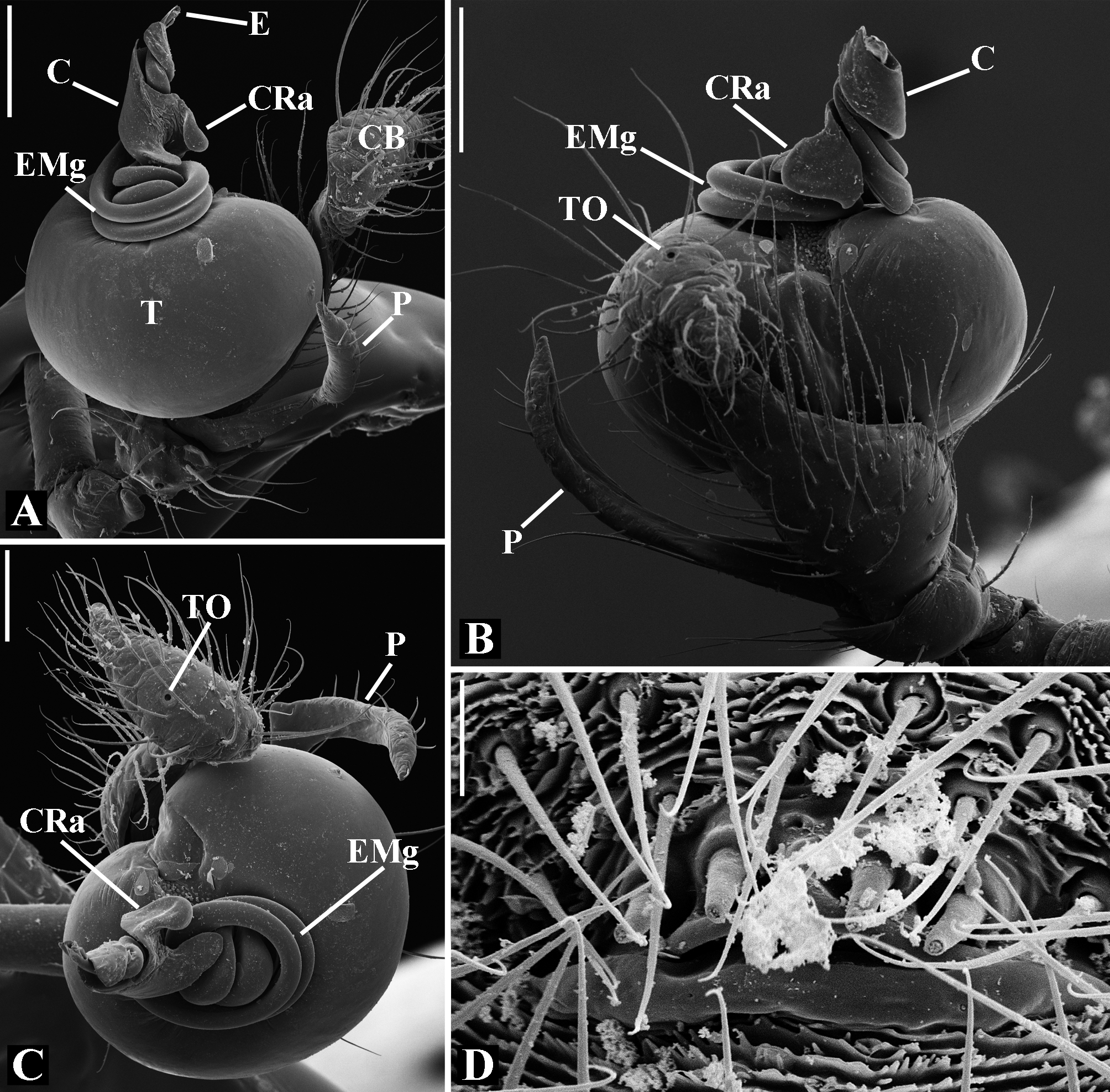
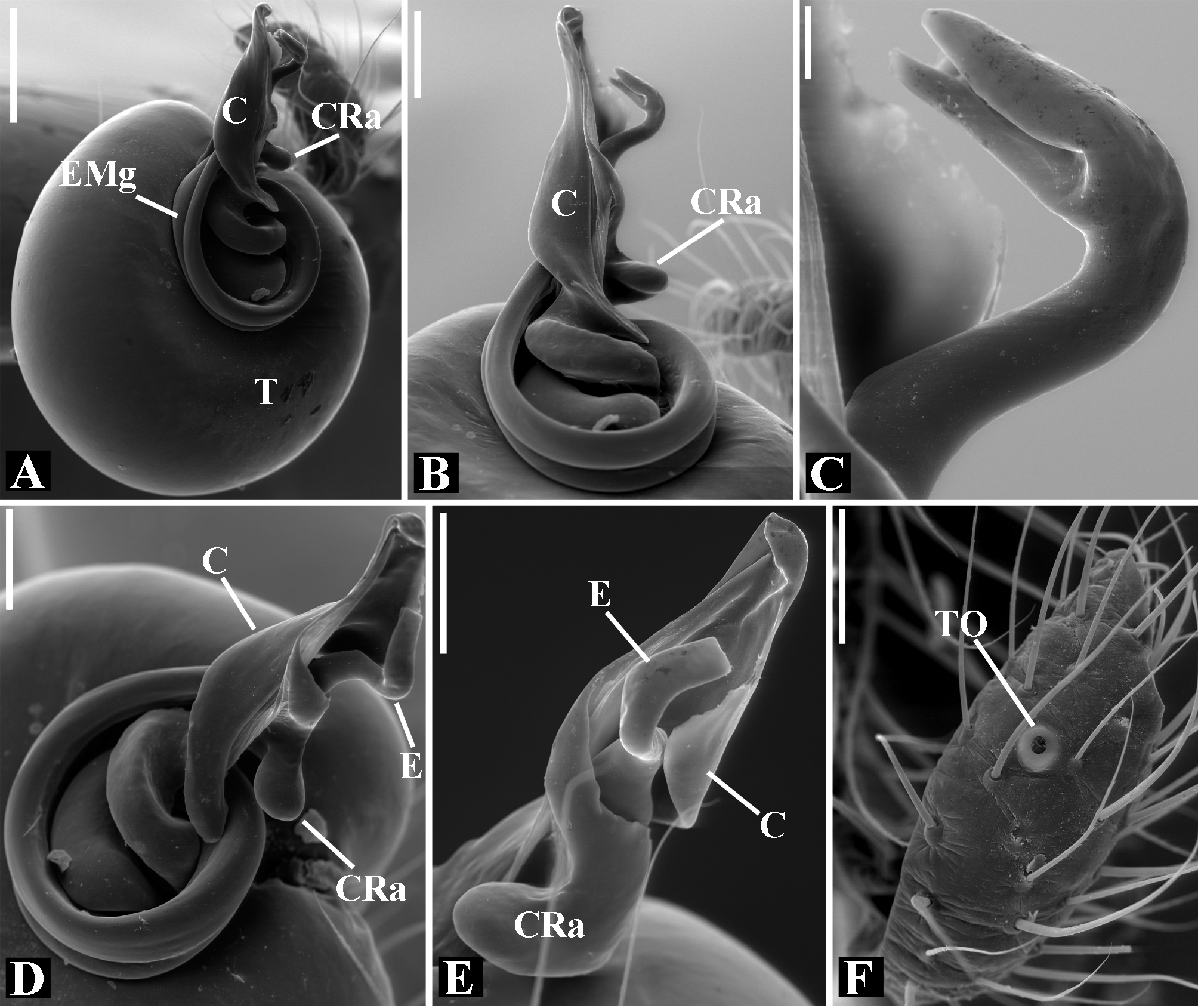
Male palpal tibia as long as wide ( Figs. 91 A View FIGURE 91 , 95 A View FIGURE 95 , 107 A View FIGURE 107 , 112 A View FIGURE 112 ) or longer ( Figs. 47 A View FIGURE 47 , 52 A View FIGURE 52 , 58 A View FIGURE 58 , 62 A View FIGURE 62 ), with three to six trichobothria on its ventral and retrolateral surfaces. Cymbium elongated with a swollen apical portion and a conspicuous tarsal organ ( Figs. 18 A View FIGURE 18 , 22E View FIGURE 22 , 27E View FIGURE 27 , 28F View FIGURE 28 , 47E View FIGURE 47 , 70K View FIGURE 70 , 79C View FIGURE 79 , 104 A View FIGURE 104 ). Paracymbium with two perpendicular portions, as seen in retrolateral view ( Figs. 11H View FIGURE 11 , 27H View FIGURE 27 , 47H View FIGURE 47 , 49B View FIGURE 49 , 99H View FIGURE 99 , 128H View FIGURE 128 ). Tegulum spherical, with a distal depression where the embolus and the conductor rest. Embolus surrounding the conductor base. Embolic medial groove limited to the inner surface of the embolus ( Figs. 13 A View FIGURE 13 , 18 A View FIGURE 18 , 23D View FIGURE 23 ) or extended towards its outer surface ( Figs. 28H View FIGURE 28 , 49F View FIGURE 49 , 54 A View FIGURE 54 , 64B View FIGURE 64 , 74F View FIGURE 74 , 92F View FIGURE 92 , 97 A View FIGURE 97 , 109 A View FIGURE 109 ). Embolus surface smooth or with filiform ( Fig. 23G, N, P View FIGURE 23 ), tooth-like ( Fig. 16D View FIGURE 16 , 23P View FIGURE 23 ) or scale-like projections ( Fig. 54L View FIGURE 54 ). Conductor emerging from the center of the tegulum as a cylindrical tube that bends towards the center of the tegulum and flattens into a lamina. The conductor lamina has its apical portion folded and carries a conspicuous prolaterally folded retrolateral apophysis ( Figs. 18B View FIGURE 18 , 23F, L View FIGURE 23 , 28F View FIGURE 28 , 54C View FIGURE 54 , 79C View FIGURE 79 , 84E View FIGURE 84 , 92G View FIGURE 92 ). Embolus partially enclosed by the apical fold of the conductor lamina and the prolaterally folded retrolateral apophysis ( Figs. 18B View FIGURE 18 , 23F View FIGURE 23 , 28F View FIGURE 28 , 43H View FIGURE 43 , 70H View FIGURE 70 , 79C View FIGURE 79 , 97D View FIGURE 97 ). Female external genitalia slit-shaped with sclerotized edges. Internal genitalia haplogyne, with the following structures: a membranous chamber, a large uterus externus and a pair of copulatory ducts leading to the spermathecae ( Figs. 12 A –B View FIGURE 12 , 17 A –E View FIGURE 17 , 23 A –C View FIGURE 23 , 28 A –C View FIGURE 28 , 53 A –E View FIGURE 53 , 70 A –E View FIGURE 70 , 92 A –E View FIGURE 92 , 129 A –C View FIGURE 129 ). The membranous chamber has filiform projections on its lateral borders that serve as muscle attachment points ( Figs. 48D View FIGURE 48 , 53E View FIGURE 53 ). In some species the spermathecae and copulatory ducts are absent ( Figs. 12 A –B View FIGURE 12 , 17 A –E View FIGURE 17 , 53B–E View FIGURE 53 , 121 A –C View FIGURE 121 ) (see also Cabra-García et al. 2014).
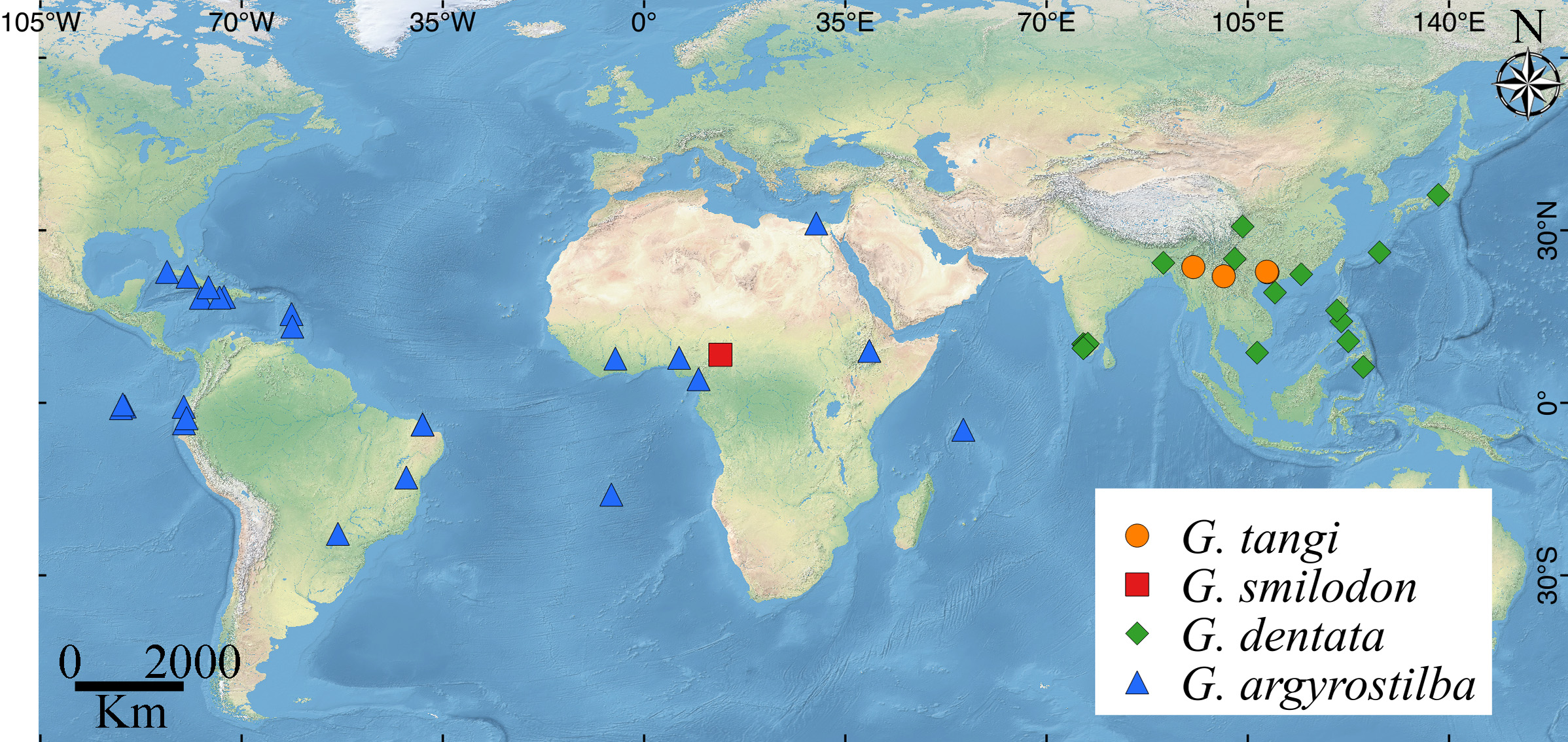
Composition: Glenognatha currently includes 27 species: G. argyrostilba ( Pickard-Cambridge, 1876) n. comb. G. australis ( Keyserling, 1883) , G. dentata ( Zhu & Wen, 1978) n. comb., G. emertoni Simon, 1887 , G. foxi ( McCook, 1894) , G. gaujoni Simon, 1895 , G. globosa (Petrunkevitch, 1925) , G. gloriae ( Petrunkevitch, 1930) , G. heleios Hormiga, 1990 , G. hirsutissima ( Berland, 1935) , G. iviei Levi, 1980 , G. lacteovittata ( Mello-Leitão, 1944) , G. minuta Banks, 1898 , G. smilodon Bosmans & Bosselaers, 1994 , G. spherella Chamberlin & Ivie, 1936 , G. tangi ( Zhu, Song & Zhang, 2003) n. comb. and 11 new species described herein.
Distribution: Glenognatha species have a broad distribution with records in Nearctic, Neotropic, Afrotropic, Indo-Malaya, Oceania and Paleartic regions. It occupies a broad altitudinal range from 6 ( G. iviei ) to 2,700 m. ( G. florezi ) ( Figs. 130–138 View FIGURE 130 View FIGURE 131 View FIGURE 132 View FIGURE 133 ).
Natural history: The biology of Glenognatha species is still poorly known and published data focus mainly on Nearctic species. Glenognatha foxi is recorded as an abundant spider in a wide variety of crops in the United States: soybean ( Young & Edwards 1990; Balfour & Rypstra 1998), alfalfa ( Young & Edwards 1990; Birkhofer et al. 2007, Welch et al. 2011), rice, sugarcane, peanuts, cotton ( Agnew et al. 1985; Heiss & Meisch 1985; Young & Edwards 1990) and citrus ( Mansour et al. 1982). Chapman et al. (2013) recorded Collembola, Aphididae and Brachycera as prey taxa for G. foxi in winter wheat. Phenology of G. foxi in Georgia was assessed by Draney (1997), who suggested a possible annual reproductive cycle with mating in the early summer. G. foxi is recorded as the most abundant aerial species in one of the two sampling stations assessed by Dean & Sterling (1985). Barrows (1919) described some aspects of the reproductive biology and habits of G. foxi . According to this author, G. foxi is common in meadows and waste-lands in hot dry situations. The mating occurs in the middle of the web with cheliceral clasp and each male palp is inserted alternately every five minutes. The overall copula lasts fifteen minutes. Nevertheless, as noted by Heiss & Meisch (1985), G. foxi also lives in moist conditions; populations in Arkansas rice crops build webs near the middle of the rice plants not far above the water.
Hormiga & Döbel (1990) and Döbel et al. (1990) provided phenology and web architecture data for G. heleios . According with these authors, G. heleios is a univoltine species in intertidal salt marshes of New Jersey, with greater abundance in short and intermediate forms of Spartina alterniflora . Webs are placed close to the soil surface with the sticky spiral very closely spaced. Some aspects of the reproductive biology of G. heleios were described by Edwards & Senske (2001). These authors recorded the insertion of only one palp and a copula duration of two hours.
Danielson-François (2006) carried on a detailed study of habits, sperm release patterns and reproductive behavior of G. emertoni . In this work, specimens were found in vegetation overhanging streams and under streamside rocks in Arizona. Avoidance behavior after disturbance and mating behavior were described. In the former, the spider dropped into the water beneath and floated for some distance until the extended legs allow climbed up some streamside vegetation. The mating behavior involved cheliceral clasp, vibratory courtship through legs movements and palps insertions.
Glenognatha lacteovittata View in CoL is recorded as a common species in alfalfa and wheat crops in Argentina ( Armendano & González 2010, 2011). G. argyrostilba View in CoL is collected preferentially in humid habitats of Santa Cruz island in Galápagos, Ecuador ( Baert et al. 1991). Glenognatha dentata View in CoL is recorded in rice crops from China ( Barrion et al. 2012), India (Sebastian et al. 2005; Sudhikumar et al. 2005) and Philippines ( Barrion & Litsinger 1995; Litsinger et al. 2006). Dupo & Barrion (2009) classify G. dentata View in CoL as a specialist predator of planthoppers nymphs in rice Asian ecosystems.
Adults and juveniles of G. mendezi View in CoL were collected in low mountain humid forests in western Colombian Andes ( Figs. 7 A –B View FIGURE 7 ). This species builds its webs in vegetation adjacent to streams about 25 cm to 2 m above ground level, although some individuals were found far away from any current in low vegetation. In both cases, the web is almost horizontal. It has closed hub and few sticky spiral turns and radii ( Fig. 8C View FIGURE 8 ). The web frame can be rectangular or triangular. Some webs were observed under leaves.
Glenognatha patriceae lives in the same habitats as G. mendezi ( Fig. 7B View FIGURE 7 ). Nevertheless, this species builds its webs a few centimeters ( 1–5 cm) above streams ( Fig. 8D View FIGURE 8 ). Rocks are used as attachment points ( Fig. 8D View FIGURE 8 ). Web aggregations with shared radii and numerous hubs were commonly observed ( Fig. 8E View FIGURE 8 ). In this structures different individuals cohabit, each occupying one hub. No aggression between conspecifics was recorded. A similar avoidance behavior, as that described for G. emertoni by Danielson-François (2006), was observed after disturbance. Diptera and Ephemeroptera were preyed during observations.
Glenognatha gaujoni ( Figs. 8 A –B View FIGURE 8 ) builds its webs a few centimeters ( 1–7cm) above streams in Amazonian rainforests ( Figs. 7C–D View FIGURE 7 ). The web is horizontal with closed hub, few radii and sticky spiral turns ( Fig. 8F View FIGURE 8 ).
Eberhard (1982) described the web building behavior of G. globosa from Colombia (the identification of the voucher was made after Eberhard’s original publication). This species determines the sticky spiral attachment point tapping forward with the internal leg I, holds the next radius near the attachment point with the outer leg III and IV, pushes the current segment with internal leg IV, has no temporary spiral, attaches the sticky spiral to each radius crossed, lays one radius to the frame and lefts the hub of the web intact after finishing the sticky spiral.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
Glenognatha Simon, 1887
| Jimmy Cabra-García & Antonio D. Brescovit 2016 |
Glenognatha
| Levi 1980: 64 |
| Berland 1935: 50 |
| Banks 1929: 90 |
| Simon 1887: 193 |