Stiphodon maculidorsalis, Maeda & Tan, 2013
|
publication ID |
https://doi.org/ 10.5281/zenodo.5352886 |
|
publication LSID |
lsid:zoobank.org:pub:3148A9D7-09A3-4180-8783-08B0DCD897E5 |
|
persistent identifier |
https://treatment.plazi.org/id/2483413E-C793-46A1-B89B-23311FBFBAB5 |
|
taxon LSID |
lsid:zoobank.org:act:2483413E-C793-46A1-B89B-23311FBFBAB5 |
|
treatment provided by |
Tatiana |
|
scientific name |
Stiphodon maculidorsalis |
| status |
sp. nov. |
Stiphodon maculidorsalis View in CoL , new species
( Figs. 1 View Fig , 8–10 View Fig View Fig View Fig , Tables 2, 3)
Material examined. — Holotype: MZB 17213 (male, 43.7 mm SL), South Painan, West Sumatra Province, Sumatra, donated by T. Sim , Sep.2004.
Paratypes: West Sumatra Province (1 male and 3 females): ZRC 51822 (1 male, 47.0 mm SL; 3 females, 37.2–40.3 mm SL), collected with holotype. Bengkulu Province (1 male and 6 females): ZRC 51836 (3 females, 49.8–54.8 mm SL), aquarium trade in Singapore (from Bengkulu), donated by Qian Hu, 22 Jan.2009 ; ZRC 51445 (1 male, 25.4 mm SL; 3 females, 28.1–32.5 mm SL), aquarium trade in Singapore (from South Bengkulu ), coll. H. H. Tan, 18 Mar.2008 .
Non-type material: Aceh Province (5 males and 4 females): ZRC 54183 (1 male, 35.0 mm SL), Kreung Susoh , Aceh Barat , coll. H. H. Ng et al., Jun.2010 ; ZRC 54185 (4 males, 38.8–42.9 mm SL; 4 females, 34.7–37.8 mm SL), Seunaloh, Kreung Sosoh , Aceh Barat , coll. H. H. Ng et al., Jun.2010 .
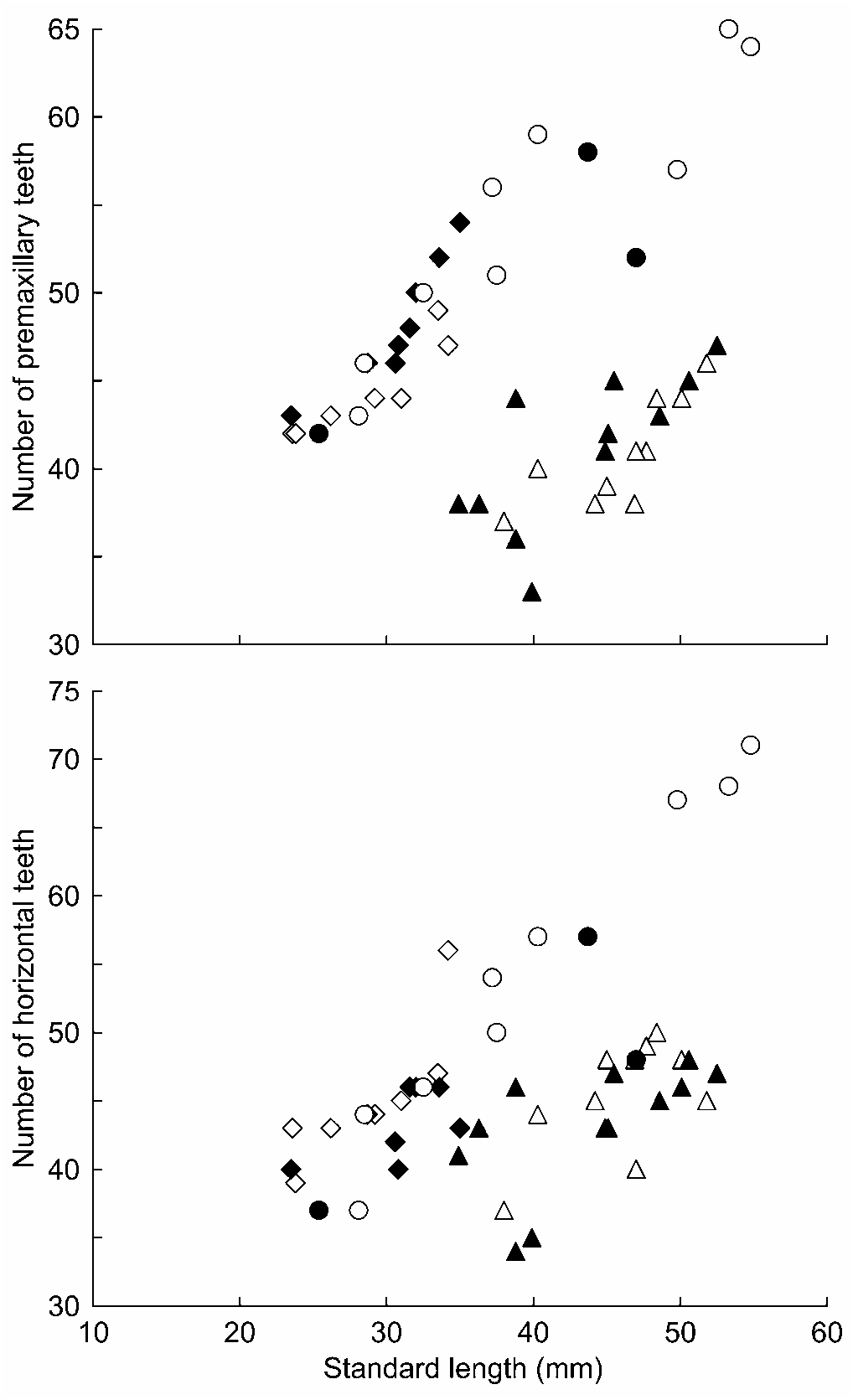
Diagnosis. — The new species is distinguished by the following character combinations: Number of soft-rays in second dorsal fin usually 9, pectoral fin usually 15; male having pointed first dorsal fin with elongate spines 4 and 5; relatively high tooth-counts (premaxillary teeth 42–46 in <30.0 mm SL; 51–56 in 30.0– 39.9 mm SL; 52–59 in 40.0– 49.9 mm SL; 64–65 in ≥ 50.0 mm SL); dentary with canine-like symphyseal teeth in both sexes; male lacking white patch behind pectoral-fin base; anterior half of nape almost naked in male; most of nape scaled and most of occipital region naked in female. Colourations in both male and female are very unique and also distinguish it from all congeners, such as black spots scattering dorsally on head and trunk of male and female, broad black bands on distal part of second dorsal fin and dorsal part of caudal fin of male; fine black spots on pectoral-fin rays of male; dusky transverse bars laterally on trunk and tail of female.
Description. — Morphometric measurements are given in Table 3. Body elongate, cylindrical anteriorly and somewhat compressed posteriorly. Head somewhat depressed with a round snout protruding beyond upper lip. Anterior nostril short tubular, posterior nostril not tubular. Mouth inferior with upper jaw projecting beyond lower jaw. Upper lip thick and smooth with small, medial cleft. Premaxillary teeth 42–65, fine and tricuspid. Dentary with canine-like symphyseal teeth, number of teeth 3 or 4 in larger males, 2 in smallest male (25.4 mm SL), usually 1 or 2 in females (but 3 in one female); dentary with a row of unicuspid horizontal teeth (37–71) enclosed in a fleshy sheath. Larger fish having more premaxillary and horizontal teeth ( Fig. 1 View Fig ). Urogenital papilla in male rectangular, posterior edge with some faint projections, not smooth; female rectangular or somewhat rounded often with two small projections at both sides of tip. but 24% of SL in smallest male, 22–24% of SL in female). Pectoral fin with 15 (n = 10) or 16 (n = 2) rays. Pelvic fin I, 5, paired fins joined together to form a strong cup-like disk with fleshy frenum.
Scales in longitudinal row 30–35 ( Table 2); scales in transverse row 10 (n = 3) or 11 (n = 9); scales in transverse row in caudal peduncle 9. Anterior half of nape almost naked in male ( Fig. 8a View Fig ); most of nape scaled in female, some scales occurring on posterior part of occipital region and the rest of occipital region usually naked ( Fig. 8b View Fig ), but sometimes a few scales on middle of occipital region. Scales on nape and occipital region usually cycloid, but sometimes some weak ctenoid scales occur posteriorly. Ctenoid scales covering Dorsal fins VI-I, 9 (n = 9) or VI-I, 10 (n = 3); in female, first dorsal fin almost semicircular and spine 2 or 3 longest; in male, first dorsal fin forming parallelogram with spines 3–5 elongate but not filamentous, except smallest male of which first dorsal-fin shape similar to female. Most posterior points of first dorsal fin of larger males (tip of spine 4 or 5) extending to base of soft-ray 4 or 5 of second dorsal fin when depressed. Anal fin I, 10 (n = 11) or I, 11 (n = 1), below second dorsal fin. In female, anterior rays (usually soft-ray 1 or 2 in second dorsal fin, soft-ray 2 or 3 in anal fin) longest in second dorsal and anal fins; in male, posterior rays longer than anterior rays (last or next to last ray longest) except for smallest male. Caudal fin with 13 (n = 11) or 14 (n = 1) branched rays within 17 segmented rays, posterior margin rounded or somewhat truncated; male with larger fin than female (caudal-fin length 27–30% of SL in larger males almost entire tail and trunk, but belly covered by cycloid scales. Pectoral-fin base naked. Small gap between posterior side of pectoral-fin base and anterior terminal of scaled area on lateral sides of trunk; some of most-anterior scales on lateral sides of trunk cycloid. Cycloid scales also occurring along second dorsal- and anal-fin base, and proximal part of caudal fin.
Cephalic sensory pore system always A, B, C, D, F, H, K, L, N, and O; pore D singular, all others paired ( Fig. 9 View Fig ). Oculoscapular canal separated into anterior and posterior canals between pores H and K. Cutaneous sensory papillae developed over lateral and dorsal surface of head ( Fig. 9 View Fig ).
Colour in preservation. — Sexual dichromatism well developed. The two larger males (43.7 and 47.0 mm SL) and smallest male (25.4 mm SL) exhibit different colouration, thus they are described separately below.
Larger males ( Fig. 10a View Fig ). Background of body and head pale brown; many black spots scattered dorsally on head and trunk; trunk and caudal region without other distinct markings, or with 3 dusky transverse bars on trunk and 6 dusky transverse bars on caudal region dorsally and laterally. First dorsal-fin membranes grey, spine 1 with 2–7 black spots, other spines without distinct marking. Distal one third of second dorsalfin rays and membranes black forming broad black band; proximal part grey with 2–4 obscure pale grey spots on each ray and middle part along black band lighter grey. Anal fin entirely greyish. Dorsal part of caudal fin black; translucent longitudinal bar immediately below this black part; middle and ventral part of caudal fin dusky with 9–10 black transverse stripes. Pectoral-fin membranes translucent; rays with fine black spots, number of spots on longest rays (rays 7 and 8) 13 or 14. Proximal part of pelvic fin pale brown, distal part somewhat dusky.
Smallest male ( Fig. 10b View Fig ). Similar to female. Number of spots on longest pectoral-fin rays (rays 7 and 8) 7.
Females ( Fig. 10c–e View Fig ). Background of body and head cream; 3 and 6–7 dusky transverse bars laterally on trunk and tail, respectively, these bars linked with those on other side by obscure dusky dorsal bars; dorsal side of body somewhat dusky; a lot of black spots scattering dorsally on head and trunk; dusky longitudinal band running along lateral midline from behind pectoral-fin base to posterior end of caudal peduncle, but this band often obscure; dusky band on upper lip and along lower margin of snout; dusky longitudinal band extending from infraorbital region to middle of pectoral-fin base, but larger females sometimes lack this band. First and second dorsal-fin membranes transparent, but sometimes pale grey; first dorsal-fin spines dusky with 0–4 translucent spots; second dorsal fin bordered by narrow transparent edge with black band running immediately inside of this transparent margin; 2–4 black spots along each of proximal two thirds of second dorsal-fin spine and soft-rays. Anal fin pale grey without clear markings or with black band running near its margin. Black rectangular blotch usually at centre of proximal part of caudal fin; black band (upside-down “L” shape) along dorsal and posterior margin of caudal fin with transparent border; 3–6 black spots on 7–9 central caudalfin rays often forming transverse bars, membrane mostly transparent. Pectoral-fin rays with black spots, number of spots on longest rays (rays 7 and/or 8) usually 5–9, but 4 in smaller females (28.1 and 28.5 mm SL, n = 2); membranes transparent. Pelvic fin translucent without pigment.
Etymology. — The name for the new species is from the combination of the Latin words maculosus, meaning spotted, and dorsalis, meaning dorsal, referring to unique spotted dorsum on head and trunk in both sexes. The new specific name is treated as an adjective.
Distribution. — The specimens of this new species were collected from Bengkulu, West Sumatra, and Aceh Provinces, Sumatra. Currently, no information about occurrence of this species from other places is known.
Remarks. — The smallest specimen (25.4 mm SL) is identified as male because it has few scales on nape and has more black spots on pectoral-fin rays than females of same size-class (<30 mm SL). This male is considered to be an immature juvenile (see remarks for S. semoni ).
The new species resembles S. multisquamus Wu & Ni, 1986 and S. aureorostrum Chen & Tan, 2005 . They have similar meristic characters, scalation, dusky transverse bars laterally on trunk and tail of female, and fine black spots on pectoralfin rays of male (Wu & Ni, 1986; Chen & Tan, 2005; Wu & Zhong, 2008; Nip, 2010). But the new species differs from S. multisquamus and S. aureorostrum in having black spots scattering dorsally on head and trunk of male and female and broad black bands on distal part of second dorsal fin and dorsal part of caudal fin of male. Stiphodon ornatus , S. atratus , S. imperiorientis , S. martenstyni , S. pelewensis , S. pulchellus , and S. weberi have similar fin-ray counts and first dorsal-fin shape in male with S. maculidorsalis (see remarks for S. ornatus ), but premaxillary teeth counts of these species are lower than that of S. maculidorsalis (except for S. martenstyni ) and their colourations of male and female are completely different.
Stiphodon gobies in aquarium trade. — More than half of the specimens of all three Stiphodon species examined in the present study were obtained from aquarium trade in Singapore. Stiphodon gobies are often sold commercially as ornamental fish (e.g., Delventhal, 2003; Mukai, 2011). The three species ( S. ornatus , S. semoni , and S. maculidorsalis ) are common and found in many pet shops often selling them on the Internet. Because S. ornatus and S. maculidorsalis have been reported only from Bengkulu, West Sumatra, and Aceh Provinces, the western slope of Sumatra is believed to be the main source of Stiphodon for the aquarium trade. There exist no expertise for the captive breeding of Stiphodon species due to difficulty in feeding to their small larvae and the long pelagic larval duration (Yamasaki & Tachihara, 2006; Yamasaki et al., 2007; Maeda & Tachihara, 2010). Therefore, all aquarium Stiphodon species should be collected from the wild.
Live Stiphodon gobies are captured for the aquarium trade from the hill stream habitats in Western Sumatra (THH, pers. obs.; see Tan, 1999, for more habitat details). Two methods are commonly used to collect riparian gobies including Stiphodon species. The first involves bending down and immersing the head with goggles and visually targeting individual gobies and scooping them using a deep but small mouthed hand net. This method is tedious and involves many hours in high velocity cold water. The second method involves three or more people using a seine net with a heavy metal chain bottom. Two persons drag this net along the rocky bottom, and one or two other person(s) at the front of the net chase fishes into the net. The fishes are then bagged and sent to a middle man who will accumulate sufficient numbers before sending to an exporter. Larger sicydiine gobies are also caught using electricity or seine net and sold as food fish, and commonly observed stringed up by the road side for sale. These larger sicydiine gobies (usually Sicyopterus ) are gutted and deep fried before consumption. They are sometimes encountered in the aquarium trade as well.
This collection of Stiphodon from the wild may not be sustainable in the long-term if brood stock is not monitored closely or the waterways and adjacent habitats become polluted. As the larvae of Stiphodon require a marine phase which will migrate back to the freshwater system, this is the most vulnerable stage at which any physical or chemical barrier will impose detrimental effects on future population. Currently, most of the lowland coastal zone in western part of Sumatra is undergoing urbanisation and modification for crop planting (THH, pers. obs.). Already in 1999, feral populations of Amatitlania nigrofasciatum (Cichlidae) , Oreochromis mossambicus (Cichlidae) and Poecilia reticulata (Poeciliidae) had been observed in a hill stream habitat near Painan, West Sumatra ( Tan, 1999).
Stiphodon species in the aquarium trade are usually not identified correctly as well as one of them is named in the present study. We hope that information of this study could provide basic taxonomic knowledge for proper management and conservation of wild Stiphodon populations in Sumatra.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |